Research Article Open Access
Quantification of Sugar Epimers in Polygalactomannans by ESI-MS/MS
Erika Ponzini1, Greta Borgonovo1, Luca Merlini2, Yves M Galante2, Carlo Santambrogio1* and Rita Grandori1*
1Department of Biotechnology and Biosciences, University of Milano-Bicocca, Piazza della Scienza 2, 20126, Milan, Italy
2Istituto di Chimica del Riconoscimento Molecolare, CNR, Via Mario Bianco 9, 20131, Milan, Italy
- *Corresponding Author:
- Carlo Santambrogio
Department of Biotechnology and Biosciences
University of Milano-Bicocca Piazza della Scienza 2
20126 Milan, Italy
Tel: 39-02-64483363
E-mail: carlo.santambrogio@unimib.it
- Rita Grandori
Department of Biotechnology and Biosciences
University of Milano-Bicocca
Piazza della Scienza 2, 20126 Milan, Italy
Tel: 39-02-64483363
E-mail: rita.grandori@unimib.it
Received date: September 03, 2015; Accepted date: September 25, 2015; Published date: October 02, 2015
Citation: Ponzini E, Borgonovo G, Merlini L, Galante YM, Santambrogio C (2015) Quantification of Sugar Epimers in Polygalactomannans by ESI-MS/MS. J Anal Bioanal Tech 6:281. doi:10.4172/2155-9872.1000281
Copyright: © 2015 Ponzini E, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
Polygalactomannans (PGMs) represent an important family of polysaccharides. They are obtained from the endosperm of leguminous plant seeds and are employed in a growing number of industrial applications as thickening agents and rheology modifiers. Each PGM has a typical mannose/galactose (M/G) ratio (from ~1 to ~5), which determines its solubility, viscosity and other physico-chemical properties. Complex polysaccharides are commonly characterized by proton nuclear magnetic resonance (1H-NMR), whereas mass spectrometry (MS) has not yet seen wide application. In this work, a new quantification method for sugar epimers in PGMs is presented, based on the characteristic fragmentation patterns of mannose and galactose in tandem MS (MS/MS) analyses. Standard galactose and mannose have been analyzed and fragmented in negative-ion mode on a hybrid quadrupole/time-of-flight (qTOF) mass spectrometer equipped with a nano electrospray ionization (ESI) source. The MS/MS spectra indicate accumulation of the same fragments for the two monosaccharides, but significant and reproducible differences in the relative intensities of the product ions. Known mixtures of mannose and galactose have been analyzed by the same procedure to test the applicability of the method for quantification purposes. The resulting peak intensities over the entire MS/MS spectrum can be deconvoluted as a linear combination of the signals from pure mannose and galactose standards, obtaining reliable and reproducible quantification of the epimers. The method has been applied to the characterization of hydrolysis products of PGMs from different species of leguminous plants, such as guar (Cyamopsis tetragonolobus), sesbania (Sesbania bispinosa) and tara (Caesalpinia spinosa), in order to assess specific susceptibility to hydrolysis conditions.
Keywords
Polygalactomannans; Electrospray-ionization mass spectrometry; Monosaccharides quantification; Collision-induced dissociation; Acidic hydrolysis
Introduction
Polysaccharides from animal (e.g., chitin, chitosan and hyaluronan), vegetal (e.g., cellulose, starch and pectin) and microbial (e.g., xanthan, gellan and curdlan) organisms are emerging as attractive materials in several industrial fields. They are employed as emulsion stabilizers, rheology modifiers, coating agents etc. in food, textile, cosmetic, biomedical and pharmaceutical branches [1-5]. Thanks to their nontoxic, biodegradable and renewable origin and their amenability to chemical and biochemical modifications, these compounds represent a promising alternative to synthetic polymers for the development of new functionalized materials [6-8].
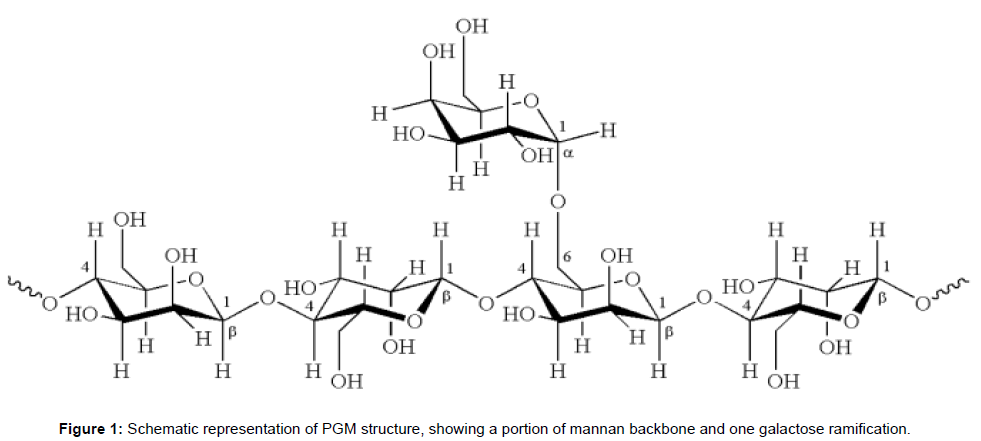
An important group of polysaccharides is represented by polygalactomannans (PGMs), mainly obtained from the endosperm of the seeds of leguminous plants, where they represent a reserve source for carbon and energy upon germination [9]. Nowadays, the most widely employed PGM for industrial purposes is obtained from the guar plant (Cyamopsis tetragonolobus), mainly cultivated in India [10]. Other important, but commercially less exploited PGMs are extracted from locust bean (Ceratonia siliqua) and fenugreek (Trigonella foenum-graecum), spontaneous plants of the Mediterranean regions, from tara (Caesalpinia spinosa), native to South America, and from sesbania (Sesbania bispinosa), native to Asia and Africa [9,11]. PGMs are high-molecular weight polymers of about 1-2 MDa [12,13]. They are normally composed of a β(1→4)-D-mannan backbone with several α(1→6) branches of single D-galactose units (Figure 1), although more complex ramifications have been described [6]. The interest in these polymers is mainly due to the possibility of obtaining solutions with unique rheological properties, like extreme viscosities and non- Newtonian behavior [13-15]. PGMs are often subjected to chemical reactions (e.g., hydroxyalkylation, carboxymethylation) [16,17] or enzymatic treatments (e.g., depolymerization by β-mannanase, oxidation by laccase or galactose oxidase) [18-20], in order to modify their solubility and viscoelastic properties, or to obtain hybrid, functionalized materials [21,22].
PGMs from distinct leguminous species generally differ in structural properties, like the amount and distribution of galactose ramifications along the backbone, resulting in different physico-chemical properties of the PGMs solutions [13]. Mannose/galactose (M/G) ratio can vary from ~1 to ~5 [6,9,14,23], affecting structural properties and solubility of the polymer. The abundance of cis-OH groups in the mannan backbone induces a strong tendency to form inter-chain hydrogen bonds, promoting precipitation and poor solubility in polar solvents. The presence of galactose ramifications enhances solubility preventing such interactions by steric hindrance. Therefore, PGMs with low M/G (i.e., with high galactose content) usually dissolve easier than others, leading to the formation of viscous, non-Newtonian fluids. For example, locust bean gum (M/G ~4) requires a boiling procedure to reach full hydration, while fenugreek (M/G ~1) and guar (M/G ~2) gums are highly soluble even in cold water [9,10]. Thanks to the nonionic nature of PGMs, the viscosity of the resulting solutions is almost constant over a wide range of temperature and pH [6,10]. The degree of galactose substitutions also affects the propensity of the polymer to form gels, although the relation to the M/G ratio is not straightforward and is strongly dependent on environmental conditions [13,24].
Computational simulations suggest that the M/G ratio is a key factor also for PGMs conformational properties [25-27]. Galactose substitutions promote chain bending via intramolecular H-bonding, favoring compact conformations and affecting structural properties like persistence length and gyration radius. As a result, PGMs with low M/G are predicted to be more compact and flexible than the variants with high M/G [27]. Experimental data on the radius of gyration are in agreement with the trend suggested by computational modeling [13,27].
Other factors, such as the pattern of galactose substitutions, have also been suggested to affect the conformational properties of PGMs [25,27]. This “fine structure” of the polymers seems to vary even among variants of the same species [28]. Different patterns have been described, such as periodic [6,28], random [28-30] or hybrid [28,31].
Several experimental techniques have been applied to the study of the physico-chemical properties of PGMs. Size-exclusion chromatography is usually employed for the estimation of the molecular-weight distributions [13,32], while 13C-NMR combined with enzymatic hydrolysis are used for the determination of the galactose distribution on the backbone [28,33-36]. The M/G ratios are commonly obtained by gas chromatography, liquid chromatography or 1H-NMR, on intact or hydrolyzed PGMs [13,37-39]. Mass spectrometry (MS) has seen only limited application to PGM analysis [20,40-45], although it has a strong potential as an approach complementary to other techniques for the characterization of complex mixtures.
In this work, a new approach to M/G quantification, based on tandem electrospray-ionization mass spectrometry (ESI-MS/MS), is proposed. The method exploits the distinct fragmentation patterns of mannose and galactose when subjected to collision-induced dissociation (CID) with an inert gas [46]. The method is applied here to the analysis of different PGMs, in order to characterize the depolymerization products and to assess the specific susceptibility to hydrolysis conditions.
Materials and Methods
Analysis of mannose and galactose standard solutions by mass spectrometry
D-(+)-mannose (Carbosynth, Compton, UK) and D-(+)-galactose (Sigma Aldrich, St. Louis, MO, USA) were dissolved in milliQ water at a final concentration of 10 μM. The stock solutions were mixed together obtaining different M/G ratios (0.11, 0.43, 1.00, 2.33 and 9). Pure mannose and galactose solutions and their mixtures were freshly prepared before each ESI-MS analysis. The samples were infused by borosilicate-coated capillaries of 1 μm internal diameter (Thermo Fisher Scientific, Waltham, MA USA) into a QSTAR-Elite hybrid quadrupole/time-of-flight (qTOF) instrument equipped with a nanoelectrospray ion source (AB Sciex, ForsterCity, CA, USA). MS and MS/ MS measurements were performed both in positive- and negative-ion mode, employing the instrumental settings indicated in the Results section. MS/MS spectra were obtained by CID of singly charged ions with molecular nitrogen. The recorded spectra were averaged over 1 minute acquisition time.
Quantification of M/G from ESI-MS/MS data
The relative intensity of each peak in MS/MS spectra can be interpreted as the sum of the corresponding peaks generated by pure mannose and galactose, weighted by the fractional amount of the sugars in the sample:
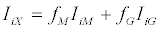 (1)
(1)
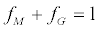 (2)
(2)
where IiX is the intensity of the generic fragment peak from the mixture, IiM and IiG are the intensities of the same peak in pure mannose and galactose samples, and fM and fG are the sugar fractional amounts. The above model is based on the assumption that mannose and galactose ions from the same sample have identical fragmentation propensities. The fG value was estimated by combining equations (1) and (2) and minimizing the following function:
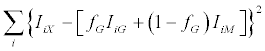 (3)
(3)
The sum was extended over the 10 most intense peaks of the MS/ MS spectrum. Setting the first derivative of the function (3) to zero, the fraction fG can be expressed as:
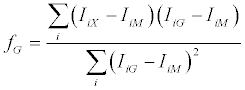 (4)
(4)
The M/G ratio is then given by:
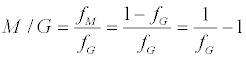 (5)
(5)
PGMs chemical hydrolysis
Flours from guar (Cyamopsis tetragonolobus), tara (Caesalpinia spinosa) and sesbania (Sesbania bispinosa) (industrial stocks from Lamberti SpA, Albizzate, Italy) employed in this work have a PGM content of 85-90%, and a Brookfield viscosity of ~5000, ~4500 e ~3000 mPa/s, respectively, in 1% (w/v) aqueous solutions. PGMs were purified by dispersing 30 g of flour in a 7:3 ethanol:water mixture (v/v) and vacuum-filtering the solution by a Büchner funnel. The resulting powder was resuspended in acetone, filtered again, and dried for 12 h at 65°C. After purification, 1.5 mg of PGM was dissolved in 300 mL of milliQ water and the pH was lowered below 2 by the addition of an acidic solution (0.5 M HCl, 26.5 M HCOOH or 13.5 M CF3COOH, according to the chosen protocol). Chemical hydrolysis was performed by autoclaving the solution at 121°C for 1.5 h. Right after the incubation in the autoclave, the solution was cooled on ice for 30 min, and neutralized by the addition of NaOH. Insoluble material was removed by centrifugation before MS analysis.
Results
Mannose and galactose fragmentation properties in the gasphase
Aqueous solutions of standard mannose and galactose were analyzed by ESI-MS in the two different ionization polarities. In positive ion-mode, the spectra of either monosaccharide are dominated by sodiated molecular ions, with only barely visible peaks corresponding to protonated species. CID experiments on sodium adducts do not lead to sugars fragmentation at any value of collision energy (CE), indicating a mere loss of the Na+ ion during the collisions with the inert gas (data not shown), in agreement with previous reports on the MS/ MS behavior of pentoses and hexoses in positive ion-mode [46].
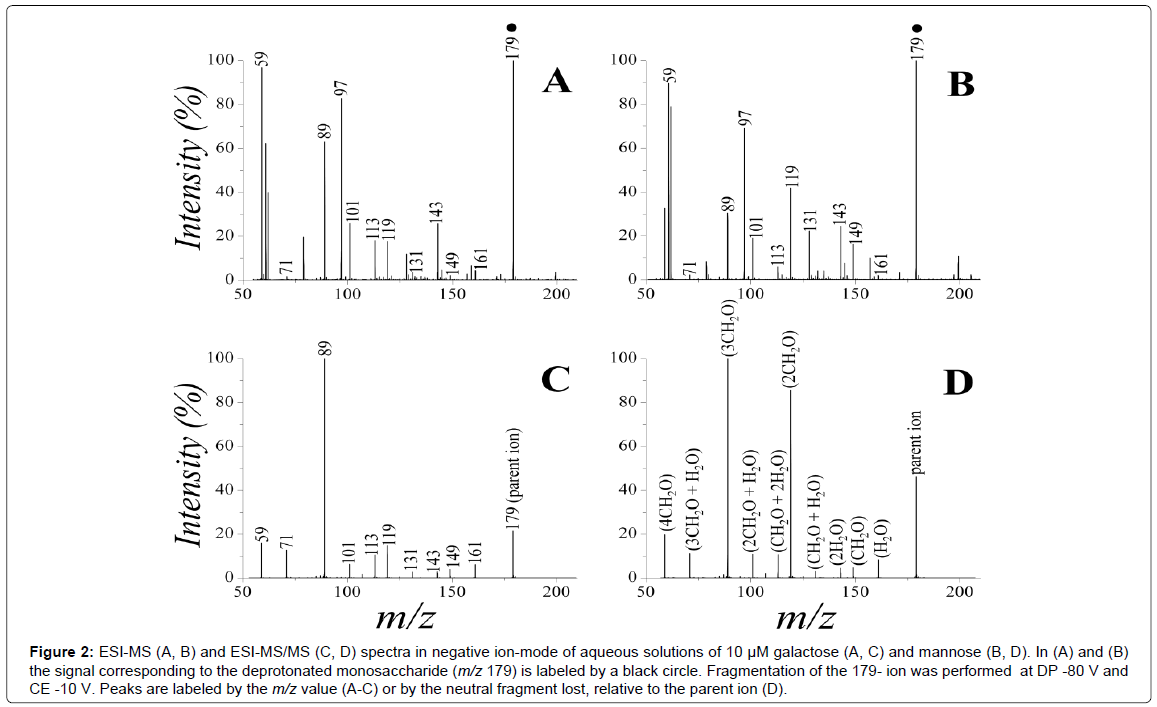
In negative ion-mode, the prevalent peak corresponds to the deprotonated form of the sugars (m/z 179) (Figure 2A and 2B). Although the isomeric nature of the two monosaccharides gives rise to identical signals, the MS/MS spectra are different for mannose or galactose (Figure 2C and 2D), due to different susceptibility of the sugars to competing fragmentation pathways [46]. The fragments obtained by CID are the same in the two cases, and result from the neutral loss of water and/or formaldehyde (Table 1 and Figure 2D) [46]. The relative intensities of the corresponding peaks, however, are dependent on the sugar nature. The main distinction for the two epimers is represented by the propensity to lose two formaldehyde molecules (producing an ion at m/z 119), that is highly pronounced for mannose and quite low for galactose. Besides this major difference, other minor variations in the fragmentation patterns are also observable. These differences in the fragmentation patterns can be exploited for deconvolution of the spectra of sugar mixtures.
Figure 2: ESI-MS (A, B) and ESI-MS/MS (C, D) spectra in negative ion-mode of aqueous solutions of 10 μM galactose (A, C) and mannose (B, D). In (A) and (B) the signal corresponding to the deprotonated monosaccharide (m/z 179) is labeled by a black circle. Fragmentation of the 179- ion was performed at DP -80 V and CE -10 V. Peaks are labeled by the m/z value (A-C) or by the neutral fragment lost, relative to the parent ion (D).
| m/z | Elemental composition | Neutral loss |
|---|---|---|
| 179 | C6H11O6- | - |
| 161 | C6H9O5- | H2O |
| 149 | C5H9O5- | CH2O |
| 143 | C6H7O4- | 2H2O |
| 131 | C5H7O4- | CH2O + H2O |
| 119 | C4H7O4- | 2CH2O |
| 113 | C5H5O3- | CH2O + 2H2O |
| 101 | C4H5O3- | 2CH2O + H2O |
| 89 | C3H5O3- | 3CH2O |
| 71 | C3H3O2- | 3CH2O + H2O |
| 59 | C2H7O2- | 4CH2O |
Table 1: Peak assignment for the MS/MS spectra (parent ion m/z 179).
Quantification of mannose and galactose content in reference mixtures
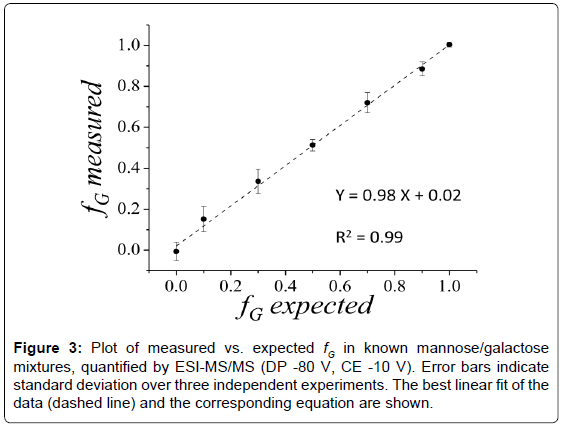
Known mixtures of mannose and galactose have been analyzed by ESI-MS/MS in the negative ion-mode by fragmentation of the 179- ion. The fractional amount of galactose (fG) has been estimated by applying equation (4) on the 10 most intense fragmentation peaks. The effectiveness of the quantification method has been evaluated by linear regression on measured vs. expected fG plots (Figure 3).
The influence of instrumental settings has been explored by varying capillary voltage (CV), declustering potential (DP) and collision energy (CE) systematically and independently. The conditions that minimize the quantification error were IS -1.1 kV, DP -80 V and CE -10 V (Figure 3). CV does not have a significant effect on fG estimation (data not shown). On the contrary, DP influences the quantification results (Figure S1A (supplementary material)), leading to an overestimation of galactose at low voltage values (i.e., -60 V or below). This behavior could be ascribed to different properties of the sugars in desolvation, declustering or in-source dissociation, affecting the transmission efficiency of the ions into the collision cell. Both sugars are prone to in-source dissociation under the here employed experimental conditions, as indicated by the spectra reported in Figure 2A and 2B. The parameter that mostly affects the quantification results is CE (Figure S1B and S1C), inducing biases at either low (i.e., -7.5 V and below) or high (i.e., -15 V and above) values. Low CE values result in poor fragmentation, while high CE values lead to preferential accumulation of the smaller fragments. In either case, quantification is hindered by the lack of several diagnostic peaks. Nevertheless, Figure 3 shows that reliable quantification is obtained upon optimization of the instrumental parameters on standard mixtures. The procedure displays good reproducibility, yielding relative small standard deviations over three independent sets of measurements on freshly prepared samples (Figure 3).
Analysis of PGMs acidic hydrolysis by mass spectrometry
The method described in the previous sections has been applied to the characterization of the products obtained by chemical hydrolysis of three PGMs: guar, tara and sesbania. Canonical protocols for acidic hydrolysis of polysaccharides at high temperature have been employed [47-50] in order to obtain a complete depolymerization of the PGMs in their monosaccharide constituents.
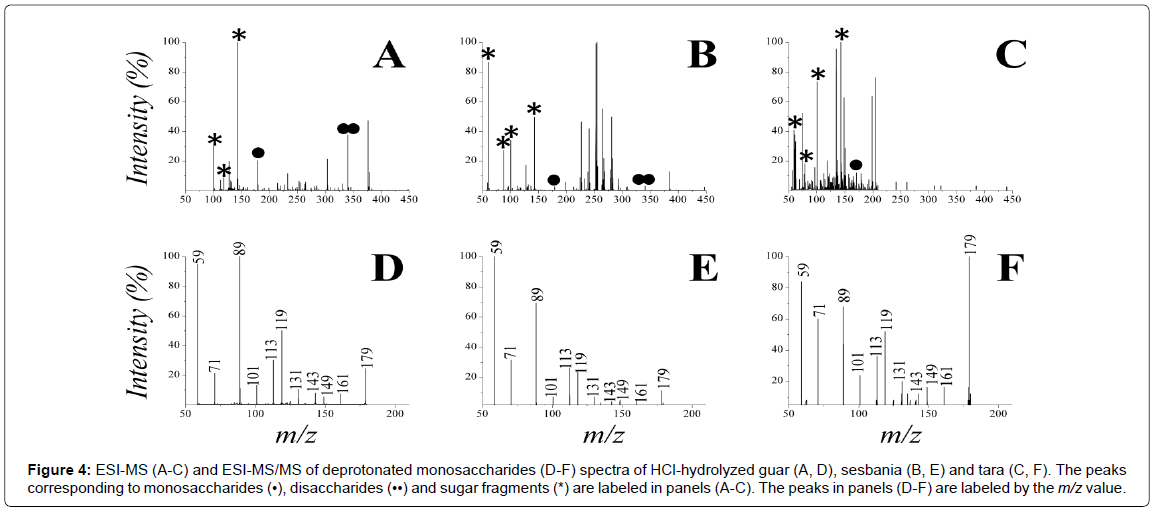
The analysis of the HCl-hydrolyzed PGMs by ESI-MS in negative ion-mode (Figure 4) shows the presence of a peak at m/z 179 for all the three samples, in agreement with accumulation of free mannose and/or galactose. However, the peak corresponding to disaccharides (m/z 341) is also present in the spectra (Figure 4A and 4B), suggesting that the hydrolysis process is highly advanced but not fully complete. The MS/ MS data for the 179- ion from the three samples are shown in Figure 4D- 4F and the quantification results are summarized in Table 2. The results reflect the known ranking in galactose content (tara<guar<sesbania) and display a fairly good agreement, especially for tara, with the M/G values reported in the literature, as assessed by other techniques.
Figure 4: ESI-MS (A-C) and ESI-MS/MS of deprotonated monosaccharides (D-F) spectra of HCl-hydrolyzed guar (A, D), sesbania (B, E) and tara (C, F). The peaks corresponding to monosaccharides (•), disaccharides (••) and sugar fragments (*) are labeled in panels (A-C). The peaks in panels (D-F) are labeled by the m/z value.
| Leguminous species | Measured M/G | M/G range from literature | References |
|---|---|---|---|
| Guar (Cyamopsis tetragonolobus) | 0.96 ± 0.08 | 1.4-1.6 | [14,23] |
| Sesbania (Sesbania bispinosa) | 0.79 ± 0.13 | 1.3-1.5 | [14] |
| Tara (Caesalpinia spinosa) | 2.33 ± 0.22 | 2.5-3.0 | [14] |
Table 2: Estimation of the M/G ratio in different PGMs by HCl hydrolysis and ESI-MS/MS. The range of M/G values from the literature, obtained by different techniques, is given for comparison.
On the other hand, a systematic underestimation of M/G seems to emerge from the data. This bias could be ascribed to incomplete hydrolysis, together with a higher susceptibility of galactose to the depolymerization conditions employed here. Due to exclusive localization of galactose in the ramifications of the PGM structure, it is conceivable that these monomers are released preferentially under suboptimal depolymerization conditions. The discrepancy between expected and measured M/G ratios becomes larger as the galactose content in the PGMs increases (i.e., M/G decreases) (Table 2), consistent with a stronger screening-effect on mannoses and/or higher availability of galactose ramifications.
Guar PGM has also been hydrolyzed by formic or trifluoroacetic acids, in order to compare different depolymerization protocols. The resulting M/G values are in keeping with those obtained by HCl (0.89 ± 0.11 with formic and 0.82 ± 0.16 with trifluoroacetic acid). Chloridric acid seems to be the most suitable acid for PGMs hydrolysis, followed by formic acid and trifluoroacetic acid.
Discussion
The distinct fragmentation properties of mannose and galactose enable deconvolution of MS/MS spectra to the end of epimer quantification from given mixtures. The method represents an interesting alternative or complementation to the techniques typically employed to this purpose (e.g., NMR and chromatography). MS is attractive in terms of speed and sensitivity and being independent of sugar derivatization. The protocol is not limited to mannose and galactose quantification, and is virtually applicable to any monosaccharides presenting detectable differences in gas-phase fragmentation, like glucose and fructose [46].
The method has been employed to monitor chemical hydrolysis of PGMs from three leguminous species, leading to M/G estimations that are in fair agreement with the values reported in the literature and reflect the different galactose content characterizing the three flours. Systematic discrepancies, however, suggest that the depolymerization process is not complete, as further evidenced by the presence of disaccharides in the hydrolysis products. It is therefore recommendable to systematically optimize the hydrolysis protocol testing the effects of incubation conditions, such as polymer concentration, temperature, stirring, incubation time type and concentration of the acid. ESIMS and ESI-MS/MS could be further employed to monitor chemical and/or enzymatic depolymerization reactions, in order to optimize hydrolysis conditions and to characterize the response of flours from different species. Another particular issue in the quantitative analysis of hydrolyzed polysaccharides is that mixtures of monosaccharides with vastly different physicochemical properties (i.e., negatively charged, neutral, deoxy etc.) are often obtained [51,52]. This is a challenge for either chromatographic or MS analysis, due to signal dispersion over multiple peaks and/or detection failure for some components of the sample. Further investigation will be needed to assess whether the present method could be extended to monosaccharide degradation products and to test its robustness towards sugar nature.
The applicability of the method is now limited to the quantification of monosaccharides, and does not deliver information on the details of the polysaccharide structure. However, the method might be extended to the analysis of oligosaccharides and the study of galactosesubstitution pattern. Differences in the fragmentation properties of disaccharides have been already described [46], and these features could be exploited for structural investigation of PGMs. Further potential applications involve monitoring and mapping PGMs modifications (e.g., oxidation, methylation, etc.), as a first step for the creation of functionalized polymers and nano-engineered materials.
Acknowledgements
This project was supported by the program “Suschem Lombardia: prodotti e processi chimici sostenibili per l’industria lombarda” Accordo Quadro Regione Lombardia-CNR, 16/07/2012 (Protocol no. 18096/RCC), and by Cariplo Foundation grant 2014-0478.
References
- Krishnaiah YS, Karthikeyan RS, Gouri Sankar V, Satyanarayana V (2002) Three-layer guar gum matrix tablet formulations for oral controlled delivery of highly soluble trimetazidine dihydrochloride. J Control Release 81: 45-56.
- Vieira IGP, Mendes FNP, Gallao MI, Brito ES (2007) NMR study of galactomannans from the seeds of mesquite tree (Prosopis juliflora (Sw) DC). Food Chemistry 101: 70-73.
- Itzincab-Mejía L, López-Luna A, Gimeno M, Shirai K,Bárzana E (2013) Enzymatic grafting of gallate ester onto chitosan: evaluation of antioxidant and antibacterial activities. Int J Food Sci Technol 48: 2034-2041.
- Kenawy el R, Worley SD, Broughton R (2007) The chemistry and applications of antimicrobial polymers: a state-of-the-art review. Biomacromolecules 8: 1359-1384.
- Das D, Ara T, Dutta S, Mukherjee A (2011) New water resistant biomaterial biocide film based on guar gum. Bioresour Technol 102: 5878-5883.
- Srivastava M, Kapoor VP (2005) Seed galactomannans: an overview. Chem Biodivers 2: 295-317.
- Rinaudo M (2008) Main properties and current applications of some polysaccharides as biomaterials. Polym Int 57: 397-430.
- Salwiczek M, Qu Y, Gardiner J, Strugnell RA, Lithgow T, et al. (2014) Emerging rules for effective antimicrobial coatings. Trends Biotechnol 32: 82-90.
- Prajapati VD, Jani GK, Moradiya NG, Randeria NP, Nagar BJ, et al. (2013) Galactomannan: a versatile biodegradable seed polysaccharide. Int J Biol Macromol 60: 83-92.
- Mudgil D, Barak S, Khatkar BS (2014) Guar gum: processing, properties and food applications-A Review. J Food Sci Technol 51: 409-418.
- Pollard MA, Fisher P, Windhab EJ (2011) Characterization of galactomannans derived from legume endosperms of genus Sesbania (Faboideae). Carbohydr Polym 84: 550-559.
- Cerqueira MA, Pinheiro AC, Souza BWS, Lima AM, Ribeiro C, et al. (2009) Extraction, purification and characterization of galactomannans from non-traditional sources. Carbohydr Polym 75: 408-414.
- Merlini L, Boccia AC, Mendichi R, Galante YM (2015) Enzymatic and chemical oxidation of polygalactomannans from the seeds of a few species of leguminous plants and characterization of the oxidized products. J Biotechnol 198: 31-43.
- Daas PJ, Grolle K, Van Vliet T, Schols HA, De Jongh HH (2002) Toward the recognition of structure-function relationships in galactomannans. J Agric Food Chem 50: 4282-4289.
- Sittikijyothin W, Torres D, Goncalves MP (2005) Modelling the rheological behaviour of galactomannan aqueous solutions. Carbohydr Polym 59: 339-350.
- Cheng Y, Prudhomme RK, Chick J, Rau DC (2002) Measurement of forces between galactomannan polymer chains: effect of hydrogen bonding. Macromolecules 35: 10155-10161.
- Risica D, Dentini M, Crescenzi V (2005) Guar gum methyl ethers. Part I. Synthesis and macromolecular characterization. Polymer 46: 12247-12255.
- Lavazza M, Formantici C, Langella V, Monti D, Pfeiffer U, et al. (2011) Oxidation of galactomannan by laccase plus TEMPO yields an elastic gel. J Biotechnol 156: 108-116.
- Cheroni S, Gatti B, Margheritis G, Formantici C, Perrone L, et al. (2012) Enzyme resistance and biostablity of hydroxyalkylated cellulose and galactomannan as thickeners in waterborne paints. Int Biodeterior Biodegrad 69: 106-112.
- Parikka K, Leppänen AS, Pitkänen L, Reunanen M, Willför S, et al. (2010) Oxidation of polysaccharides by galactose oxidase. J Agric Food Chem 58: 262-271.
- Gliko-Kabir I, Yagen B, Baluom M, Rubinstein A (2000) Phosphated crosslinked guar for colon-specific drug delivery. II. In vitro and in vivo evaluation in the rat. J Control Release 63: 129-134.
- Cunha PL, Castro RR, Rocha FA, de Paula RC, Feitosa JP (2005) Low viscosity hydrogel of guar gum: preparation and physicochemical characterization. Int J Biol Macromol 37: 99-104.
- Crescenzi V, Dentini M, Risica D, Spadoni S, Skjåk-Braek G, et al. (2004) C(6)-oxidation followed by C(5)-epimerization of guar gum studied by high field NMR. Biomacromolecules 5: 537-546.
- Bresolin TM, Milas M, Rinaudo M, Reicher F, Ganter JL (1999) Role of galactomannan composition on the binary gel formation with xanthan. Int J Biol Macromol 26: 225-231.
- Petkowicz CLO, Reicher F, Mazeau K (1998) Conformational analysis of galactomannans: from oligomeric segments to polymeric chains. Carbohydr Polym 37: 25-39.
- Wu Y, Cui W, Eskin NAM, Goff HD (2009) An investigation of four commercial galactomannans on their emulsion and rheological properties. Food Res Int 42: 1141-1146.
- Wu Y, Li W, Cui W, Eskin NAM,Goff HD (2012) A molecular modeling approach to understand conformation efunctionality relationships of galactomannans with different mannose/galactose ratios. Food Hydrocolloids 26: 359-364.
- Daas PJ, Schols HA, de Jongh HH (2000) On the galactosyl distribution of commercial galactomannans. Carbohydr Res 329: 609-619.
- McCleary BV (1979) Modes of action of beta-mannanase enzymes of diverse origin on legume seed galactomannans. Phytochemistry 18: 757-763.
- McCleary BV, Clark AH, Dea ICM, Rees DA (1985) The fine structures of carob and guar galactomannans. Carbohydr Res 139: 237-260.
- Hoffman J, Svensson S (1978) Studies of the distribution of the d-galactosylside-chains in guaran. Carbohydr Res 65: 65-71.
- Pettolino FA, Hoogenraad NJ, Stone BA (2002) Application of a mannan-specific antibody for the detection of galactomannans in foods. Food Hydrocoll 16: 551-556.
- Gorin PAJ, Iacomini M (1985) Structural diversity of d-galacto-d-mannan components isolated from lichens having ascomycetous mycosymbionts. Carbohydr Res 142: 253-267.
- Taravel FR, Mazeau K, Tvaroska I (1995) Conformational analysis of carbohydrates inferred from NMR spectroscopy and molecular modeling. Applications to the behavior of oligo-galactomannan chains. Braz J Med Biol Res 28: 723-732.
- Joshi H, Kapoor VP (2003) Cassia grandis Linn. f. seed galactomannan: structural and crystallographical studies. Carbohydr Res 338: 1907-1912.
- Cunha PLR, Vieira IGP, Arriaga AMC, de Paula RCM, Feitosa JPA (2009) Isolation and characterization of galactomannan from Dimorphandra gardneriana Tul seeds as a potential guar gum substitute. Food Hydrocoll 23: 880-885.
- Ganter JL, Heyraud A, Petkowicz CL, Rinaudo M, Reicher F (1995) Galactomannans from Brazilian seeds: characterization of the oligosaccharides produced by mild acid hydrolysis. Int J Biol Macromol 17: 13-19.
- Ramesh HP, Yamaki K, Ono H, Tsushida T (2001) Two-dimensional NMR spectroscopic studies of fenugreek (Trigonella foenum-graecum L.) galactomannan without chemical fragmentation. Carbohydr Polymers 45: 69-77.
- Muschin T, Yoshida T (2012) Structural analysis of galactomannans by NMR spectroscopy. Carbohydr Polymers 87: 1893-1898.
- Guo S, Mao W, Yan M, Zhao C, Li N, et al. (2014) Galactomannan with novel structure produced by the coral endophytic fungus Aspergillus ochraceus. Carbohydr Polym 105: 325-333.
- Simões J, Maricato E, Nunes FM, Domingues MR, Coimbra MA (2014) Thermal stability of spent coffee ground polysaccharides: galactomannans and arabinogalactans. Carbohydr Polym 101: 256-264.
- Chen Y, Mao W, Wang B, Zhou L, Gu Q, et al. (2013) Preparation and characterization of an extracellular polysaccharide produced by the deep-sea fungus Penicillium griseofulvum. Bioresour Technol 132: 178-181.
- Shang M, Zhang X, Dong Q, Yao J, Liu Q, et al. (2012) Isolation and structural characterization of the water-extractable polysaccharides from Cassia obtusifolia seeds. Carbohydr Polym 90: 827-832.
- Parikka K, Leppänen AS, Xu C, Pitkänen L, Eronen P, et al. (2012) Functional and anionic cellulose-interacting polymers by selective chemo-enzymatic carboxylation of galactose-containing polysaccharides. Biomacromolecules 13: 2418-2428.
- Moreira AS, Coimbra MA, Nunes FM, Simões J, Domingues MR (2011) Evaluation of the effect of roasting on the structure of coffee galactomannans using model oligosaccharides. J Agric Food Chem 59: 10078-10087.
- March RE, Stadey CJ (2005) A tandem mass spectrometric study of saccharides at high mass resolution. Rapid Commun Mass Spectrom 19: 805-812.
- Laopaiboon L, Nuanpeng S, Srinophakun P, Klanrit P, Laopaiboon P (2009) Ethanol production from sweet sorghum juice using very high gravity technology: effects of carbon and nitrogen supplementations. Bioresour Technol 100: 4176-4182.
- Sun Y, Cheng J (2002) Hydrolysis of lignocellulosic materials for ethanol production: a review. Bioresour Technol 83: 1-11.
- Baldaro E, Gallucci M, Formantici C, Issi L, Cheroni S, et al. (2012) Enzymatic improvement of guar-based thickener for better-quality silk screen printing. Color Technol 128: 315-322.
- Kupiainen L, Ahola J, Tanskanen J (2012) Distinct Effect of Formic and Sulfuric Acids on Cellulose Hydrolysis at High Temperature. Ind Eng Chem Res 51: 3295-3300.
- Anumula KR (2000) High-sensitivity and high-resolution methods for glycoprotein analysis. Anal Biochem 283: 17-26.
- Behan JL, Smith KD (2011) The analysis of glycosylation: a continued need for high pH anion exchange chromatography. Biomed Chromatogr 25: 39-46.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 15448
- [From(publication date):
December-2015 - Jul 13, 2025] - Breakdown by view type
- HTML page views : 10761
- PDF downloads : 4687