Quantification of Organochlorine Pesticide Residues in Chewable Part of Chata edulus in Gurage Zone, SNNPR, Ethiopia
Received: 05-Jan-2018 / Accepted Date: 12-Jan-2018 / Published Date: 16-Jan-2018 DOI: 10.4172/2155-9872.1000395
Abstract
This study was conducted with the objective of profile of some pesticide residue on chewable part of Catha edulis. The samples were collected from Gurage zone of different sites, the three different dominant Wore; Cheha, Ezha, Enemorna Ener woreda. because most of the Catha edulis sold in Gurage zone was distributed form these places, and DDT was sprayed on them. Chewable parts of Catha edulis were bought from the local farms in each site. The collected sample was placed in plastic materials until sample preparation and analysis by GC-MS. The percentage recovery obtained for DDT and its metabolite in the present study for the QuEChERS extraction method was in the range of reference put by international organization. 4, 4’-DDT and its metabolite (4, 4’-DDE and 4, 4’- DDD) were determined in Catha edulis samples obtained from Ezha and Enamor woreda. Accordingly, the average concentration of 4, 4’-DDT was 5.77 ± 0.18 mg/kg in the Ezha woreda and 4.77 ± 0.17 mg/kg in the Enamorna Ener woreda. The average concentrations of the first DDT metabolite, 4, 4’-DDD was 2.59 ± 0.09 mg/kg in Catha edulis sample of Ezha and 1.41 ± 0.05 mg/kg in Enamor while the average concentration of the second DDT metabolite, 4, 4’-DDE is 0.05 ± 0.01 mg/kg in Ezha and 0.07 ± 0.02 mg/kg in Enamor woreda respectively. Value of DDT and its metabolite determined in Catha edulis samples obtained from selected woredas of Gurage zone is somewhat high. This indicates that, the farmers who cultivate the Catha edulis plant in the indicated areas of Gurage zone had been using the DDT and spraying it on their Catha edulis plant to control different pests. Hence, this finding is expected to be an alarm so that similar study could be carried out.
Keywords: QuEChERS extraction method; DDT; Catha edulis; GCMS
Introduction
In light of increment of population, the demand for food production is high. The livelihoods of rural farmers focus on Conventional agricultural practices with few viable options. Thus, industrial agriculture practice focuses on maximizing production while minimizing labor requirements. The intention is to support farmers in rural areas that depend on subsistence farming. With this regard, the use of machinery, fertilizers, and pesticides are heavily relying upon with often little to no regard to the well-being of the farmers or their surrounding environments [1].
Pesticides, herbicides, and insecticides are used by a variety of farmers across the globe to meet the demand of high-yields. Each year, some five billion kilograms of active pesticide ingredients are added to farms. These chemicals have many adverse effects including; decreasing the health and fertility of soil, resulting in erosion and the pollution of nearby water sources; illness of the surrounding environment and animals in those environments (and even illness in humans); A dependency on these pesticides and an eventual loss in arable land; Short-term effectiveness; insects can develop resistance to pesticides [2]. Modern agriculture is supported by the wide spread application of pesticide to guarantee productivity and quality. However, the misuse and abuse of these chemicals may lead to environmental damage and human health. Pesticide residues in environment have become a public concern problem [3,4].
Catha edulis is an evergreen, mild-narcotic, flowering tree that mostly grows in East Africa. It was first cultivated in Ethiopia and Yemen, and then spread to Kenya, Somalia, Malawi, Uganda, Tanzania, Arabia, Afghanistan, Congo, Zimbabwe, Zambia, South Africa and Madagascar. The habit of chewing Catha edulis has been common for many centuries. The earliest records with more factual bases showed the Arabic source indicated the ancient use of Catha edulis was used for medicinal purposes as well as a phrodisial; through it was also used for recreational purposes. It is used for avoiding sleepiness, euphoriatic effects, to boast efficiency of work and to increase sexual performance. Chewing Catha edulis is the most common mode of administration; it has been taken as a tea and occasionally smoked. In Ethiopia, processed leaves and roots are used to treat influenza, cough, gonorrhea, asthma and other chest problems. The roots are used for stomach ache and an intrusion made from them is taken to treat boils. Local cultivators of Catha edulis were described based on geographical location, growth habit and physical appearance that mean color of the leaf, stem sizes and potency of effect. It has been known for a long time that different kinds of Catha edulis have different degrees of pharmacological action. Different types of marker systems have been used for genetic analysis and genotyping including morphological, cytological, biochemical and DNA markers [5].
DDT is one of the most pervasive of the persistent organic pollutants (POPs) agro-chemicals with widespread negative impacts on biodiversity and human health throughout the world. It is taken up from the soil by plants, kills most invertebrates, particularly insects, and accumulates in the fatty tissues of animals, including humans, and leads to disruptions of normal breeding, particularly in animals that are high up the food chain such as birds of prey, and in mammals that are hunters. DDT is found almost everywhere in the world, even far from where it has been used as an insecticide. In the tropics, it is highly volatile but in colder climates, particularly around the Arctic Circle it gets deposited and taken up by animals in their food. Since the 1980s, the international community through the United Nations Environment Program has developed for controlling, or rather banning the use of DDT except under special circumstances, is known as the Stockholm Convention on POPs. All developed countries have now completely banned the use of DDT. Ethiopia has also developed a National Implementation Plan (NIP) to eliminate or minimize the use of these chemicals. However, it is still allowed to use DDT, but only for controlling the malaria carrying mosquito through spraying of houses once or twice a year. These spraying campaigns are not ad hoc. They are planned to give maximum protection to people living in areas where there are regular outbreaks of malaria [6].
In Ethiopia, from 1950s to about 2000, DDT has been sprayedoutdoors (for agricultural use) as well as indoors for malaria control by reducing the density and longevity of vector mosquitoes using IRS.
Though, different studies were conducted on Catha edulis socioeconomic and few study at country level no study is conducted yet on concentration based profile of OCP residue in this study area. More over with the survey we did farmers are using DDT for pest control and product increment of Chata edulus plant. In light of this survey we come up with the need assessment of three woredas of the zone, are well known site in using DDT on Catha edulis sample and source for production of Catha edulis even at country level for market. With this regard, it was aimed to conduct OCP profile of chewable part of Chata edulis [7].
Therefore, the main objective of this research work was to determine concentration profile of OCP in chewable part of Catha edulis cultivated in the zone.
Materials And Methods
Description of study area
Gurage zone was the sample areas where large population of Catha edulis is cultivated. The study area is around 170 Km far from the capital city of Ethiopia, Addis Ababa to southwest direction. Geographically, Guraghe Zone is located between 7.8°C -8.5°C North latitude and 37.5°C- 38.7°C East longitude of the equator. Gurage zone has a total area 5932 km2. It has 13 woredas with a total population estimated about 1343246 (CSA). The zone comprises altitudes ranging from 1,001 to 3,500 meters above sea level (m.a.s.l). It is classified into three agro-climatic zones: Dega (high altitude) covers 28.3% of the area and ranges between 2,500-3662 m.a.s.l, Woinadega (mid-altitude) at 1,500-2,500 m.a.s.l, encompasses about 64.9% of the area, and Kolla (lowland) at 1,000-1,500 m.a.s.l covers 6.8% of the area. The mean annual temperature of the zone ranges between 13-30°C and the mean annual rainfall ranges 600-1600 mm. The rainfall pattern in the Gurage Zone is bimodal in which 80% of rain falls in the Kremt period of June to August whereas 20% in the Belg period of February to May. According to the land utilization data of the region 298,369 ha cultivated land, 67,678 ha forest, bushes and shrub covered land, 70,249.31 ha grazing land, and 14,234 ha of land is covered by others.
Instruments
Gas chromatograph, GC (model 7890B manufactured by Agilent technology) coupled with Triple Quadrupole mass spectrometer, MS (model 7000C manufactured by Agilent technology) were used for pesticide residue analysis.
Chemical and reagents used
Reagents that were used in the analysis were all analytical grade. Buffered acetonitrile (1% HAc) anhydrous magnesium sulfate (MgSO4), primary secondary amine (PSA) and analytical standards Chromatographic grade obtained from sigma Aldrich were used for extraction of pesticide residue.
Sample collection
The samples were collected from Gurage zone of different sites, three different dominant Woreda namely Cheha, Ezha and Enemor woreda, where chewable part of Catha edulis distributed within the zone and country at large. Chewable parts of Catha edulis were bought from the local farms of dominant Woreda in each zone. From each Woreda again three sites were selected. From each sites 10 samples were selected randomly and homogenized to represent the bulk sample. The collected sample was placed in plastic materials until sample preparation and analysis. Catha edulis samples analyzed for DDT and its metabolite residues were collected from those areas which farmers spray DDT. This sample also used for mineral analysis. The total selected samples for analysis was 120 Catha edulis samples.
Sample preparation
Sample extraction for determination of pesticides: Extraction was used with modification used by Ashraf et al. [8] started with 5 g of fresh Catha edulis leaves. After oven drying and grounding by mortar, 1 g of the powder was mixed with 5 ml of NaOH, sonicated for 3 min, and then left for 30 min at room temperature. Three extraction cycles were performed on the original sample with 4 ml of extraction solvent in each cycle acetone/ethylacetate/n-hexane (1:2:1) for three minutes vortex followed by two minutes centrifugation at 2000 g. The three extracts were combined and dehydrated with 1 g of sodium sulfate, filtered, and then reduced to 5 ml at room temperature.
QuEChERS methods protocols: A portion of the sample was extracted using a QuEChERS Extraction Salt and Acetonitrile (0.1% Chromatographic Grade Acetic Acid in Chromatographic Grade Acetonitrile both obtained from Sigma Aldrich) and centrifuged. The supernatant was treated using QuEChERS Dispersive Kit to remove interferences in the matrix and centrifuged. 0.5 mL of the supernatant was taken and 1 μl of the sample was injected to the GC-MSMS system. The sample chromatogram was evaluated against a calibration curve obtained from a 7 point calibration made using pure analytical standards (Chromatographic Grade obtained from Sigma Aldrich) for quantization purposes. The procedures of QuEChERS method used for pesticide residue analysis are written in Table 1.
| Method 1 | 15 g of Catha edulis t sample+15 mL of Acetonitrile (+IS)+6 g MgSO4+1.5 g NaCl | 3 mL toluene was added to the solution and shaked for 1 min. |
| Shake for 1 min, centrifuged. | It was centrifuged at 4500 rpm for 5 min. | |
| The extract was transferred to tube contain C18 (500 mg)+MgSO4 (1200 mg) | 6 mL solution was transferred to a glass tube, evaporated to dryness. | |
| 9 mL extract was transferred to tube containing PSA (400 mg), GCB (200 mg) and MgSO4 (1200 mg) |
1 mL Toluene+QC IS was added and transferred to ALS vial for GC/MS analysis | |
| Method validation was checked. | ||
| Method 2 | Weigh 10 g of Sample (50 mL Teflon-Tube) | 1 mL aliquot of supernatant was transferred to a 2 mL d-SPE tube (MgSO4, PSA and C18). |
| 10 mL of acetonitrile was added, acidified with HAc for samples with pH>5 | The sample was vortexed for 1 min and centrifuged for 2 min at high rpm (5000). | |
| Shake vigorously 1 min | Purified supernatant was filtered through a 0.2 µm syringe filter directly into a sample vial. | |
| Add 4 g MgSO4 and 1 g NaCl | Analyze samples by GC/MS. | |
| Method validation was checked. | ||
| Shake vigorously 1 min Shake min | ||
| Add ISTD-Solution | ||
| Shake 30 s and centrifuge Shake centrifuge | ||
| Citrate buffered salt was added to centrifuge tube, shake for 2 min and centrifuged for 5 min at 3000 rpm. | ||
| Method-3 | 15 g Catha edulis +100 μL of IS (TPP) and QC in 50 ml centrifuge tube, solution was spiked and vortexed for 1 min. |
Vortexed 1 min, centrifuged at 13000 rpm for 2 min for 2 mL tubes and at 4000 rpm for 1 min for 15 mL tubes. |
| 15 mL of CAN contain 1% HAC was added, caped and shaked vigorously for 1 min, | 500 μL of extract was transferred to auto sampler vial | |
| Centrifuged at 4000 rpm for 5 min, 1 mL of upper CAN layer was transfered to bond AOAC dispersive SPE (2 mL) and 8 mL Bond Elut AOAC dispersive SPE (15 mL) tube. |
Analyzed by GC/MS. | |
| Method validation was checked. |
Among these methods, the first method (Method-1) had good percentage recovery and it was selected as QuEChERS method of extraction techniques for analysis of DDT and its metabolite.
Table 1: Method development of QuEChERS.
Gas Chromatography/Mass spectroscopy/GC-MS analysis
Separation and determination of DDT residue was carried out using Gas Chromatograph (Model 7890B manufactured by Agilent Technologies) Coupled with Triple Quadrupole Mass Spectrometer (Model 7000C manufactured by Agilent Technologies). A DB-XLB column (60 m × 0.25 mm ID, 0.25 um film thickness, 5% phenyl methyl poly siloxane). The GC conditions were set as described by Huang et al. [9] m with minor modifications. The oven temperature was programmed initially at 60°C for 1 min, then raised to 140°C at 12°C min-1, and finally raised to 280°C at 8°C min-1. Pure Helium was used as carrier gas with a constant column head pressure of 50 kPa and 1 μL sample was injected in to split less injector with injection port temperature of 260°C for analysis. The mass selector detector was operated in the EI-SIM mode to determine DDT and its metabolite. The electron energy was 70 eV and the ion source, and the interface temperature was maintained at 230°C. The electron multiplier voltage was 1 kV and solvent delay was set to 15 min [9].
Results And Discussion
Method validation for pesticide analysis
Accuracy and validity were also the most quoted terms to express the extent of errors in a given results. In this study the accuracy and validity of optimized procedure was evaluated by analyzing the extracts of spiked samples. Spike recoveries were determined by adding pesticide to Catha edulis sample at fortification level indicated and recovery assay were done in triplicate (Table 2).
| Parameters | Original Catha edulis Sample mean (mg/kg) |
Khat sample Spiked amount (mg/kg) |
Mean recovery | % Recovery |
|---|---|---|---|---|
| 4,4’-DDD | 2.59 | 0.8 | 3.43 | 101.66 ± 0.093 |
| 4,4’-DDE | 0.05 | 0.8 | 0.81 | 95.42 ± 0.093 |
| 4,4’-DDT | 5.77 | 0.8 | 6.5 | 91.25 ± 0.252 |
Table 2: Recovery test for the Determination of Organochlorine Pesticides According to AOAC 2007.01.
There are many different permutations of the QuEChERS approach, some of which serve a useful purpose to improve results or practical efficiency for the given analyte(s)/matrix (es) applications, but some others have differences only due to personal preferences. The 3 versions of QuEChERS we compare in this study particularly stand out because they have been extensively evaluated in many labs for a wide range of pesticides in many fruits and vegetables.
Three methods of QuEChERS extraction technique was employed for the present study by changing the quantity of sample and extraction salt, volume of solvent, parameters like round per minute (rpm) for centrifugation, time for shaking the solution. The validity of each method was checked by calculating the percentage recovery by spiking 0.08 mg/kg of standard pesticides and compared each other. The percentage recovery for method two and three were not in the range of reference put by international organization, 90 ± 10% for the validity of any method. The first method was taken because; its percentage recovery is in the range put by reference value indicated. As it is shown in Table 3, the recoveries of the detected pesticide residue in the spiked Catha edulis samples for the first QuEChERS method were, 91.25 ± 0.252, 95.42 ± 0.093 and 101.66 ± 0.093 for 4,4-DDT, 4,4’-DDE and 4,4’-DDD respectively. The percentage recovery obtained for DDT and its metabolite in the present study for the first QuEChERS extraction method was in the range of reference put by international organization indicated above.
| Parameters | Ezha woreda(mg/kg) | Enenor na Ener(mg/kg) | Cheha woreda (mg/kg) |
|---|---|---|---|
| Aldrin | ND | ||
| α-BHC | ND | ND | ND |
| β-BHC | ND | ND | ND |
| δ-BHC | ND | ND | ND |
| γ-BHC | ND | ND | ND |
| α-Chlordane | ND | ND | ND |
| γ-Chlordane | ND | ND | ND |
| 4,4’-DDD | 2.59 ± 0.09 | 1.41 ± 0.05 | ND |
| 4,4’-DDE | 0.05 ± 0.01 | 0.07 ± 0.02 | ND |
| 4,4’-DDT | 5.77 ± 0.18 | 4.77 ± 0.17 | ND |
| Dieldrin | ND | ND | ND |
| Endosulfan I | ND | ND | ND |
| Endosulfan II | ND | ND | ND |
| Endosulfan Sulfate | ND | ND | ND |
| Endrin | ND | ND | ND |
| Endrin Ketone | ND | ND | ND |
| Heptachlor | ND | ND | ND |
| Heptachlor Epoxide | ND | ND | ND |
| Methoxychlor | ND | ND | ND |
Table 3: Average concentration (mean ± SD, n=3) of pesticide residue in Catha edulis sample.
Concentration of pesticide residue
The concentration of pesticide residue, together with DDT and its metabolite were determined in Catha edulis sample, which was collected from selected woredas of Gurage zone. In the present study, most of the pesticide expected was not detected. Only DDT and its metabolite were detected. As shown in Table 4, about sixteen pesticide residues such as aldrin, α-BHC, γ- BHC, β- BHC, δ-BHC, α-chlordane, γ-chlordane, dieldrin, endosulfan I, endosulfan II, endosulfan sulfate, endrin, endrin ketone, heptachlor, epoxide, methoxychlor and heptachlor were not detected in the samples of all woredas in the Gurage zone by the GC/MS instrument (the concentrations were less than 0.01 mg/kg which is the instrument detection limit). This confirms that, there is no application of those classes of pesticides which were not detected.
| Sampling location | 4,4’-DDT (mg/kg) | 4,4’-DDE (mg/kg) | 4,4’-DDD (mg/kg) | Total DDT (mg/kg) |
|---|---|---|---|---|
| Ezha Woreda | 5.77 ± 0.18 | 0.05 ± 0.01 | 2.59 ± 0.09 | 8.4133 ± 0.19 |
| Enemor Woreda | 4.77 ± 0.17 | 0.07 ± 0.02 | 1.41 ± 0.05 | 6.253 ± 0.12 |
| Cheha Woreda | ND | ND | ND | ND |
ND indicates that the concentration was not detected; Superscripts with different letters denote a significant difference (p=0.05) between sites.
Table 4: Mean concentration of DDT and its metabolites (mg/kg) in the extract of Catha edulis samples (mean ± SD, n=3).
However, 4, 4’-DDT and its metabolite (4, 4’-DDE and 4, 4’- DDD) were determined in Catha edulis samples obtained from Ezha and Enamor woreda. Accordingly, the average concentration of 4,4’- DDT was 5.77 ± 0.18 mg/kg in the Ezha woreda and 4.77 ± 0.17 mg/ kg in the Enamor woreda. The average concentrations of the first DDT metabolite, 4, 4’-DDD was 2.59 ± 0.09 mg/kg in Catha edulis sample of Ezha and 1.41 ± 0.05 mg/kg in Enamor while the average concentration of the second DDT metabolite, 4, 4’-DDE is 0.05 ± 0.01 mg/kg in Ezha and 0.07 ± 0.02 mg/kg in Enamor woreda respectively.
In the present study, the value of DDT and its metabolite determined in Catha edulis samples obtained from selected woredas of Gurage zone is somewhat high. This indicates that, the farmers who cultivate the Catha edulis plant in the indicated areas of Gurage zone had been using the DDT and spraying it on their Catha edulis plant to control different pests. They also believe that, spraying DDT on the Catha edulis plant is not only to control pests, but also used to increase the production of the chewable leaf of the plant.
In the present study, the value of DDT and its metabolite determined in Catha edulis samples obtained from selected woredas of Gurage zone is somewhat high. This indicates that, the farmers who cultivate the Catha edulis plant in the indicated areas of Gurage zone had been using the DDT and spraying it on their Catha edulis plant to control different pests. They also believe that, spraying DDT on the Catha edulis plant is not only to control pests, but also used to increase the production of the chewable leaf of the plant.
Mean concentration of DDT and its metabolites
As reported on literature [10], the ratio of DDE/DDT or DDT/DDE has been used to elaborate whether the pesticide residues detected are from past or recent pesticide use. In the present study, as it is shown in Table 4, the concentration of 4,4’-DDT (parent compound) was higher than the concentrations of each metabolite (4,4’-DDE, 4,4’-DDD). This indicates that, there is recent application of DDT on Catha edulis plant in the study area, which is similar to a report made in 2014. The average concentration of total DDT determined in the present study (Table 4), were more than the recent maximum residue limit (MRL) of the FAO/ WHO (0.1-0.2 mg/kg) for different agricultural food items [11], or European Commission (EC) MRL value of 0.050 mg/kg and Japanese MRL values of 0.2 mg/ kg for different food items [11]. From this report, it can be suggested that there is a resent application of DDT on Catha edulis sample which actually supported by survey we did during sample collection. Most of farmers told as they just sprayed DDT on Catha edulis plant and other crops to control pests as well to improve production of the yield.
Comparison of total DDT with literature report elsewhere
According to the report by Daba et al. and Shamsu et al., the concentration of total DDT in chewable parts of Catha edulis leaves were investigated in some parts of Ethiopia. Compared to the concentration of total DDT obtained in Catha edulis from Galamso (141.2-973.0 μg/kg), Aseno (194.3-999.0 μg/kg), Bada Buna (173.9- 686.9 μg/kg) and Sidama (16.7-44.8 μg/kg), the concentration of total DDT determined in Catha edulis samples of the present study (Gurage Zone), is very high (6253-8413.3 μg/kg). The concentration of total DDT in the present study is tenfold higher than the concentration determined in the four parts of Ethiopia. This indicates that, there is a recent application of DDT on Catha edulis leaf samples in the present study (Gurage zone). This value is much higher than values reported elsewhere in earlier studies and this confirms that, the existence of a higher quantity of DDT from a fresh input in the two woredas. While collecting the Catha edulis samples in Gurage zone, the researcher had interviewed the farmers who had been produced in Catha edulis in the study area. The farmers owing the Catha edulis plant responded that, they had using pesticide specially DDT for controlling pests and also, they believed that spraying DDT on the young leaf of Catha edulis would increases the production status of Catha edulis for their economic point of view.
On the other hand, the total DDT was also determined in different samples rather than Catha edulis samples in Ethiopia and other countries.
As studied by Gebremichael et al. in Ethiopia, the concentration of total DDT was determined in human milk. The samples were collected from human milk of Asendabo, Jimma, and Serbo and checked for the total concentration of DDT. The results obtained were 17170, 14460, and 6420 μg/kg in human milk samples of Asendabo, Jimma, and Serbo respectively. These results also show that, there was high accumulation of DDT in the area. The soils in Awash basin was also checked for the levels of DDT and the results of the total DDT was in the range of 22- 230 ng/g. The total DDT in soil samples from some areas of Africa by Ngabe, Kishimba and Manirakiza was ranged from 1.4 to 71, 1.5 to 180 and 120 ng/g respectively. The total DDT was again determined in different vegetables in Ghana from Ghanian market by Crentsil and the results of total DDT in Papaya, Banana, Mango, Tomato, Cabbage and Onion were 12, 38, 20, 35 and 50 μg/kg respectively. And, researchers determined the total DDT from vegetables of Kirklareli such as banana pepper, marrow squash, cherry tomato, lettuce, onion, purslane green Beas and cucumber and the results obtained were 42.6, 70.63, 14.66, 30.55, 46.12, 81.38, 41.05, 82.79 μg/kg respectively
The high concentration of DDT in different samples of the country can be recognized as health officers to control malaria secretly distribute DDT and perhaps applied on Catha edulis plant. DDT is also being exported from abroad by some illegal venders through smuggling while majority of sources are from the country itself (which is formulated in Adami Tulu). Many unknown pesticides are also being sold on local markets through smuggling [7], The same is true in the Gurage zone.
Recommended Daily Allowance (RDA) of total DDT in food
The Joint Food and Agriculture Organization of the United Nations FAO/WHO Codex Alimentarius Commission have set the acceptable daily intake (ADI) for total DDT to be 0.010 mg/kg [11]. The ADI is explained based on a body weight of a person who intake DDT as an individual in a population may be exposed daily over his or her lifetime without appreciable health risk [11]. To know the exposure of chewers to DDT and its metabolites, the estimated daily intake (EDI) of these pesticides was determined from the measured concentrations. It was calculated based on the assumption that the average Catha edulis consumption of an adult having a body weight of 60 kg was 0.1 kg/ day. The mean values for daily intakes of DDT and its metabolites were estimated from the following equation [12-16].
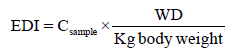
Csample=mean concentration of pesticide in Catha edulis sample (mg/kg); WD=Dry weight of Catha edulis consumed (kg/day).
According to the present study, the EDI of DDT ranges from 0.010 to 0.014 mg/Kg which is comparable with the range recommended by FAO/WHO ADI value. Nevertheless, this value gives information only about DDT exposure associated with Catha edulis chewing. Perhaps farmers in the study area might also use DDT for other agricultural activities which intern increases the actual daily intake (ADI).
Conclusion
It is concluded that, though other classes of pesticides were not detected, DDT and its metabolite were detected in the two sites of the study area. Even though the concentration of DDT and its metabolite is high, the estimated daily intake is comparable with the daily intake put by FAO and WHO. The high level of DDT in our investigation confirms that, there is recent application of DDT in Catha edulis samples of the area. Moreover, this investigation is in line with the survey we did before sample collection. With the survey, it is understood that, most of the farmers in zone are unknowingly use DDT and other types of pesticide on their farming activities. Hence, this finding is expected to be an alarm so that similar study could be carried out. Beside to this, the following points are recommended:
1. There should be further investigation to monitor the level of DDT and its metabolite even in other food sample in the zone.
2. There should be health education and Catha edulis information programs regarding to possible health risks associated with use of DDT and other pesticides on Catha edulis .
3. Risk reduction and safer Catha edulis use, such as encouraging the sellers and users to wash the leaves before consumption would minimize the risk from pesticides.
4. Communicate and enforce farmers about the safe use of pesticides and establish reference laboratories to support field testing activities.
5. Formal or informal training for farmer sprayers on safety handling and proper use of pesticides, including periodic review of equipments is needed.
6. Regular training for health extension, development agents, and woreda experts on precaution that should be taken while working with pesticides.
7. Awareness to farmers, sprayers, authorities, and retailers on toxicity of using DDT and other pesticides to humans, animals as well as environmental health.
8. Coordination among zone Health and Agriculture office.
Acknowledgements
The authors are grateful to Bless Agri Food Laboratory Services PLC where all laboratory is carried out. We are also grateful to Wolkite University, research and development directorate office for funding this project.
References
- Dreistadt S (2007) Biological Control and Natural Enemies of Invertebrates. UC ANR 74: 1-7.
- Ritter L, Gousheleff N, Arbuckle T, Cole D, Raizenne M (2006) Addressing the Linkage between Exposure to Pesticides and Human Health Effects - Research Trends and Priorities for Research. Journal of Toxicological and Environmental Health 9: 441-456.
- Gameel S (2013) The side effect of some pesticide alternatives on the population densities of the natural enemies of two piercing-sucking insect pests at the new valley province, Egypt. Plant Protection Research Institute, Giza 5: 1-6.
- Paleerat A (2005) Farmers awareness of danger caused by pesticide use in growing hua rue pepper, Tambonhua rue, Amphoe Muang, Ubonratchani Province. MSc Thesis, Mahidol University.
- Ali M, Masood A (2015) Determination of Iron, Cobalt, Chromium and Copper Metals. In: Commercially Available Catha edulis (Catha Edulis Forsk) In Arba Minch, Ethiopia. Journal of Engineering Research and Applications 5: 66-74.
- Institute for Sustainable Development (2009) Information Dissemination on the Status of Pesticide residues in fruits and vegetables.
- DDT Use in the Ethiopian Rift Valley CTA (1989) Pesticides: Compounds, use and hazards. AGRODOK, p: 29.
- Abdulaziz M (2010) An assessment of possible health risks of using DDT and Farmers’ Perception towards toxicity of pesticides used on Catha edulis (Catha edulis). Haromaya Woreda, Ethiopia. Addis Ababa University.
- Hussen A, Megersa N, Westbom R, Retta N, Mathiasson L, et al. (2006) Optimization of pressurized liquid extraction for the determination of p,p’-DDT and p,p’-DDE in aged contaminated Ethiopian soil. Anal Bio Anal Chem 386: 1525-1533.
- Huang Z, Chen B, Yao S (2007) Simultaneous Determination of 102 Pesticide Residues in Chinese Teas by Gas Chromatography–Mass Spectrometry. Journal of Chromatography B 853: 154-162.
- El-Nahhal Y (2004) Contamination and safety status of plant food in Arab countries. J Appl Sci 4: 411-417.
- FAO/WHO Food Standards Codex Almintarius (2013) Pesticide residues food and feeds. Geneva, Switzerland.
- Anastassiades M, Lehotay SJ, Stajnbaher D, Schenck FJ (2003) Fast and easy multiresidue method employing acetonitrile extraction/partitioning and dispersive solid-phase extraction for the determination of pesticide residues in produce. AOAC Int 86: 412.
- Lehotay SJ, Mastvoska K, Yun SJ (2005) Evaluation of Two Fast and Easy Methods for Pesticide Residue Analysis in Fatty Food Matrixes. J AOAC Int 88: 630-638.
Citation: Hailemariam T, Belete T, Hailu T, Ayele D, Ligani S (2018) Quantification of Organochlorine Pesticide Residues in Chewable Part of Chata edulus in Gurage Zone, SNNPR, Ethiopia. J Anal Bioanal Tech 9: 395. DOI: 10.4172/2155-9872.1000395
Copyright: © 2018 Hailemariam T, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 5214
- [From(publication date): 0-2018 - Dec 23, 2024]
- Breakdown by view type
- HTML page views: 4473
- PDF downloads: 741
