Quantification of Hemoglobin Concentration in Whole Blood using Raman Spectroscopy
Received: 28-Oct-2022 / Manuscript No. jabt-22-78585 / Editor assigned: 31-Oct-2022 / PreQC No. jabt-22-78585 / Reviewed: 14-Nov-2022 / QC No. jabt-22-78585 / Revised: 18-Nov-2022 / Manuscript No. jabt-22-78585 / Accepted Date: 24-Nov-2022 / Published Date: 25-Nov-2022 QI No. / jabt-22-78585
Abstract
Rapid and accurate quantification of hemoglobin concentration in human blood samples is imperative to diagnose anemia. There are many methods that currently exist to do this, including manual and automatic laboratory methods and point-of-care devices. However, many of these methods necessitate the use of chemical reagents which can be expensive to purchase, store, and dispose of. Raman spectroscopy offers an alternative method of quickly and accurately determining hemoglobin concentration without the need for reagents. Here, we present a Raman based hemoglobin model that is valid from 1.7-24.8 g/dL. The method described here has a standard deviation of 1.3 g/dL and is shown to be precise within this standard deviation. This method was validated using 82 natural and artificial samples and demonstrates good agreement with current methods of hemoglobin quantification.
Keywords
Raman spectroscopy; Whole Blood; Anemia
Introduction
Hemoglobin (Hgb) is a protein found in red blood cells (RBCs) that transports oxygen throughout the body. Hgb concentration is commonly measured during routine hematological tests of a patient’s blood and is used to diagnose anemia, a medical condition in which there is an insufficient number of healthy RBCs present in the body. The normal range of Hgb varies for adults as a function of age, sex, and ethnicity [1]. However, the upper and lower limits are often separated only by sex. According to the National Heart, Lung, and Blood Institute, the normal range of Hgb for males is 14-17 g/dL and for females is 12-15 g/dL [2]. Causes of abnormally high Hgb values include smoking tobacco products, dehydration, and certain forms of cancer. Abnormally low Hgb levels are indicative of anemia and can result from iron deficiency, vitamin deficiency, abnormally shaped RBCs (e.g. sickle cell anemia), among other causes. Current laboratory methods for measuring Hgb concentration can be divided into manual and automatic procedures. One such manual method is Drabkin’s method in which blood is diluted with a potassium ferricyanide and potassium cyanide solution and forms hemiglobincyanide [3]. The absorbance of a 540 nm light by this compound is measured using colorimetry and compared to a standard to determine Hgb concentration [3]. One major disadvantage to this method is that it involves the use of dangerous chemicals, and even newer methods that utilize less dangerous reagents, such as alkaline hematin or sodium lauryl sulfate, still require some degree of biohazardous waste removal [4-6]. Similar to the manual method described above, automated methods of determining Hgb concentration typically involve mixing blood with a reagent and using colorimetry to measure concentration [6]. However, automated procedures generally use different reagents, handle a much higher throughput, and provide information on additional blood analytes [6-7]. One major disadvantage of this method is the cost of training and operation. Several Point-of-Care (PoC) procedures are available as well, however, they tend to be less accurate than laboratory methods [6, 8-10]. There is a need for an accurate, inexpensive method of determining Hgb concentration without the use of any potentially harmful reagents. To this end, many studies have been published exploring the potential of Raman spectroscopy for the rapid, inexpensive, and safe analysis of blood and blood components [11-15]. Raman spectroscopy is a rapid, non-destructive analytical technique used to determine both qualitative and quantitative properties of a wide variety of materials. Specific to the medical field, Raman spectroscopic studies of bodily fluids such as urine [16], saliva [16-18], and blood [11-13, 16, 19-20] have been conducted for more than four decades. The technique utilizes the inelastic scattering of light to identify the presence of particular chemical bonds and was first described in 1928 [21]. Photons, generally with a wavelength in the visible or near-infrared spectrum, are made incident upon a material of interest. The majority of these photons are scattered elastically in a process known as Rayleigh scattering. However, a small proportion of incident photons are scattered inelastically, such that they have a vibrational energy that is either greater or less than that of the incident photon. This energy difference is equivalent to the vibrational energy of a particular chemical bond in the material of interest and may be used to identify the presence of that bond. A complete Raman spectrum is unique to that material and can be used to identify unknown materials. One major advantage of Raman spectroscopy in the analysis of blood is that it does not require the use of reagents, which makes it a cheaper and safer alternative to many common medical instruments and techniques. In the current study, the Raman spectral properties of whole blood measured from samples of participants with varying sex, age, and ethnicity were used to develop a regression model relating Raman spectral properties to Hgb concentration. This model was then tested to assess the effectiveness of using Raman spectroscopy to measure Hgb concentration in whole blood.
Materials and Methods
Samples
To develop a model relating Raman spectral properties to Hgb concentration, 251 whole blood samples were collected from June to July 2022. Twenty-six of these samples were lost due to operator errors such as improper volume used or bubbles present in the sample. All samples were collected from participants by one phlebotomist at five different collection sites over five different days and were measured by three operators on site. Participants were guided through the informed consent process, two vials of blood were drawn, and the participants were given compensation for their time and assistance. Figure 1 provides the distribution of age, sex, ethnicity, and Hgb concentration of these samples. The Hgb concentrations ranged from 9.5-17.2 g/ dL and show a normal distribution with the majority of sample concentrations between 12.5-14.5 g/dL. To extend the measured Hgb range to adequately cover a clinically significant range, two tubes of blood were collected from each of four participants in August 2022. One of the two tubes was placed in a centrifuge for fifteen minutes at 3,500 RPM to separate RBCs from serum. The Hgb concentration of the second tube was artificially increased or decreased by adding RBCs or serum, respectively, from the first tube. RBCs and serum were never mixed between participant samples. This procedure produced an additional 49 concentrations that were added to the 225 successful measurements and used to develop a Raman based Hgb model. A separate validation data set of 46 natural and 36 artificially adjusted whole blood samples were collected in September 2022 to test the effectiveness of the Raman based Hgb model. These samples are hereafter referred to as the “validation data set”. The validation data set was collected over five days at three different collection sites and measured by six different operators. The Hgb concentration of the artificially adjusted samples was altered via the addition of RBCs or serum as described above. Information on the samples used in the validation data set is given in Table S1 of the Appendix. The detection limits for the Raman method described in this study were assessed using five additional samples diluted to <2 g/dL Hgb concentration and five serum “blank” samples. Detection limits samples were refrigerated between measurement days and allowed to reach thermal equilibrium with the laboratory before measurements were collected. Hgb concentration, in units of g/dL, was determined for all samples described above using either or both the Sysmex XN-10 Automated Hematology Analyzer located at AdventHealth Tampa in Tampa, Florida or the HemoCue Hb 301 System located at Kaligia Biosciences, LLC in Largo, Florida. The Sysmex XN-10 uses the sodium lauryl sulfate method and the HemoCue uses spectrophotometry to determine Hgb concentration. All natural whole blood samples measured by Raman were venous drawn collections in either BD Vacutainer® 6 mL No Additive Plus Tubes or Covidien™ Monoject™ 10 mL K3EDTA Blood Collection Tubes. Samples measured by Sysmex XN-10 or HemoCue were venous drawn collections in either Covidien™ Monoject™ 10 mL K3EDTA Blood Collection Tubes or Vacuette® Tube 2 mL or 3 mL K3E K3EDTA collection tubes. All artificially altered samples were venous drawn and collected in Covidien™ Monoject™ 10 mL K3EDTA Blood Collection Tubes. No measurable difference was found between Raman measurements from blood collected in additive free tubes compared to K3EDTA tubes. Samples collected in additive free tubes were measured within ten minutes of collection to avoid clotting. Samples collected in K3EDTA tubes were measured within 48 hours of collection.
| Sample Number | Age | Sex | Ethnicity | HemoCue Hgb (g/dL) | Sysmex Hgb (g/dL) | RBA Hgb (g/dL) | Number of Measurements | Difference between RBA and HemoCue | Difference between RBA and Sysmex |
|---|---|---|---|---|---|---|---|---|---|
| 319 | 24 | Male | Mixed | 15.7 | 15.2 | 15.9 | 2 | 0.2 | 0.7 |
| 321 | 32 | Female | Asian | 11.9 | 11.8 | 10.3 | 2 | 1.6 | 1.5 |
| 322 | 28 | Male | White | 16.2 | 16.3 | 16.8 | 2 | 0.6 | 0.5 |
| 323 | 37 | Male | Black | 16.2 | 16.2 | 16.7 | 2 | 0.5 | 0.5 |
| 324 | 47 | Male | White | 14.7 | 14.6 | 14.5 | 10 | 0.2 | 0.1 |
| 325 | 23 | Female | White | 13.5 | 13.2 | 12.1 | 2 | 1.4 | 1.1 |
| 326 | 45 | Female | White | 15.2 | 14.9 | 14.2 | 2 | 1 | 0.7 |
| 327 | 48 | Male | White | 14.2 | 13.8 | 17.5 | 3 | 3.3 | 3.7 |
| 328 | 65 | Female | White | 12.1 | 11.9 | 12.1 | 2 | 0 | 0.2 |
| 329 | 72 | Male | White | 16.8 | 16.5 | 16.3 | 2 | 0.5 | 0.2 |
| 330 | 70 | Female | White | 13.1 | 12.7 | 13.4 | 3 | 0.3 | 0.7 |
| 331 | 42 | Male | White | 16.8 | 16.9 | 17.4 | 2 | 0.6 | 0.5 |
| 332 | 53 | Male | White | 15.3 | 15 | 18.2 | 2 | 2.9 | 3.2 |
| 333 | 50 | Male | White | 15.9 | 15.5 | 15.7 | 1 | 0.2 | 0.2 |
| 334 | 46 | Female | White | 14.8 | 14.7 | 14 | 10 | 0.8 | 0.7 |
| 335 | 43 | Female | White | 14.2 | 13.9 | 15 | 2 | 0.8 | 1.1 |
| 336 | 60 | Male | White | 17.2 | 17.1 | 15.5 | 1 | 1.7 | 1.6 |
| 337 | 57 | Female | White | 12.9 | 12.6 | 11.9 | 3 | 1 | 0.7 |
| 338 | 60 | Male | White | 13.5 | 13.3 | 14.7 | 2 | 1.2 | 1.4 |
| 339 | 38 | Male | White | 16.4 | 15.7 | 17.8 | 2 | 1.4 | 2.1 |
| 340 | 61 | Male | White | 12.8 | 12.5 | 12.2 | 2 | 0.6 | 0.3 |
| 365 | 30 | Female | Mixed | 13.5 | 13.3 | 12.9 | 11 | 0.6 | 0.4 |
| 366 | 80 | Male | Pakistani | 13.4 | 13.3 | 13.4 | 3 | 0 | 0.1 |
| 367 | 28 | Female | White | 13.3 | 13.5 | 13.2 | 3 | 0.1 | 0.3 |
| 378 | 23 | Male | White | 15.3 | 15.5 | 14.8 | 2 | 0.5 | 0.7 |
| 380 | 57 | Female | White | 12.4 | 11.9 | 12.7 | 2 | 0.3 | 0.8 |
| 381 | 54 | Male | White | 13.7 | 13.5 | 15.6 | 3 | 1.9 | 2.1 |
| 382 | 64 | Male | White | 15.1 | 14.9 | 14.2 | 2 | 0.9 | 0.7 |
| 383 | 44 | Male | White | 13.4 | 13 | 13.3 | 2 | 0.1 | 0.3 |
| 384 | 51 | Male | White | 13.8 | 13.4 | 15.2 | 2 | 1.4 | 1.8 |
| 385 | 61 | Male | White | 15.2 | 14.6 | 15.4 | 2 | 0.2 | 0.8 |
| 386 | 63 | Male | Black | 15.2 | 15.1 | 14.4 | 2 | 0.8 | 0.7 |
| 387 | 31 | Male | White | 14.3 | 13.9 | 16.8 | 4 | 2.5 | 2.9 |
| 388 | 57 | Male | White | 16.4 | 16.1 | 17.1 | 3 | 0.7 | 1 |
| 389 | 62 | Male | White | 13.7 | 13.5 | 13.6 | 3 | 0.1 | 0.1 |
| 390 | 55 | Male | Mixed | 14.6 | 14.1 | 13.9 | 2 | 0.7 | 0.2 |
| 391 | 55 | Female | White | 13.4 | 13.3 | 13.4 | 2 | 0 | 0.1 |
| 392 | 63 | Male | Black | 13.2 | 13.4 | 11.9 | 2 | 1.3 | 1.5 |
| 393 | 27 | Male | White | 15.4 | 15.1 | 17.8 | 3 | 2.4 | 2.7 |
| 394 | 50 | Male | Hispanic | 16.7 | 16.5 | 17.8 | 2 | 1.1 | 1.3 |
| 395 | 27 | Male | Hispanic | 16.3 | 16 | 16 | 2 | 0.3 | 0 |
| 396 | 24 | Female | White | 13 | 12.6 | 13.3 | 10 | 0.3 | 0.7 |
| 397 | 27 | Male | Asian | 15.1 | 15.1 | 13.8 | 4 | 1.3 | 1.3 |
| 398 | 36 | Female | Asian | 12.1 | 11.8 | 12.7 | 2 | 0.6 | 0.9 |
| 399 | 41 | Male | Asian | 14.3 | 14 | 13.7 | 3 | 0.6 | 0.3 |
| 400 | 25 | Male | White | 15.9 | 15.6 | 15.3 | 3 | 0.6 | 0.3 |
| 326 dil | 45 | Female | White | 9.2 | N/A | 10.8 | 2 | 1.6 | N/A |
| 326 dil2 | 45 | Female | White | 6.4 | N/A | 7.7 | 2 | 1.3 | N/A |
| 326 spk | 45 | Female | White | 17.9 | N/A | 18 | 2 | 0.1 | N/A |
| 337 dil | 48 | Male | White | 10.7 | N/A | 9.2 | 2 | 1.5 | N/A |
| 337 dil2 | 48 | Male | White | 8.7 | N/A | 7.5 | 2 | 1.2 | N/A |
| 327 dil | 48 | Male | White | 10.4 | N/A | 13.1 | 3 | 2.7 | N/A |
| 327 dil2 | 48 | Male | White | 8 | N/A | 11.6 | 2 | 3.6 | N/A |
| 327 spk | 48 | Male | White | 17.7 | N/A | 22.4 | 2 | 4.7 | N/A |
| 328 dil | 65 | Female | White | 11 | N/A | 11.9 | 2 | 0.9 | N/A |
| 328 dil2 | 65 | Female | White | 7.8 | N/A | 7.8 | 2 | 0 | N/A |
| 328 spk | 65 | Female | White | 18.2 | N/A | 18.2 | 2 | 0 | N/A |
| 330 dil | 70 | Female | White | 9.4 | N/A | 10.1 | 10 | 0.7 | N/A |
| 330 dil2 | 70 | Female | White | 5.6 | N/A | 6.1 | 2 | 0.5 | N/A |
| 330 spk | 70 | Female | White | 17.7 | N/A | 18.1 | 2 | 0.4 | N/A |
| 334 dil | 46 | Female | White | 10.5 | N/A | 10.5 | 2 | 0 | N/A |
| 334 dil2 | 46 | Female | White | 9 | N/A | 9.2 | 2 | 0.2 | N/A |
| 334 spk | 46 | Female | White | 17.4 | N/A | 17.3 | 2 | 0.1 | N/A |
| 335 dil | 43 | Female | White | 11.1 | N/A | 12.1 | 2 | 1 | N/A |
| 335 dil2 | 43 | Female | White | 7.7 | N/A | 7.6 | 2 | 0.1 | N/A |
| 329 dil | 72 | Male | White | 10.2 | N/A | 10.4 | 2 | 0.2 | N/A |
| 329 dil2 | 72 | Male | White | 7.5 | N/A | 7.5 | 2 | 0 | N/A |
| 329 spk | 72 | Male | White | 17.6 | N/A | 17.5 | 2 | 0.1 | N/A |
| 331 dil | 42 | Male | White | 10.5 | N/A | 8.9 | 10 | 1.6 | N/A |
| 331 dil2 | 42 | Male | White | 8.6 | N/A | 7.2 | 2 | 1.4 | N/A |
| 331 spk | 42 | Male | White | 18.5 | N/A | 18.1 | 2 | 0.4 | N/A |
| 333 dil | 50 | Male | White | 9.5 | N/A | 10 | 2 | 0.5 | N/A |
| 333 dil2 | 50 | Male | White | 6.9 | N/A | 6.9 | 2 | 0 | N/A |
| 332 dil | 53 | Male | White | 9.6 | N/A | 10.9 | 2 | 1.3 | N/A |
| 336 dil | 60 | Male | White | 10.6 | N/A | 10.5 | 2 | 0.1 | N/A |
| 336 dil2 | 60 | Male | White | 8.5 | N/A | 8.6 | 2 | 0.1 | N/A |
| 337 dil | 57 | Female | White | 10.7 | N/A | 9.2 | 2 | 1.5 | N/A |
| 337 dil2 | 57 | Female | White | 8.7 | N/A | 7.5 | 2 | 1.2 | N/A |
| 338 dil | 60 | Male | White | 11 | N/A | 10.4 | 2 | 0.6 | N/A |
| 338 dil2 | 60 | Male | White | 8.3 | N/A | 8.1 | 2 | 0.2 | N/A |
| 339 dil | 38 | Male | White | 10.4 | N/A | 11.3 | 2 | 0.9 | N/A |
| 340 dil | 61 | Male | White | 12 | N/A | 14.7 | 2 | 2.7 | N/A |
Table S1: Sample information for the validation data set. RBA is the Rapid Biofluid Analyzer used to collect all Raman spectral measurements.
Measurement Procedure
For each sample, 340 μL of whole blood were pipetted into a Roche AssayCup polypropylene sample container. Measurements were collected through the opening in the container to prevent measurement of polypropylene along with the blood sample. If polypropylene was identified in the Raman spectrum of a sample, that measurement was excluded. Polypropylene was most commonly observed in measurements when an insufficient volume of blood (i.e. <340 μL) was used. To prevent bubble from forming in the sample, the reverse pipetting method was applied. Raman spectra were collected using the Rapid Biofluid Analyzer™ developed by Kaligia Biosciences, LLC. This device utilizes a Wasatch Photonics WP 830-R-SR-LMM- 50-KAL spectrometer to collect Raman spectra and the Kaligia Optical Spectrum Analysis (OSA) software to process Raman spectra. Excitation was provided by a Wasatch Photonics 830 nm wavelength stabilized TO-56 diode laser with a power output at the source of 100 mW. This wavelength was chosen to maximize the signal while minimizing the fluorescence of the biological material. Before measuring a sample, the background “dark noise” was collected to account for hot pixels within the CCD. Measurements of whole blood samples collected from June to July 2022 were collected for eight second intervals in nine cycles. The number of collection cycles was then reduced to three for all subsequent measurements. This was done to reduce the amount of time that the sample was exposed to the laser to prevent photodegradation of the sample. While not common, it was observed that over exposure to the laser could cause damage to the sample. This photodegradation was visible by inspection of the sample volume, as well as in the resulting Raman spectrum. The damaged blood showed extreme fluorescence that would often saturate the detector. Photodegradation was more often observed in samples with Hgb concentrations greater than ~15 g/dL and was not observed in samples with less than ~12 g/dL Hgb. No damaged measurement cycles were used in this study, and if a majority of measurement cycles showed evidence of photodegradation, the sample was either excluded or remeasured. Reducing the number of collection cycles was found to reduce the frequency of sample photodegradation by the laser. No difference was found between results calculated using nine cycles compared to three cycles. All samples were measured between one and eleven times to assess the performance of the Raman method of quantifying Hgb concentration.
Results and Discussion
Raman Spectrum of Whole Blood
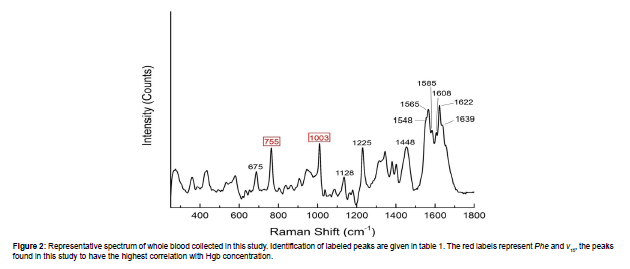
Figure 2 shows an example spectrum of whole blood collected in this study using an excitation wavelength of 830 nm. The vibrational modes and representations of the characteristic Raman peaks of whole modes and representations of the characteristic Raman peaks of whole blood shown in Figure 2 are given in Table 1. For a more detailed list of the whole blood Raman peaks, refer to Makhnii 2016 and references therein [22]. Many of the Raman peaks present in whole blood are assigned to the chemical bonds found in hemoporphyrins such as Hgb. It is important to note that the positions and intensities of these peaks depend upon the excitation wavelength [11]. This is due to resonance effects with particular bonds in hemoporphyrins that lead to increased or decreased Raman scattering [23].We found that the peaks representing the “breathing” of the pyrrole ring structure (ν15) and phenylalanine ring structure (Phe) nominally located at 755 cm-1 and 1003 cm-1, respectively, provide the strongest correlation with Hgb concentration. Figure 3A shows the relationship between Hgb concentration in whole blood and the peak intensity of the ν15 and Phe peaks for the 225 natural samples and 49 artificially adjusted samples. This set of 274 samples is hereafter referred to as the “calibration data set”. In general, as Hgb concentration increases, the peak intensity of both Phe and ν15 increases. This is expected as higher concentrations of Hgb would yield a larger number of molecules with the appropriate bonds to produce these peaks. However, Raman peak intensity is also affected by other factors, such as laser power, collection time, and how well focused the laser is on the sample. While careful control of the measurement parameters will mitigate this, it is often more useful to observe peak intensity ratios. As shown in Figure 3B, the ν15/Phe intensity ratio shows a significant correlation with Hgb concentration. Much of the variability observed in the individual peak intensities, especially the variability observed in the Phe peak (Figure 3A), is also reduced by using the intensity ratio. This relationship between Hgb concentration and ν15/Phe intensity ratio is used to calibrate the Raman based Hgb model. The model shown in Figure 3B has a standard deviation (SD) of 1.3 g/dL is valid from 1.7-24.8 g/dL.
| Peak Position (cm-1) | Description |
|---|---|
| 675 | ν7 (Pyrrole Breathing) † |
| 755 | ν15 (Pyrrole Breathing) † |
| 1003 | Phenylalanine ring breathing* |
| 1128 | ν5 (Cβ-methyl Stretch) ● |
| 1225 | ν13 (Cm-H bending mode) † |
| 1448 | Amino acid bending* |
| 1548 | ν11 (Cβ-Cβ stretch) † |
| 1565 | ν19 (Cα-Cm and Cα-Cβ stretch mixed with Cm-H bending mode) † |
| 1585 | ν37 (Asymmetrical Cα-Cm vibration) † |
| 1608 | Lower frequency ν(C=C) vinyl mode● |
| 1622 | Higher frequency ν(C=C) vinyl mode● |
| 1639 | ν10 (Cα-Cm and Cα-Cβ stretch) † |
Table 1: Major whole blood peak positions recorded using an excitation wavelength of 830 nm and associated vibrational mode/components represented by the peaks. Note that the descriptions given here were determined from an excitation wavelength of 735 nm and as such the peak positions do not match exactly what is recorded in the literature. This is discussed in further detail in the text. Cα, Cβ, and Cm indicate the alpha, beta, and meso-carbon atom positions in porphyrins, respectively [32]. The bolded entries are the ones used in this study to determine Hgb concentration [31] [32] [33].
Detection Limits, Precision, and Linearity
The Limit of Blank (LoB) and Limit of Detection (LoD) were determined following the method described in CLSI EP17-A2 Evaluation of Detection Capability for Clinical Laboratory Measurement Procedures; Approved Guideline – Second edition [24]. A sample with no Hgb will not have peaks associated with Hgb and thus the LoB would be 0.0 g/dL and all measurements would ideally reflect this. However, there is some error associated with the OSA software used to calculate Hgb based on the Raman model. The LoB and LoD described here are reflective of the device and analytical software used. Note that the OSA software used does not generally provide a Hgb concentration for blank samples and instead provides an error, however we adjusted it to proceed with Hgb calculation for this assessment. To determine the LoB, five serum samples were measured four times a day each for three days to produce 60 “blank” measurements. A nonparametric analysis method was chosen to determine the Lob in which the 60 measurements were ordered from smallest to largest and the LoB is determined based on a critical rank position. This rank position is determined using the following equation [24]:
Where B is the number of measurements and PctB is the percentile for the distribution of blank samples. For this study, the 95th percentile was chosen, giving a rank position of 57.5. As there are no half ranks, the average of the 57th and 58th rank positions is used to determine a LoB of 0.4 g/dL. Table S2 in the Appendix gives the observed blank sample measurement results. To determine the LoD, five samples were diluted with serum to produce Hgb concentrations between 0.9-1.9 g/dL. Similar to the LoB experiment, these five samples were measured four times a day each for three days to produce 60 low level samples [24]. The Hgb concentration of each low level sample, determined by the HemoCue Hb 301 System, for each measurement day is given in Table S3 in the Appendix. A parametric analysis approach was used to determine the LoD [24]. The LoD may be calculated using the following equation [24]:
Day |
Replicate | Sample 380 | Sample 383 | Sample 384 | Sample 387 | Sample 388 |
|---|---|---|---|---|---|---|
| Blank 1 | Blank 2 | Blank 3 | Blank 4 | Blank 5 | ||
| 1 | 1 | 0.3 | 0.4 | 0.4 | 0.4 | 0.3 |
| 2 | 0.3 | 0.4 | 0.4 | 0.4 | 0.3 | |
| 3 | 0.3 | 0.4 | 0.4 | 0.4 | 0.3 | |
| 4 | 0.3 | 0.4 | 0.4 | 0.4 | 0.3 | |
| 2 | 1 | 0.3 | 0.4 | 0.4 | 0.4 | 0.3 |
| 2 | 0.3 | 0.3 | 0.4 | 0.3 | 0.3 | |
| 3 | 0.3 | 0.4 | 0.3 | 0.4 | 0.3 | |
| 4 | 0.3 | 0.3 | 0.4 | 0.4 | 0.3 | |
| 3 | 1 | 0.3 | 0.4 | 0.4 | 0.4 | 0.3 |
| 2 | 0.3 | 0.4 | 0.4 | 0.4 | 0.3 | |
| 3 | 0.3 | 0.4 | 0.4 | 0.4 | 0.3 | |
| 4 | 0.3 | 0.4 | 0.4 | 0.4 | 0.3 |
Table S2: Observed blank sample results measured using the RBA (Units in g/dL). The original samples used to make the blank solutions were randomly selected from the validation data set. All samples were confirmed to be 0.0 g/dL by the HemoCue Hb 301 system.
Sample Number |
Hgb Day 1 (g/dL) | Hgb Day 2 (g/dL) | Hgb Day 3 (g/dL) |
|---|---|---|---|
| 380 | 1.9 | 1.8 | 1.8 |
| 383 | 0.9 | 0.9 | 0.9 |
| 384 | 1.7 | 1.7 | 1.7 |
| 387 | 1.4 | 1.4 | 1.4 |
| 388 | 1.5 | 1.5 | 1.5 |
Table S3: Low level sample concentrations. The original samples used to make the low level solutions were randomly selected from the validation data set. These are the same samples used to make the blank solutions. All Hgb concentrations provided were determined by the HemoCue Hb 301 system.
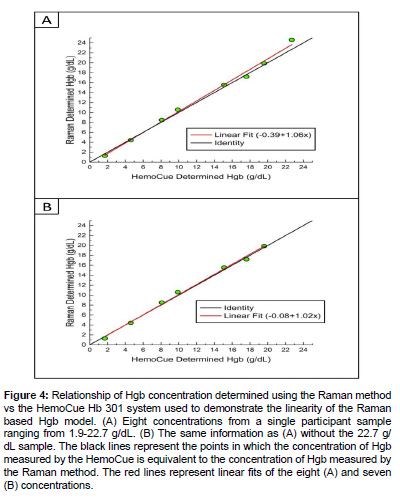
Where L is the number of measurements, J is the number of samples, ni is the number of measurements of a given sample i, and SDi is the standard deviation of a given sample i. Equation 2 gives a LoD for the Raman method of 0.6 g/dL. This LoD is well below the normal Hgb range for both males and females. Table S4 in the Appendix gives the observed low level sample measurement results. Precision was analyzed by measuring up to ten replicates of seven different samples. Table 2 summarizes the results of the precision analysis. Sample 387 showed the highest SD of 1.2 g/dL, however this sample coagulated more quickly than any other sample and only four replicate measurements were able to be collected. The SD of the precision analysis samples is less than the SD of the Raman method described here (1.3 g/dL). This demonstrates the method’s ability to provide consistent results for a given sample within the SD of the method. The linearity of the model was assessed following the guideline EP6-A Evaluation of the Linearity of Quantitative Measurements Procedures: A Statistical Approach; Approved Guideline [25]. One venous K3EDTA blood sample was diluted to eight different Hgb concentrations (1.7, 4.6, 8.1, 9.9, 15.1, 17.6, 19.6, and 22.7 g/dL). Figure 4A shows the Hgb concentration of these samples determined using the Raman method vs the HemoCue. The 22.7 g/dL sample shows the largest difference between the two measurement methods. Figure 4B shows the Hgb concentration determined using the Raman method vs the HemoCue without the 22.7 g/ dL sample. Figure 4B shows a more linear fit with the coefficient closer to unity and the intercept closer to zero compared to Figure 4A. As such, the Raman method demonstrates linearity over the range 1.7-19.6 g/dL. This range is sufficient for the analysis of most human blood samples.
Figure 4: Relationship of Hgb concentration determined using the Raman method vs the HemoCue Hb 301 system used to demonstrate the linearity of the Raman based Hgb model. (A) Eight concentrations from a single participant sample ranging from 1.9-22.7 g/dL. (B) The same information as (A) without the 22.7 g/ dL sample. The black lines represent the points in which the concentration of Hgb measured by the HemoCue is equivalent to the concentration of Hgb measured by the Raman method. The red lines represent linear fits of the eight (A) and seven (B) concentrations.
Day |
Replicate | Sample 380 | Sample 383 | Sample 384 | Sample 387 | Sample 388 |
|---|---|---|---|---|---|---|
| Low 1 | Low 2 | Low 3 | Low 4 | Low 5 | ||
| 1 | 1 | 1.3 | 0.9 | 1.5 | 1 | 1 |
| 2 | 1.3 | 0.7 | 1.3 | 1 | 1 | |
| 3 | 1.4 | 0.9 | 1.2 | 1 | 1.1 | |
| 4 | 1.3 | 0.9 | 1.1 | 0.9 | 1 | |
| 2 | 1 | 1.3 | 0.8 | 1.4 | 1.1 | 1 |
| 2 | 1.3 | 0.8 | 1.3 | 0.9 | 1 | |
| 3 | 1.3 | 0.8 | 1.2 | 1.1 | 1.2 | |
| 4 | 1.4 | 0.8 | 1.3 | 1 | 1.1 | |
| 3 | 1 | 1.5 | 0.8 | 1.5 | 1.1 | 1 |
| 2 | 1.4 | 0.8 | 1.4 | 0.9 | 1 | |
| 3 | 1.3 | 0.9 | 1.3 | 1.1 | 1.1 | |
| 4 | 1.1 | 0.8 | 1.1 | 1 | 0.9 |
Table S4: Observed low level sample results measured using the RBA (Units in g/dL). The original samples used to make the low level solutions were randomly selected from the validation data set. These are the same samples used to make the blank solutions.
Sample Number |
Hgb (g/dL) | Replicate Number | SD (g/dL) | Notes |
|---|---|---|---|---|
| 324 | 14.7 | 10 | 0.8 | |
| 334 | 14.8 | 9 | 1.0 | One replicate was damaged by laser |
| 365 | 13.5 | 10 | 0.7 | |
| 387 | 14.3 | 4 | 1.2 | Sample Coagulated Quickly |
| 396 | 13.0 | 10 | 1.0 | |
| 330 dil | 9.4 | 10 | 0.5 | |
| 331 dil | 10.5 | 10 | 0.7 |
Table 2:: Precision analysis results. All samples have a SD less than the SD of the model. Note that samples 330 dil and 331 dil are artificially altered samples.
Performance Validation
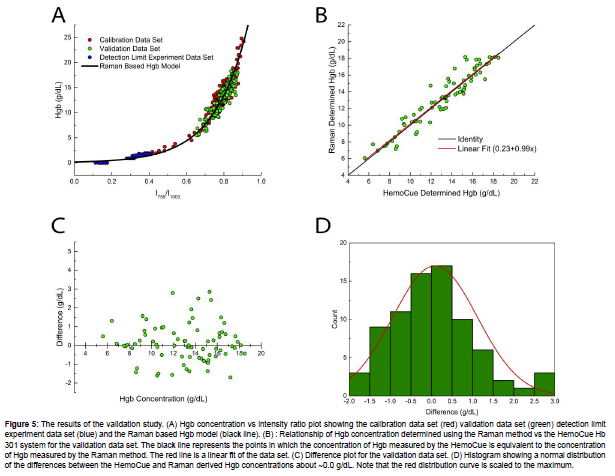
A performance validation test was performed using H15-A3 Reference and Selected Procedures of the Quantitative Determination of Hemoglobin in Blood; Approved Standard – Third Edition [26] and EP09c Measurement Procedure Comparison and Bias Estimation Using Patient Samples – 3rd Edition [27] as guidelines for experimental setup and data analysis. To assess if any measurements were outliers, the generalized extreme studentized deviate (ESD) method was used [27]. One natural sample (Sample 327) and two artificial samples made from this natural sample (Samples 327 spk and 327 dil2) were found to show aberrant behavior and were deemed as outliers. A third artificial sample that was made using Sample 327 was also removed from analysis. It is currently unknown as to why this sample showed aberrant behavior compared to the other samples, or why this behavior was observable in all artificially adjusted samples made from it. Figure 5A shows the calibration, detection limits, and validation data sets along with the Raman based Hgb model. The validation data set falls in the expected region showing consistent behavior with the calibration data set samples. Figure 5B shows the Hgb concentration of the validation data set determined using the Raman method vs the HemoCue results. The SD of the validation data set shown in Figure 5B is 1.0 g/dL, which is similar to the SD of the calibration data set. A linear fit of the data shown in Figure 5B has an intercept at 0.23, a slope of 0.99, and a Pearson’s coefficient (r) value of 0.952. Figure 5C shows a difference plot that may be used to estimate bias. This is accomplished using the following equation [27]:
Where is the bias, yi is the Raman measured Hgb concentration in g/dL, xi is the HemoCue measured Hgb concentration in g/dL, and N is the number of measurements. For the distribution shown in Figure 5C, the average bias is 0.09 g/dL. Finally, Figure 5D shows a histogram with a bell-shaped normal distribution about a difference between measurement procedures of ~0.0 g/dL Hgb. The results from the validation data set show that the Raman method agrees well with an already established PoC medical device and is able to consistently measure the Hgb concentration of whole blood over a clinically significant concentration range. This demonstrates significant prospects for the measurement of Hgb concentration in the medical field using Raman. Due to the ease of use, cost effectiveness, and rapid results calculation of Raman spectroscopy, it could be used as a rapid screening method for anemia in PoC facilities or as a more rigorous laboratory method for quantification of Hgb.
Figure 5: The results of the validation study. (A) Hgb concentration vs intensity ratio plot showing the calibration data set (red) validation data set (green) detection limit experiment data set (blue) and the Raman based Hgb model (black line). (B) : Relationship of Hgb concentration determined using the Raman method vs the HemoCue Hb 301 system for the validation data set. The black line represents the points in which the concentration of Hgb measured by the HemoCue is equivalent to the concentration of Hgb measured by the Raman method. The red line is a linear fit of the data set. (C) Difference plot for the validation data set. (D) Histogram showing a normal distribution of the differences between the HemoCue and Raman derived Hgb concentrations about ~0.0 g/dL. Note that the red distribution curve is scaled to the maximum.
Advantages and Limitations
Raman spectroscopy offers a variety of advantages over currently existing manual laboratory methods of determining Hgb concentration. Raman spectroscopy requires no reagents, making it a safer alternative to methods that utilize harmful reagents such as cyanide [3]. Additionally, the purchase, maintenance and disposal of expensive reagents are not needed, and thus the method requires less cost to run. The method described in this study does not require extensive training for technicians. The technician only needs to pipette 340 μL of blood into the sample container. Raman spectrometers are available in several different sizes and can fit in most labs easily. The smaller versions are even portable. For example, the Rapid Biofluid Analyzer device in this study only weighs ~12 lbs. The technique described in this study uses 340 μL of blood, which allows for more hematological tests to be run with a single venous drawn blood sample. Finally, Raman spectroscopy has the potential to measure several analytes at the same time. This is described in the next section in more detail. There are some limitations to using Raman spectroscopy to analyze Hgb. The main limitation of Raman spectroscopy is the resolution of the data. The proportion of inelastically scattered photons is significantly lower than the proportion of elastically scattered photons. This means that Raman signals are relatively weak and require the use of expensive gratings and CCDs to accurately resolve. As a result, the initial cost of purchasing the instrument can be high. This is, however, offset by the low cost of running the instrument. Raman spectroscopy also requires consistent measurement parameters to produce consistent results. The laboratory conditions must be controlled (e.g., climate) and the collection parameters, such as time, excitation wavelength, focus, etc., must be constant. One main limitation of the method described in this study is that the throughput is limited due to the manual nature of the technique. The procedure and method described above is intended as an alternative to manual laboratory based or PoC methods of determining Hgb concentration which are used in facilities with smaller throughputs. The samples themselves can be measured and processed in less than two minutes, thus the amount of time that a patient must wait for results will depend more upon the length of the queue and less upon the length of analysis. This can be mitigated with auto-sampling devices and similar technological upgrades.
Prospects with Other Analytes
The Raman method has the potential to quantitatively measure several analytes in addition to Hgb. When using whole blood, the resonance of the heme structure is so strong that it limits the reasonably detectable analytes to hemoporphyrins [11]. We found correlations in the Raman spectral properties of whole blood with Hgb, hematocrit (Hct), and RBC. However, if blood serum is used, several other analytes become reasonably detectable. These analytes include glucose, cholesterol, triglyceride, albumin, and total protein [13, 28- 33]. Some studies have also found success measuring these analytes in whole blood; however the signal quality and models developed tend to be worse than those obtained using serum [11].
Conclusion
Diagnosing anemia requires quantification of hemoglobin concentration in human blood samples that are both rapid and accurate. Common methods for quantifying hemoglobin concentrations have numerous disadvantages such as the use of potentially harmful reagents, high operation costs, and can be less accurate. The Raman method described in this study required no reagents, was developed based on 274 samples and validated using 82 samples that were measured and processed in less than two minutes. The model is calibrated over the range 1.7-24.8 g/dL and shows excellent linearity over the range 1.9- 19.6 g/dL. It has a(n) SD of 1.3 g/dL, Lod is 0.6 g/dL, average bias of 0.09 g/dL, and demonstrates precision within the SD. The Raman method provides a useful alternative to current manual laboratory based and PoC methods for determining Hgb concentration. Future work will assess additional analytes and additional biofluids.
Acknowledgement
This work was privately funded by Kaligia Biosciences, LLC and all authors are employees of Kaligia Biosciences, LLC. No additional incentives were provided to the authors by Kaligia Biosciences, LLC during this project or the construction of this research article. The authors wish to thank Elliot Morales and Jennica Sublett for serving as operators on the Rapid Biofluid Analyzer during the calibration portion of this project and Muhammad Asif, Abdul Wahab, and Tariq Iqbal for their work developing the OSA software.
References
- Beutler E, Waalen J (2006) The definition of anemia: what is the lower limit of normal of the blood hemoglobin concentration? Blood 107: 1747-1750.
- US Department of Health and Human Services. (n.d.). Blood tests - blood tests. National Heart Lung and Blood Institute.
- Sundermann FW (1956) Status of clinical hemoglobinometry in the United States. Am J Clin Pathol 43: 9-15.
- Wolf HU, Lang W, Zander R (1984) Alkaline haematin D-575, a new tool for the determination of haemoglobin as an alternative to the cyanhaemiglobin method. II. Standardisation of the method using pure chlorohaemin. Clin Chim Acta 136: 95-104.
- Shah VB, Shah BS, Puranik GV (2011) Evaluation of non cyanide methods for hemoglobin estimation. Indian J Pathol Micr 54: 764-768.
- Karakochuk CD, Hess SY, Moorthy D, Namaste S, Parker ME, et al. (2019) Measurement and interpretation of hemoglobin concentration in clinical and field settings: a narrative review. Ann NY Acad Sci 1450: 126-146.
- Kang SH, Kim HK, Ham CK, Lee DS, Cho HI (2008) Comparison of four hematology analyzers, CELL-DYN Sapphire, ADVIA 120, Coulter LH 750, and Sysmex XE-2100, in terms of clinical usefulness. Int J Lab Hem 30: 480-486.
- Whitehead Jr RD, Zhang M, Sternberg MR, Schleicher RL, Drammeh B, et al. (2017) Effects of preanalytical factors on hemoglobin measurement: A comparison of two HemoCue point-of-care analyzers. Clin Biochem 50: 513-520.
- Ingram CF, Lewis SM (2000) Clinical use of WHO haemoglobin colour scale: validation and critique. J Clin Pathol 53: 933-937.
- Osborn ZT, Villalba N, Derickson PR, Sewatsky TP, Wagner AP, et al. (2019) Accuracy of Point-of-Care Testing for Anemia in the Emergency Department. Resp Care 64: 1343-1350.
- Dybas J, Alcicek FC, Wajda A, Kaczmarska M, Zimna A, et al. (2022) Trends in biomedical analysis of red blood cells - Raman spectroscopy against other spectroscopic, microscopic and classical techniques. Trend Anal Chem 146: 116481-116508.
- Atkins CG, Buckley K, Blades MW, Turner RFB (2017) Raman spectroscopy of blood and blood components. Appl Spectrosc 71: 767–793.
- Berger AJ, Koo T-W, Itzkan I, Horowitz G, Feld MS, et al. (1999) Multicomponent blood analysis by near-infrared Raman spectroscopy. Appl Opt 38: 2916-2926.
- Wood BR, Caspers P, Puppels GJ, Pandiancherri S, McNaughton D, et al. (2007) Resonance raman spectroscopy of red blood cells using near-infrared laser excitation. Anal Bioanal Chem 387: 1691–1703.
- Virkler K, Lednev IK (2010) Raman spectroscopic signature of blood and its potential application to forensic body fluid identification. Anal Bioanal Chem 396: 525-534.
- Jaychandran S, Meenapriya PK, Ganesan S (2016) Raman spectroscopic analysis of blood, urine, saliva and tissue of oral potentially malignant disorders and malignancy-A diagnostic study. Int J Oral Craniofac Sci 2: 11–14.
- Zermeño-Nava J, Martínez-Martíne MU, Rámirez-de-Ávila AL, Hernández-Arteaga AC, García-Valdivieso MG, et al. (2018) Determination of sialic acid in saliva by means of surface-enhanced Raman spectroscopy as a marker in adnexal mass patients: Ovarian cancer vs benign cases. J Ovarian Res 11: 1–9.
- Radzol ARM, Lee KY, Mansor W (2013) Nonstructural protein 1 characteristic peak from NS1-saliva mixture with surface-enhanced Raman spectroscopy. 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC): 2396–2398.
- Premasiri WR, Lee JC, Ziegler LD (2012) Surface-Enhanced Raman Scattering of Whole Human Blood, Blood Plasma, and Red Blood Cells: Cellular Processes and Bioanalytical Sensing. J Phys Chem B 116: 9376-9386.
- Enejder AMK, Koo T-W, Oh J, Hunter M, Sasic S, et al. (2004) Blood analysis by Raman spectroscopy. Opt Lett 27: 2004-2006.
- Raman CV (1928) A new radiation. Indian J Phys 2: 387-298.
- Makhnii T, Ilchenko O, Reynt A, Pilgun Y, Kutsyk A, et al. (2016) Age-related changes in FTIR and Raman spectra of human blood. Ukrainian Journal of Physics 61: 853–862.
- Wood BR, Langford SJ, Cooke BM, Lim J, Glenister FK, et al (2004) Resonance Raman Spectroscopy Reveals New Insight into the Electronic Structure of β-Hematin and Malaria Pigment. J Am Chem Soc 126: 9233-9239.
- CLSI (2012)Evaluation of detection capability for clinical laboratory measurement procedures; approved guideline – second edition. CLSI document EP17-A2 Wayne PA Clinical and Laboratory Standards Institute.
- NCCLS (2003) Evaluation of the linearity of quantitative measurements procedures: a statistical approach; approved guideline. NCCLS document EP6-A Wayne PA NCCLS.
- NCCLS (2000) Reference and selected procedures of the quantitative determination of hemoglobin in blood; approved standard – third edition. NCCLS document H15-A3 Wayne PA NCCLS.
- CLSI (2018) Measurement procedure comparison and bias estimation using patient samples – 3rd ed. CLSI guideline EP09c. Wayne PA Clinical and Laboratory Standards Institute.
- Rohleder D, Kocherscheidt G, Gerber K, Kiefer W, Köhler W, et al. (2005) Comparison of mid-infrared and Raman spectroscopy in the quantitative analysis of Serum. J Biomed Opt 10: 031108 1-10.
- Qi D, Berger AJ (2007) Chemical concentration measurement in blood serum and urine samples using liquid-core optical fiber Raman spectroscopy. Appl Optics 46: 1726-1734.
- Borges R, Navarro RS, Giana HE, Tavares FG, Fernandes AB, et al. (2015) Detecting alterations of glucose and lipid components in human serum by near-infrared Raman spectroscopy. Res Biomed Eng 31: 160–168.
- Casella M, Lucotti A, Tommasini M, Bedoni M, Forvi E, et al. (2011) Raman and SERS recognition of β-carotene and haemoglobin fingerprints in human whole blood. Spectrochim Acta A 79: 915-919.
- Abe M, Kitagawa T, Kyogoku Y (1978) Resonance Raman spectra of octaethylporphyrinato‐ni(ii) and meso‐deuterated and 15N substituted derivatives. II. A normal coordinate analysis. J Chem Phys 69: 4526–4534.
- Hu S, Smith KM, Spiro TG (1996) Assignment of Protoheme Resonance Raman Spectrum by heme labeling in myoglobin. J Am Chem Soc 118: 12638–12646.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: Fazlin F, Sublett DM, Zhdanov AY, Bunch ZKM, Warren CA, et al. (2022) Quantification of Hemoglobin Concentration in Whole Blood using Raman Spectroscopy. J Anal Bioanal Tech 13: 482.
Copyright: © 2022 Fazlin F, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Open Access Journals
Article Usage
- Total views: 2713
- [From(publication date): 0-2022 - Apr 04, 2025]
- Breakdown by view type
- HTML page views: 2354
- PDF downloads: 359