Quantification of Saxagliptin Hydrochloride in Human Plasma and Dosage Forms by HPLC-MS/MS Method and Its Application to a Bioequivalence Study
Received: 08-Feb-2021 / Accepted Date: 20-Feb-2021 / Published Date: 27-Feb-2021
Abstract
Saxagliptin is an orally active, highly potent, selective and competitive dipeptidyl peptidase (DPP)-4 inhibitor used in the treatment of type 2 diabetes mellitus at doses of 2.5 or 5 mg once daily. The purpose of this study was to evaluate the bioequivalence and to investigate the pharmacokinetic properties of two formulations containing 5 mg saxagliptin in 30 healthy Egyptian volunteers. The two formulations were: A: Saxaliza 5 mg film coated tablets, manufactured by Hochster pharmaceutical industries(Egypt) and B: Onglyza 5 mg tablets, manufactured by AstraZeneca pharmaceuticals (USA). All these necessitate the need for a biometric tool to prove the drug pharmaceutical equivalence or bioequivalence.
Keywords
Saxagliptin; Bioequivalence; Plasma; HPLC/MS/MS
Introduction
Saxagliptine is (1S,3S,5S)-2-[(2S)-2-amino-2-(3-hydroxyadamantan- 1-yl) acetyl]-2-azabicyclo [3.1.0] hexane-3-carbonitrile with molecular formula C18H25N3O2 (Table 1). Saxagliptin is an orally dynamic hypoglycemic (anti-diabetic sedate) of the unused dipeptidyl peptidase-4 (DPP-4) inhibitor lesson of drugs. FDA affirmed on July 31, 2009. Expanded concentrations of the incretin hormones such as glucagon-like peptide-1 (GLP-1) and glucose-dependent affront tropic polypeptide (GIP) are discharged into the circulation system from the little digestive system in reaction to dinners [1]. The major metabolite of Saxagliptin is additionally a DPP4 inhibitor, which is one-half as strong as Saxagliptin. Solid CYP3A4/5 inhibitors and inducers will modify the pharmacokinetics of Saxagliptin and its dynamic metabolite [2]. Saxagliptin is disposed of by both renal and hepatic pathways. Taking after a single 50 mg measurements of 14C-saxagliptin, 24%, 36%, and 75% of the measurements was excreted within the pee as Saxagliptin, its dynamic metabolite, and add up to radioactivity, individually. 22% of the managed radioactivity was recouped in feces, the division of the Saxagliptin dosage excreted in bile and/or unabsorbed medicate from the gastrointestinal tract. Taking after single verbal measurements of ONGLYZA 5 mg to sound subjects, the cruel plasma terminal half-life (t½) for Saxagliptin and its dynamic metabolite was 2.5 and 3.1 hours, individually [3]. A few strategies have been detailed for examination of saxagliptin either single or in combination with other drugs in measurement shapes and biological fluids [4-16]. Within the display work a touchystraightforward, quick and precise HPLC-MS/ MS strategy was created, optimized and approved for the assurance of saxagliptin hydrochloride in human plasma. The points of interest of this strategy include shorter run time that’s required to attain highthroughput examination, required for clinical, pharmacokinetic and bioequivalence thinks about. Saxagliptin is rapidly absorbed after oral administration, and its pharmacokinetics are modestly affected when it is administered following a high-fat meal. Saxagliptin is not appreciably bound to plasma proteins and is eliminated by a combination of metabolism (CYP3A4/5) and renal excretion. The pharmacokinetics of saxagliptinis not altered by sex, age, body weight or race. Hepatic impairment does not result in any clinically meaningful alterations in the pharmacokinetics of saxagliptin [17]. Basic test arrangement steps and lower constrain of quantitation allowed pertinence of the proposed strategy to assess lower concentrations of the considered drugs in human plasma. This strategy was valuable to gauge the concentration of saxagliptin hydrochloride in human plasma tests collected from sound grown-up volunteers. Application of this test to a bioequivalence think about taking after verbal organization of saxagliptin hydrochloride is depicted.
| Analyte | Internal standard | |
|---|---|---|
| Saxagliptin | Vildagliptin | |
| CAS number | 75847-73-3 | 87333-19-5 |
| Molecular weight | Average: 315.41 Monoisotopic: 315.194677059 |
Average: 303.3993 Monoisotopic: 303.194677059 |
| Chemical formula | C18H25N3O2 | C17H25N3O2 |
| IUPAC name | (1S,3S,5S)-2-[(2S)-2-amino-2-(3-hydroxyadamantan-1-yl)acetyl]-2-azabicyclo[3.1.0]hexane-3-carbonitrile | (2S)-1-{2-[(3-hydroxyadamantan-1-yl)amino]acetyl}pyrrolidine-2-carbonitrile |
Table 1: Analyte and internal standard molecules description.
Materials and Methods
Chemicals, reagents and standards
Saxagliptin hydrochloride working pure standard was purchased from AstraZeneca Pharmaceuticals LP, 4601 Highway 62 East Mount Vernon, Indiana, 47620 USA. The purity was found to be 99.70 ± 0.56 (n=5) [4]. Vildagliptin hydrochloride (IS) pure standard was purchased from Galaxo Co. Its purity was 100.735 ± 0.66 (n=5) according to a reported method [5]. Water for chromatography (Sharlau, Spain) Acetonitrile, methanol, formic acid, ethyl acetate and dichloromethane, HPLC grade, were purchased from Sigma Aldrich Chemie GmbH, Steinheim-Germany. Double-distilled water was obtained from 1aquatron, UK. Blank plasma obtained from the Holding Company for Biological Products & Vaccines (VACSERA), Giza, Egypt and was stored at -80°C.
Pharmaceutical formulation
The two formulations included in this study were: Treatment A: saxaliza® 5 mg film coated tablets, manufactured by Hochster pharmaceutical industries.(Egypt) and treatment B: Onglyza® 5 mg tablets, manufactured by AstraZeneca pharmaceuticals (USA).
Apparatus and software
Prominance HPLC shimadzu associated to Mass spectrometer API 4000, MDS Sciex, Toronto, Canda. Explanatory adjust; BOECO BB1- 31, 4-d, Germany, pre-use standard calibration was done day by day. Vortex; VWR VV3 S540 Universal West Charter-USA. Centrifuge: Changsha Xiangzhi Rebellious, Hunan, China. Vacuum Pump; TID-15, Apparao Plant, Choolai, Chennai, India.pH meter; Corning pH meter, UK, pre-application celebrative alteration was done every day utilizing standardized buffers of distinctive pH-values (4.00-11.00).
Calibrators and quality control samples
Primary stock solutions (100 μg/mL) of saxagliptin and IS (vildagliptin) were prepared, separately, for preparation in methanol and stored at -20°C. All of these solutions were found to be stable for 5 Weekes. Further dilutions were made in methanol for the primary stock solutions to produce secondary stock solutions of 300 ng/mL for saxagliptin and 200 ng/mL for IS on the day of analysis. These stocks were used to prepare the calibration curves (Table 2). Calibrators and quality control samples were freshly prepared before sample analysis. Different working standard solutions of saxagliptin were prepared from their corresponding primary and secondary stock solutions to prepare the calibrators and quality control samples. Calibrators and QC samples were prepared by spiking 950 μL of control human plasma with 25 μL of saxaglitin on the day of analysis, as illustrated in Table 2.
| Prepared samples | Plasma volume | Adding 25 µL of SAX working standard solution (ng/mL) | Final volume | Final plasma concentration (ng/mL) |
|---|---|---|---|---|
| Calibrators | 950 µL | 20 | 1000 µL | 1.5 |
| 40 | 3 | |||
| 800 | 15 | |||
| 8000 | 150 | |||
| 40000 | 300 | |||
| 800000 | 750 | |||
| 160000 | 900 | |||
| 200000 | 1050 | |||
| 280000 | 1500 | |||
| QCL | 60 | 4.5 | ||
| QCM | 120000 | 600 | ||
| QCH | 240000 | 1200 |
Table 2: Preparation of calibration and quality control samples for Saxagliptin (SAX).
Sample preparation
Stock Arrangement of Vildagliptin (IS) arrangement (100 mcg/ mL): exchange 10.1 mg of Vildagliptin to a 100 mL volumetric jar. To the volumetric jar Include 60 ml of Methanol, Vortex for 10 minutes until totally broken up, Total volume to 100 mL with Acetonitrile. (100 mcg/ml saxagliptin). IS arrangement (100 ng/mL) Weaken 0.1 ml of arrangement to 100 mL volumetric flask and total with Acetonitrile? For calibration: include 50 μL of Saxagliptin from fitting concentration of working arrangement to 50 μL Vildagliptin IS (100 ng mL) to 400 ul clear plasma. Vortex the blend for 30 seconds, at that point include 3ml of Dichloromethane, Vortex for 3 minutes at that point, Centrifuge on 4500 RPM, at 5°C for 5 minutes. Dissipate dissolvable in test utilizing concentrator at 80°C, reconstitute dried test with 150 uL Acetonitrile. Put reconstituted test in HPLC vials for infusion. For volunteers: include 50 μL Vildagliptin IS (100 ng mL) to 450 μL clear plasma. Vortex the blend for 30 seconds, at that point include 3 mL of Dichloromethane, Vortex for 3 minutes, Centrifuge on 4500 RPM at 5°C for 5 minutes Vanish dissolvable in test utilizing concentrator at 80°C, reconstitute dried test with 150 μL Acetonitrile put in HPLC vials for infusion, Concentrations of Saxagliptin in obscure tests were calculated by alluding to the arranged calibration bend.
Method validation
Bio-analytical method validation includes all the procedures required to demonstrate that the bio-analytical method, employed for the accurate determination of Saxagliptin in plasma, is fit the purpose of the present bioequivalence study. The following principle provides the necessary basis for developing a full validation protocol. The parameters required to ensure acceptable performance are: stability, linearity, linear working range and limits of quantification, sensitivity, precision & accuracy. Method validation challenges the method, and assesses the extent to which possible environmental, matrix, material; procedural or individuals’ variables affect the determination of Saxagliptin in the plasma matrix, from the time of collection up to analysis time. The International Conference of Harmonization (ICH) [18] covers the principles and practices of validation of analytical procedures.
Selectivity/Specificity: The selectivity was tried by capacity of the proposed strategy to segregate the analytes from endogenous components within the plasma network. Figure 1 appears the chromatograms of a) drug-free human plasma, (b) spiked plasma at lower restrain of quantitation and (c) plasma test from a subject at 2 h after organization of one tablet containing 5 mg SAX. Moreover, none of the commonly utilized drugs by human volunteer’s impedance at the maintenance times of analytes and IS. Selectivity was tried utilizing six clear plasma tests gotten from sound volunteers: the chromatograms were found to be free on curiously crests.
Calibration curve: Plotting of the crest zone proportion of the move combine of analytes to that of IS against the ostensible concentration of calibration guidelines was done to get calibration bends. The concentrations utilized for saxaglitine bend were 0.1, 0.2, 1, 2, 5, 7 and 10 ng/mL, whereas 0.3, 4 and 8 ng/mL were utilized for its LQC, MQC and HQC, separately. Clear test and zero tests were run with each calibration bend. The acknowledgment model for each back-calculated standard concentration was ±15% deviation from the ostensible esteem. The calibration bends were direct within the considered run for each analyte (Table 2).
Precision and accuracy: The exactness, as the relative standard deviation, was 0.82-5.84 % for SAX. Exactness, deviation between the genuine measured values communicated in rates. The recuperation measured against genuine sums is at that point calculated. Ordinarily a least of three judgments at each of three concentrations over the planning run is done (Tables 3 & 4).
| N | Added (ng/mL) | Measured (ng/mL) | Bias (%) | RSD (%) |
|---|---|---|---|---|
| 15 | 0.25 | 0.251 | 0.4 | 0.82 |
| 15 | 1.5 | 1.52 | 2.6 | 4.48 |
| 15 | 3000 | 2805 | -13 | 1.62 |
| 15 | 6000 | 5796 | -6.8 | 5.84 |
Table 3: Intra-assay precision and accuracy of SAX.
| N | Added (ng/mL) | Measured (ng/mL) | Bias(%) | RSD (%) |
|---|---|---|---|---|
| 20 | 0.25 | 0.252 | 0.8 | 10.68 |
| 20 | 1.5 | 1.502 | 0.2 | 16.82 |
| 20 | 3000 | 2880 | -8.0 | 12.96 |
| 20 | 6000 | 580.6 | -5.0 | 9.3 |
Table 4: Inter-assay precision and accuracy of SAX.
Recovery: The extraction efficiencies of SAX and IS from human plasma were surveyed by comparing the reactions of the analytes extricated shape QC tests at diverse levels with the reaction of analytes from a post-extracted plasma test at the same concentration level [19].
Matrix effect: The impact of plasma constituents on the ionization of SAX and IS was assessed by comparing the reaction of the post-extracted plasma standard QC tests (n=4) with the reaction of analytes from flawless tests at the same concentrations [20].
Dilution integrity: The SAX spiked human plasma tests were arranged at the examined concentrations. These arrangements were assisting weakened with pooled human plasma 4-fold in six reproduces and analyzed.
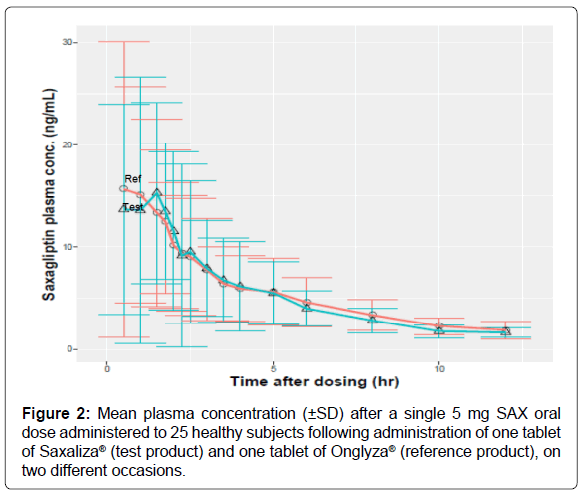
Stability experiments: The steadiness of the analyte is regularly basic in natural tests indeed over a brief period of time. Corruption isn’t unordinary indeed when all safeguards are taken to maintain a strategic distance from particularly known solidness issues of the analyte. It is hence vital to verify that there’s not test corruption between the time of collection of the test and their examination that would compromise the result of the consider. Soundness assessment is done to appear that the concentration of analyte at the time of investigation compares to the concentration of the analyte at the time of testing (Figure 2). An fundamental viewpoint of strategy approval is to demonstrate that analyte(s) is (are) steady within the natural lattice and in all solvents experienced amid the test work-up prepare, beneath the conditions to which ponder tests will be subjected [21].
Pharmacokinetic/bioequivalence study and statistical analysis
The bioequivalence study was performed on one tablet of saxaliza 5 mg (Hochter pharmaceutical industries, Egypt) and one tablet of onglysa 5 mg (Astrazeneca pharmaceuticals, USA) after a single oral dose administration of each to healthy adult volunteers under fasting conditions. The study was an open-label, balanced, randomized two-treatment, two periods, two sequence, cross-over, and single-dose bioequivalence study in 30 healthy adult Egyptian volunteers under fasting conditions with a wash-out period of 10 days. Volunteers signed a written informed consent statement after they were informed about objectives and possible risks involved in the study. The study was conducted as per US Food and Drug Administration guidelines. A single dose of test (saxaliza) and reference (onglyza) was orally administered with 240 mL of water after a recommended wash out period of 10 days. Blood samples were collected at the following intervals: 0.0 (directly prior to dosing), 15, 30, 45 minutes, 1, 1.5, 2, 3, 4, 6, 8, 12, 18, 24, 36 and 48 hours after dose administration. Blood samples were collected into tubes containing EDTA. Pharmacokinetic parameters such as Cmax, Tmax, Auc0.∞, t½ were analyzed using phoenix WinNonlin version 6.3.0 (Pharsight corporation, Mountain view. California). Saxagliptin& S-hydroxy-saxagliptin (active metabolitel Blood samples: 0, 15, 30, 45 min, 1, 1.5, 2, 3, 4, 6, 8, 12, 18, 24, 36 and 48 EDTA -20°C washout: 7-10 days.
Results and Discussion
Method development
Test planning may be a basic step in bioanalysis. Numerous solvents were tried for liquid–liquid extraction, to be specific butyl methyl ether, ethyl acetic acid derivation, diethyl ether and n-hexane. Precipitation method, utilizing methanol and acetonitrile, was too tried for evaluating SAX in human plasma tests. Ideal extraction of SAX from human plasma was best accomplished utilizing acetonitrile as an accelerating dissolvable for plasma tests. A volume of 5 μL was at that point infused into the LC–MS/MS system. The electrospray ionization procedure was worked within the positive particle mode for the different response checking examination. The Q1 full-scan mass spectra of SAX and IS appeared transcendent protonated forerunner [M+H]+ particles at m/z 630.21 to m/z 376.03 for SAX and m/z 788.56 to m/z 599.46 for IS. A few parameters were tried and optimized to make strides crest shape, range and determination for the analytes utilizing Clear UPLC BEH C18 (50×2.1 mm, 1.7 μm) expository column. Acetonitrile and methanol were tried with ammonium formate, ammonium acetic acid derivation and 0.1% fluid formic corrosive in several extents. In a high-throughput strategy, it is of awesome esteem to utilize isocratic elution that permits the exclusion of an equilibration step after the conclusion of the slope. Optimized chromatographic conditions were accomplished utilizing an isocratic mode of acetonitrile –0.1% and formic corrosive (50:50, v/v) at a stream rate of 0.3 mL/min. The IS ought to be eluted at about the same time as the analyte; hence it can compensate for any variance in ionzation that happens, and permits a brief examination time. SAX and IS were eluted in a contract extend of maintenance times (0.50–0.59 min), which encourages the emolument of lattice impacts (Figure 1). The reproducibility of maintenance times for the analytes, communicated as CV, was ≤ 0.61% for 100 infusions on the same column.
Method validation
Selectivity: Figure 1 outlines (a) drug-free human plasma, (b) spiked plasma at lower constrain of quantitation and (c) plasma test from a subject at 2 h after organization of one tablet containing 5 mg SAX. In expansion, conceivable impedances from commonly utilized drugs by human subjects was tried at the maintenance time of SAX and IS. Six distinctive clear plasma tests, gotten from sound subjects, were utilized to test for selectivity. The gotten chromatograms were found to be free of any remote crests.
Linearity and limit of quantification: The calibration curves were linear in the studied range for each analyte. The mean equation of the calibration curve (n=6) were y=0.0659x +0.0106, r=0.9997, for saxagliptin, where y represents analyte/internal standard peak area ratio and x represents the analyte concentration in ng/ml. the limit of quantitation was 0.1ng/ml for saxagliptin (Table 2).
Precision and accuracy: The exactness, characterized by the relative standard deviation, was 10.68 % at LOQ for SAX, whereas the precision, characterized as the deviation between the genuine and the measured esteem communicated as rates, was 0.9 at these concentrations (n=6). Results of intra-day accuracy and exactness through diverse QC levels are appeared in Table 3. The exactness (RSD) extended from 0.82 to 5.84% and the precision was inside 92.98-103.1% for the analytes. Additionally for inter-day tests, Table 4, the accuracy shifted from 9.3 to 16.82% and the exactness was inside 95.8-101.5%.
Extraction recovery: The cruel extraction recuperation for the analyzed SAX was calculated at all QC levels. It shifted from 93.2 to 967.1% in arrange. The cruel extraction recuperation for the IS was calculated as 92.5%.
Matrix effects: Consider of the network impact was done by comparing the analyte crest zones from plasma tests invigorated with SAX at diverse three concentration levels (15, 750 and 1500 ng/mL) as well as the IS at 50 ng/mL post-extraction, with tests from flawless arrangements at the same concentrations. The lattice impact was checked by application of the post-column implantation convention [21]. This was done to think about impact of plasma components on the ionization design of analytes and IS. The network figure (MF) was calculated for each part of framework for the examined analyte. The IS normalized MF was too evaluated by isolating the MF of the analyte by that of the IS. The CV of the IS-normalized MF, calculated from six parts of lattice, was < 5%, showing no critical framework impacts. Normalized MF was found to be 0.97 for SAX relative to IS at moo and tall QC. This comes about showing no noteworthy obstructions at the maintenance times of each SAX and IS. No upgrade or concealment of ionization was watched that was encouraged by elution of analyte and IS in a contract elution time.
Dilution accuracy: Spiked human plasma samples prepared at concentrations 16ng/mL for saxagliptin was diluted with pooled human plasma 4-fold in six replicates and analyzed. The precisions (CV) for dilution integrity were between 0.9 and 4.6% while the accuracy results were within 91.1-98.6% for the analyte.
Sample stability
Short term stability: Short term stability of saxagliptine in plasma samples at the three QC levels was studied for a period of 12 h at room temperature (25°C). The results are shown in Table 5, where the samples were stable under the studied conditions.
| Parameter | Bias (%), RSD (%) |
|---|---|
| (a) Short-term stability of analyte in matrix at room temperature | |
| Spiked concentration level 1a | -3.66, 1.66 |
| Spiked concentration level 2a | -7.29, 4.66 |
| (b) Post-preparative stability at 4°C | |
| Spiked concentration level 1a | -6.76, 1.54 |
| Spiked concentration level 2a | -8.20, 2.54 |
| (c) Long-term stability of analyte in matrix at -20°C | |
| Spiked concentration level 1a | -5.02, 3.54 |
| Spiked concentration level 2a | -4.46, 1.74 |
| (d) Freeze-thaw stability | |
| Spiked concentration level 1a | -4.60, 7.54 |
| Spiked concentration level 2a | -.1.14, 3.6 |
| Spiked concentration for SAX: Level 1, 1.5 ng/mL; Level 2, 6000 ng/mL an = 6 |
|
Table 5: Stability of SAX in matrix by the proposed method.
Post preparative stability: Three sets of spiked samples at the QC levels of the analytes were analyzed and left in the auto sampler at 4°C where for 2 days. The samples were analyzed using a freshly prepared calibration samples. The processed samples were stable as shown in Table 5.
Long-term stability: Long-term stability was checked for frozen plasma samples at -80°C after 9 weeks of storage. The samples were stable under these conditions and results are shown in Table 5.
Freeze and thaw stability: Plasma samples at the three QC concentration levels of saxagliptin were prepared. Samples were stored at -20°C and subjected to three freeze-thaw cycles. During each cycle triplicate 1ml aliquots was processed and analyzed, and the results averaged. NO significant loss during repeated thawing and freezing e=was observed, as shown in Table 5.
Application to biological samples
The proposed strategy was connected for assurance of saxagliptin in plasma tests from a bioequivalence consider that was affirmed by the moral committee. An open-label, randomized, single-dose think about with two-way cross-over plan was items; in 30 solid grown-ups volunteers the cruel age of the gather was 29 a long time (range18-48) and cruel weight was 75 kg (extend 55-92). Each subject gotten a tablet from the test item (Saxaliza® tablets) and a tablet from the reference item (Onglyza® tablets.) beneath fasting conditions, in a randomized design with a washout period of 2 weeks. Thirty solid volunteers completed the cross-over handle and blood tests of all volunteers were analyzed. Saxagliptin has long disposal half-life. Figure 2 appears the cruel plasma concentrations of saxagliptin; the blunder bars show standard deviation at person time Focuses. Table 6 appears the pharmacokinetic parameters of saxagliptin taking after verbal organization of one tablet Saxaliza® (test item) and one tablet of Onglyza® (reference item). With respect to saxagliptin, from log-transformed information, the consider uncovered that AUCO-72, AUCO-inf and Cmax were found to be 96.34% and 105.73%; 95.18% and 108.18% and 108.44% and 95.81% and 107.80% individually. The parametric 90% certainty interims of the cruel values for the test /reference proportion were, in each case, inside the bioequivalence satisfactory run, 80-125%, for the pharmacokinetic parameters AUCo-72, AUCo-inf and Cmax comes about of this bioequivalence ponder demonstrated the proportionality of the tow considered items in terms of the rate of retention as demonstrated by AUCo-72 and AUCo-inf (Table 6).
| Parameter | Test product | Reference product (occasion I) | Reference product (occasion II) |
|---|---|---|---|
| Cmaxng/mL | |||
| Mean | 1102 ± 493.2 | 1103.2 ± 460.43 | 1090.54 ± 464.43 |
| Range | 309.2-1969.8 | 312.43-2000.45 | 320.43-1540.54 |
| tmax(h) | |||
| Median | 6.0 | 6.0 | 7.0 |
| Range | 2.50-100 | 3.0-12.0 | 3.0-12.0 |
| t 1/2 (h) | |||
| Mean | 27.6 ± 8.12 | 28.01 ± 7.76 | 24.43 ± 4.62 |
| Range | 16.9-55.66 | 17.04-51.06 | 18.28-34.78 |
| AUC0-72 (ng h/mL) | |||
| Mean | 6814.76 ± 2987.39 | 6212.43 ± 2432.35 | 7686.45 ± 2575.34 |
| Range | 2015.11-2546.44 | 995.54-12456.56 | 1810.54-14699.34 |
| AUC0-∞ (ng h/mL) | |||
| Mean | 8005.34 ± 4039.15 | 6540.43 ± 3370.52 | 7054.22 ± 4039.54 |
| Range | 2102.35-2483.43 | 1614.45-11568.99 | 1655.31-13543.65 |
Table 6: Pharmacokinetic parameters of SAX following oral administration of one tablet of Saxaliza 5 mg film coated tablets (test product), and one of Onglyza® tablets (reference product) under fasting conditions.
Conclusion
The created, optimized and approved HPLC-MS/MS strategy permits assurance of saxagliptine in human plasma. The accuracy and precision of the strategy are inside the restrain required for bioanalytical measures. The basic test planning steps, moo constrain of quantitation for analyte and brief run time, required to realize highthroughput investigation, permit the utilize of this strategy for clinical pharmacokinetic and bioequivalence considers. The created strategy was effectively connected to a bioequivalence think about in human volunteers.
References
- https://www.accessdata.fda.gov/drugsatfda_docs/nda/2010/200678Orig1s000ClinPharmR.pdf
- https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/
- https://www.ema.europa.eu/en/documents/scientific-guideline/draft-note-guidance-investigation-bioavailability-bioequivalence_en.pdf
- Bhadauria RS, Agarwal V (2019) Development and validation of UV spectroscopic method for simultaneous estimation of dapagliflozin and saxagliptin in marketed formulation. J Drug Del And Thera 9:1160-1164.
- Moneeb MS (2013) Spectrophotometric and spectrofluorimetric methods for the determination of saxagliptin and vildagliptin in bulk and pharmaceutical preparations. Bulletin of Faculty of Pharmacy, Cairo University 51:139-150.
- Lotfy HM, Mohamed D, Elshahed MS (2019) Novel univariate spectrophotometric determination of the recently released solid dosage form comprising dapagliflozin and saxagliptin via factorized response spectra: Assessment of the average content and dosage unit uniformity of tablets. SpectrochimicaActa Part A: Molecular and Biomolecular Spectroscopy 222:117-120.
- Abdel-Aziz O, Ayad MF,Tadros MM (2015) Compatible validated spectrofluorimetric and spectrophotometric methods for determination of vildagliptin and saxagliptin by factorial design experiments. SpectrochimicaActa Part A: Molecular and Biomolecular Spectroscopy 140:229-240.
- Shah PA, Shah JV, Sanyal M, Shrivastav PS (2017) LC–MS/MS analysis of metformin, saxagliptin and 5‐hydroxysaxagliptin in human plasma and its pharmacokinetic study witha fixed‐dose formulation in healthy Indian subjects. Biomed Chromatogr 31:1-11.
- Caglar S, Alp AR. A (2014) Validated high performance liquid chromatography method for the determination of saxagliptin and metformin in bulk, a stability indicating study. J Anal Bioanal Tech 12:2-5.
- Singh N, Bansal P, Maithani M, Chauhan Y (2018) Development and validation of a stability-indicating RP-HPLC method for simultaneous determination of dapagliflozin and saxagliptin in fixed-dose combination. New J Chem 42:2459-2466.
- Aswini R, Eswarudu MM, Babu PS (2018)A novel RP-HPLC method for simultaneous estimation of dapagliflozin and saxagliptin in bulk and pharmaceutical dosage form. Int J Pharm Sci and Tech 13:5161-5167.
- Deepan T, Dhanaraju MD (2018) Stability indicating HPLC method for the simultaneous determination of dapagliflozin and saxagliptin in bulk and tablet dosage form. Curr Issues Pharm Med Sci 31:39-43.
- El-Kimary EI, Hamdy DA, Mourad SS,Barary MA (2016) HPTLC determination of three gliptins in binary mixtures with metformin. J Chrom Sci 54:79-87.
- Sirvidya S, Switha E,Veeresham C (2015) Development and Validation of a High Performance Thin Layer Chromatographic Method for Quantitative Analysis of Saxagliptin. Amer J Anal Chem 6:797-806.
- Abdalah NA, Ibrahim HF (2019)Electrochemical determination of Saxagliptin hydrochloride with MWCNTs/CuO/4′aminobenzo-18-crown-6-ether composite modified carbon paste electrode. Microchem J 147:487-496.
- Preston MR, Robert J, Ruslan K, Aleksander C, Jose CJ,et al. (2012)Dipeptidyl peptidase-4 inhibition with saxagliptin enhanced Nitric Oxide release and reduced blood pressure and sICAM-1 levels in hypertensive rats. J Card Pharmacol 60:467-473.
- David WB (2017) Clinical pharmacokinetics and pharmacodynamics of saxagliptin, a dipeptidyl peptidase-4 Inhibitor. Clin Pharmacokinet 56:11-24.
- https://www.gmp-compliance.org/
- Dams R, Huestis MA, Lambert WE, Murphy CM (2003) Matrix effect in bio-analysis of illict drugs with LC-MS/MS: Influence of ionization type, sample preparation and bio fluid. J Amer Soc Mass Spec 14:1290-1294.
- Van Eckhaut A, Lanckmans K, Sarre S, Smolders I, Michotte Y (2009) Validation of bioanalytical LC-MS/MS assays: Evaluation of matrix effects. J Chromatogr B 877:2198-2207.
- Sauvage FL, Gaulier JM, Lachatre G, Marquet PA (2006)Fully automated turbulent-flow liquid chromatography-tandem mass spectrometry technique for monitoring antidepressants in human serum. Ther Drug Moni 28:123-130.
Citation: Salem H, Badr K (2021) Quantification of Saxagliptin Hydrochloride in Human Plasma and Dosage Forms by HPLC-MS/MS Method and Its Application to a Bioequivalence Study. Clin Pharmacol Biopharm 10: 212.
Copyright: © 2021 Salem H, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 1769
- [From(publication date): 0-2021 - Apr 05, 2025]
- Breakdown by view type
- HTML page views: 1074
- PDF downloads: 695