Research Article Open Access
Quantification of Dimethylacetamide and its Primary Metabolite Monomethylacetamide in Plasma Using Robust LC-MS Method
Fadwa Benkessou1, Ibrahim El Serafi1, Brigitte Twelkmeyer2, Manuchehr Abedi-Valugerdi1 and Moustapha Hassan1,3*
1Division of Experimental Cancer Medicine (ECM), Department of Laboratory Medicine (LABMED), Karolinska Institutet (KI), SE-141 86 Stockholm, Sweden
2Department of Clinical Science, Intervention and Technology (CLINTEC), H9, x, Karolinska Universitetssjukhuset, K 32 141 86 Stockholm, Sweden
3Clinical Research Center (KFC), Karolinska University Hospital Huddinge, SE-141 86, Stockholm, Sweden
- *Corresponding Author:
- Moustapha Hassan
Clinical Research Center (KFC)
Karolinska University Hospital Huddinge, SE-141 86, Stockholm, Sweden
Tel: +46858583862
E-mail: moustapha.hassan@ki.se
Received date: July 16, 2016; Accepted date: August 07, 2016; Published date: August 15, 2016
Citation: Benkessou F, Serafi IE, Twelkmeyer B, Abedi-Valugerdi M, Hassan M (2016) Quantification of Dimethylacetamide and its Primary Metabolite Monomethylacetamide in Plasma Using Robust LC-MS Method. J Anal Bioanal Tech 7:327. doi:10.4172/2155-9872.1000327
Copyright: © 2016 Benkessou F, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
N,N-Dimethylacetamide (DMA) is an excellent solvent that is highly used in the production of synthetic fibres as well as in the pharmaceutical industry. It is present as a solvent in the intravenous formulation of busulphan, a drug used in high doses as myeloablative treatment prior to hematopoietic stem cell transplantation (SCT). DMA was shown to cause hepatotoxicity as well as neurotoxicity, as revealed throughout several studies including phase I study. In the present investigation we developed an LC-MS based method to detect and quantify DMA and its primary metabolite N-monomethylacetamide (MMA) simultaneously in human plasma, using a C-18 ODS-AQ/S-5 µm 12 nm separation column. The lower limits of quantification (LLOQs) for DMA and MMA were 1.8 µM and 8.6 µM, respectively. The limit of detection (LOD) for DMA and MMA were 0.53 µM and 2.52 µM, respectively. The recovery of DMA from plasma ranged from 97-101% and for MMA from 76-100%. The stability for DMA and MMA was assessed through freeze-thaw cycles and storage at different temperatures (RT, 4°C and -20°C for three days); the results have shown <7.9% CV for DMA and <14.1% for MMA. The inter-day and intra-day variation assay accuracy and precision was <6.3% for DMA and <8.6% for MMA. The calibration was linear within the ranges 1 to 4000 µM. The method was applied to follow the kinetics and to quantify DMA and its metabolite MMA in 49 plasma samples from 2 patients undergoing SCT and treated with intravenous busulphan that contain DMA. The present method is simple, robust and showed good selectivity with high accuracy, precision and reproducibility. Moreover, it can be utilized to determine DMA and its metabolite in workers, patients and environment and hence avoid toxic exposure.
Keywords
Dimethylacetamide;Monomethylacetamide;Dimethylacetamide-d9;LC-MS; Plasma;Busulphan; Stem cell transplantation
Introduction
Dimethylacetamide (DMA) is apolar solvent used in chemical and pharmaceutical industries.DMA is used in the production ofman-made fibers, films and industrial coating (2-25% of tonnage).Even though DMA is classified as a class 2 chemical, it is abundantly present in pharmaceuticals and agrochemicals (65-70% of tonnage) [1].In pharmaceuticals, DMA is used during the manufacturing process of some antibiotics (Cefadroxil, cefalexin, and cefradine); it is also used as a solvent of highly lipophilic drugs such as busulphan (Bu).The i.v.formulation of busulphan containing DMA is used in high doses during the conditioning treatment prior to hematopoietic stem cell transplantation (SCT) in order to avoid variability in bioavailability and minimize the inter-individual variation, especially in pediatric patients[2-4].
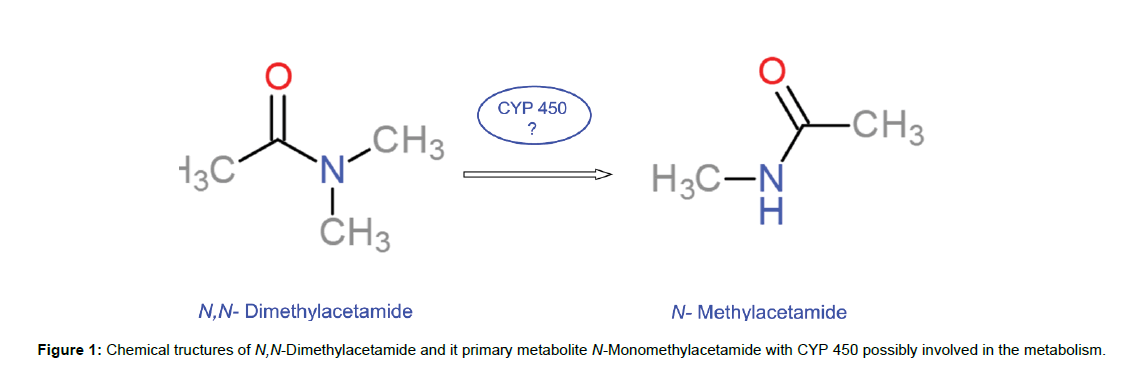
DMA is metabolized to its primary metabolite N-monomethylacetamide (MMA) in the liver possibly through the family of cytochromes P450(Figure 1).DMA metabolism was studied by microsomal incubations with purified liver microsomes from rats pretreated with acetone and ethanol, as inducers of cytochrome P450 2E1 (CYP2E1); the experiment proved that CYP2E1 is involved in DMA demethylation into MMA and acetamide[5].
DMA toxicity was studied in animals, for instance in rodents, where hepatotoxicitystarted to occur at a dose of 300-350mg/kg [6-8]. Once more in rodents, studies on reproductive toxicity of DMA and its primary metabolite MMA displayed embryotoxicity manifested in teratogenicity [9,10].A Phase I study of DMA was performed in patients by intravenous administration at a dose range of 100-450mg/kg/day for 4 to 5 days;hepatotoxicity and neurotoxicity were revealed when the dose given to patients reached 400mg/kg/day for 3 days [11]. Pediatric patientsbeing administered i.v.busulphan as part of their conditioning regimenreceivean average of 249.6mg/kg of DMA, which presents more than 62% of the daily toxic dose studied in humans; in view of that,DMA might be contributing to the liver and central nervous system (CNS) toxicity seen in those patients.Conversely, a pharmacokinetic study of DMA has shown no accumulation of the solvent in the children receiving intravenous busulphan. The pharmacokinetic analysis showed a clearance of 86.9 mL/h/kg that increased to 298 mL/h/kg on the fourth day and a volume of distribution of 469 mL/kg, while the mean initial half-life was 3.74 hours, which decreased to 0.829 hours after 96 hours [11-13].To our knowledge, no pharmacokinetic parameters have been reported for MMA as of today.
To our knowledge, there is no method for the simultaneous determination of DMA and its metabolite. However, two methods were reported for quantification of DMA in plasma; the first quantification was carried out using gas chromatography – mass spectrometry (GC-MS) while the second method was based onLC-MS [14,15]. The determination ofMMAwas previously monitored only in the urine of 27 industrial workers exposed to DMA using gas chromatography [16].In the present method, we used liquid chromatography-mass spectrometry (LC-MS/MS) for the detection and the quantification of DMA and MMA in human plasma. Compared to the published methods using LC-MS or GC-MS, the present method offers higher sensitivity, lower limit of detection for DMA and MMA, one calibration curve, and quantification of both DMA and MMA simultaneously in the same sample. The present method may be utilized for pharmacokinetic and/or metabolic studies as well as for studies to protect environment and public health from exposure to DMA.
Materials and Methods
Chemicals and reagents
N,N-Dimethylacetamide (99.9%) and N-Methylacetamide (99%) were purchased from Sigma-Aldrich CHEMIE GmbH (Steinheim, Germany); the internal standard used for this study,2[H]9-N,N-dimethylacetamide (DMA-d9) (99%), was obtained from Deutero GmbH (Kastellaun, Germany). Formic acid and acetonitrile (hypergrade for LC-MS) used in the mobile phases were from Merck KGaA (Darmstadt, Germany). The water (18 MW) used in the preparation of different solutionsduring the analysis was purified usinga water purification system (PURLAB Ultra, ELGA, High Wycombe, UK). Pooled human plasma from healthy individuals was provided by the blood and transfusion center at Karolinska University Hospital-Huddinge,and then stored immediately at -20°C until needed for experiments.
Instrumentation
The analysis was performed on a Finnigan TSQ Quantum Ultra triple quadrupole mass spectrometer with electrospray ionization (ESI) (Thermo FinniganSan Jose,CA, USA),coupled to a high performance liquid chromatography (HPLC) system consisting of a 1100 thermostatedautosampler (Agilent,Waldbronn, Germany), a 1100 binary pump (Hewlett Packard,Waldbronn, Germany), and a 1100 degasser (Agilent, Tokyo,Japan). The mass spectrum was tuned to the following operating conditions: a positive ion mode with an ion spray voltage of 5000 V, sheath and auxiliary gas pressure of 20 and 5 arbitrary units, respectively. The capillary temperature of the ion source was set at 237°C. The column used for the separation was YMC Pack ODS-AQ/S-5µm, 12nm maintained at 21°C preceded by a guard-column ODS-AQ /S-5µm with apore size of 12nm from YMC (Kyoto, Japan). Data analysis and instrument control were carried through Xcalibur™ software from Thermo Scientific (21 CFR Part 11, 1997).
The mobile phases used for sample separation in the LC-MS were acetonitrile (Phase A) and water (phase B).Both solutions contain 0.1% formic acid.
Sample preparation
All the stock solutions of the DMA, MMA, and DMA-d9 and the initial concentrations used for the experiments were prepared fresh daily in water and acetonitrile (1:1, v/v). The stock solution concentrations were 400 mM for DMA and MMA, and 300 µM for DMA-d9. A volume of 100µL of human plasma spiked with DMA and MMA was added to a 1.5mL tube followed by 200 µL of the internal standard. Protein precipitation was performed by adding 100µL acetonitrile; the samples were vortexed for 30 seconds, and then centrifuged at 22136×gfor 10 minutes. The organic phase was transferred to the LC-MS vials to be further analyzed.
For patient samples; the blood samples were collected at 5, 15 and 30 minutes and 1, 2, 4, 6, and 8 hours after the end of 2-hours infusion. Samples were centrifuged at 200 g, plasma was separated and stored at -20°C until assay. The samples were prepared as described above andDMA and MMA were quantified in 49 samples after the administration of dose number 1, 4, and 8 of the drug.
The validation procedure was carried out according to the FDA guideline for bioanalytical method validation “Guidance for Industry-Bioanalytical Method Validation, US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER) and Center for Veterinary Medicine (CVM), May 2001, http://www.fda.gov/cder.guidance.index.htm[17,18].
Results
Instrumental optimization
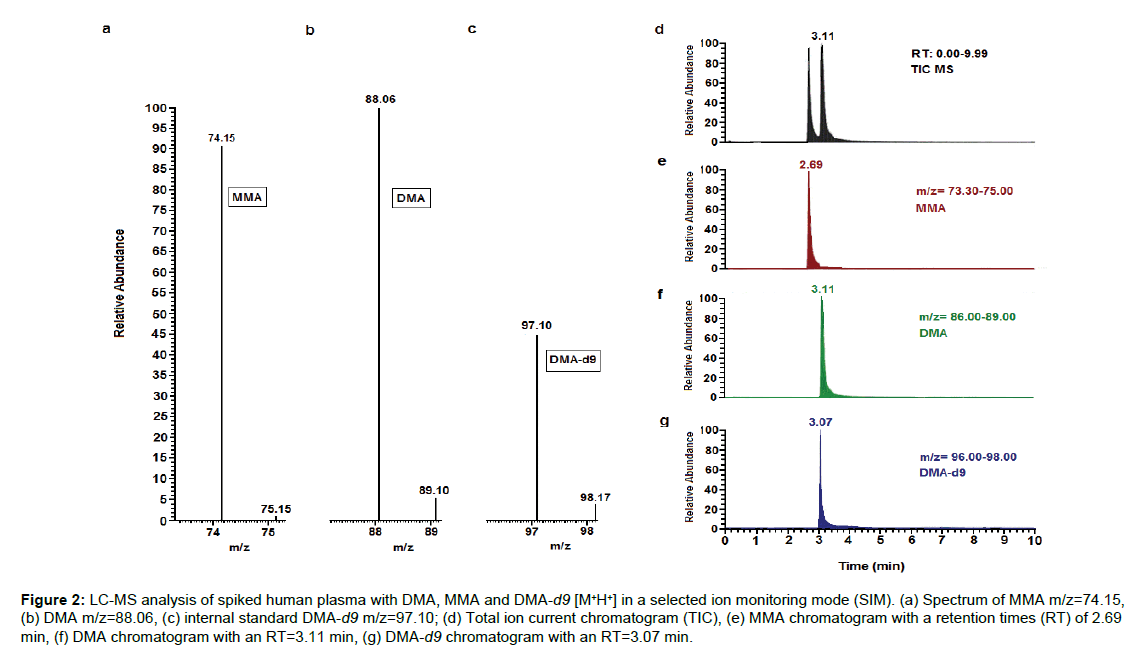
In order to establish optimal chromatography parameters for detection of DMA, MMA and DMA-d9, we performed a total ion chromatogram (TIC) to generally identify our compounds after a constant infusion of the three analytes to the mass spectrometer. Selected ion monitoring (SIM) was used next to monitor the ions of interest using the selected m/zvalues for each compound. Calibration of the LC-MS was finally defined to be conducted on a SIM and in positive ionization mode with a flow rate of 0.7mL·min-1and a changing gradientfrom 25%to5% for phase Aand from75% to95% for phase B; the gradient was returned back to the starting conditions by the end of each run for 5 minutes for the column re-equilibration. The molecular ions for the three compounds were m/z 74.15, 88.06 and 97.10 with retention times of 2.69 min, 3.11 min, and 3.07 min for MMA, DMA, and DMA-d9, respectively (Figure2).
Figure 2: LC-MS analysis of spiked human plasma with DMA, MMA and DMA-d9 [M+H+] in a selected ion monitoring mode (SIM). (a) Spectrum of MMA m/z=74.15, (b) DMA m/z=88.06, (c) internal standard DMA-d9 m/z=97.10; (d) Total ion current chromatogram (TIC), (e) MMA chromatogram with a retention times (RT) of2.69 min, (f) DMA chromatogram with an RT=3.11 min, (g) DMA-d9 chromatogram with an RT=3.07 min.
Method validation
Selectivity: The selectivity was assured by running spiked plasma with DMA, MMA, and DMA-d9 as well as blank plasma prepared by following all the steps of sample preparation. The three compounds were successfully selected by their molecular ions and there was no interference detected between the plasma with the analytes and the drug free plasma.
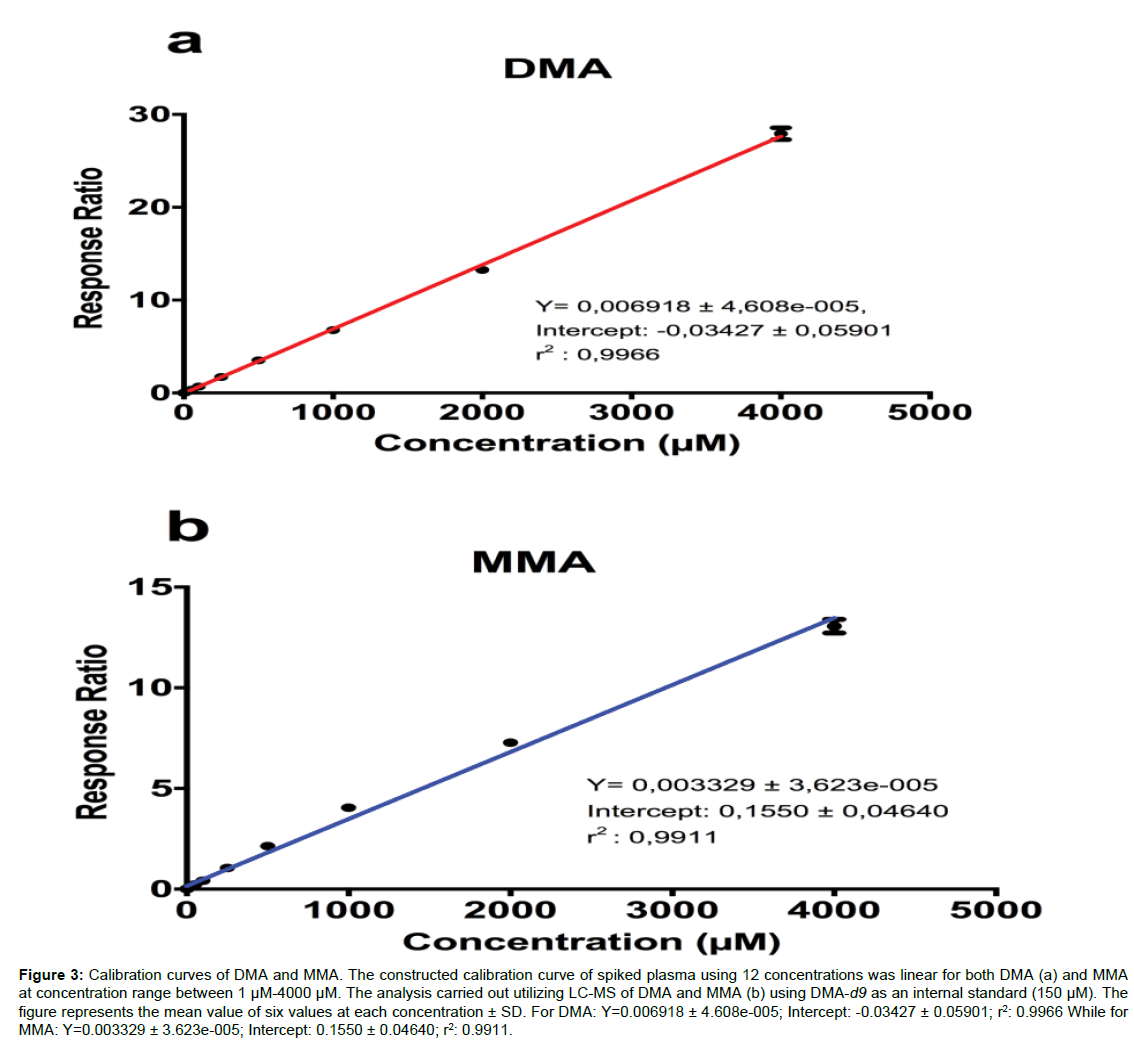
Calibration curve, LOD, and LLOQ: The final concentrations used in the standard curve for DMA and MMA ranged between 1µM and 4000µM (Figure 3); the calibration curve linearity was assessed by analyzing 12 points (1, 2.5, 5, 10, 25, 50, 100, 250, 500, 1000, 2000, and 4000µM) for both DMA (Figure 3a) and MMA (Figure 3b) along with 150µM as a final concentration used forDMA-d9. The limit of detection (LOD) for DMA and MMA were 0.53 µM and 2.52 µM, respectively (3 times signal/noise); the lower limit of quantification (LLOQ) for both compounds was calculated as 10 times signal to noise ratio, andwere 1.8µMfor DMA and 8.6µM for MMA.
Figure 3: Calibration curves of DMA and MMA.The constructed calibration curve of spiked plasma using 12 concentrations was linear for both DMA (a) and MMA at concentration range between 1µM-4000µM. The analysis carried out utilizing LC-MS of DMA and MMA (b) using DMA-d9 as an internal standard (150 µM).The figure represents the mean value of six values at each concentration± SD. For DMA:Y=0.006918 ± 4.608e-005; Intercept: -0.03427 ± 0.05901; r2: 0.9966While for MMA: Y=0.003329 ± 3.623e-005; Intercept: 0.1550 ± 0.04640; r2: 0.9911.
Accuracy and reproducibility: The accuracy of the method was assessed by running quality control (QCs) plasma samples (n=6, for each level) at low, medium and high concentrations (10, 100and 1000µM), the variation for the accuracy test was <3% for DMA and <8.2% for MMA. We have similarly evaluated the reproducibility of the method by running spiked plasma with DMA and MMA at three different known concentrations (10 µM, 250 µM and 1000 µM) and analyzing the samples(n= 6 for each concentration) within the period of the study. The deviation between samples was identified to be <5.5% for DMA and <7%for MMA (Table 1).
| DMA | MMA | ||||||
|---|---|---|---|---|---|---|---|
| Concentration | Mean (µM) | STDV | CV% | Mean | STDV | CV% | |
| (µM) | (µM) | (µM) | (µM) | ||||
|
Accuracy |
10 |
10.71 |
0.31 |
2.92 |
11.98 |
0.8 |
6.69 |
|
|
100 |
106.25 |
0.78 |
0.74 |
111.27 |
9.08 |
8.16 |
|
|
1000 |
1004.19 |
23.23 |
2.31 |
1174.9 |
78.31 |
6.67 |
|
Reproducibility |
10 |
11.15 |
0.17 |
1.51 |
10.79 |
0.23 |
2.14 |
|
|
250 |
271.79 |
14.74 |
5.42 |
238.47 |
16.47 |
6.9 |
|
|
1000 |
1041.14 |
38.68 |
3.72 |
1070.56 |
49.16 |
4.59 |
*Spiked Plasma with low, medium and high DMA and MMA concentrations to study the accuracy and the reproducibility of the method. The results for accuracy are
showing <3% CV for DMA and <8.2% for MMA while for reproducibility the variation is <5.5% for DMA and <7% for MMA.
Table 1: Results of Accuracy and Reproducibility of DMA and MMA in Human Plasma (n=6).
Precision, stability and recovery: In order to evaluate the inter- and intra-day variation, we quantified both compounds in multiple aliquots prepared from a freshly taken patient plasma sample used for therapeutic drug monitoring (TDM) for Busilvex (i.v.formulation ofbusulphan containing DMA). The results have shown <6.3% for DMA and <8.6% for MMA % coefficient of variation. In order to mimic the conditions that a biological sample goes through, we have investigated the stability of DMA and MMA in actual freshly taken patient samples used for TDM for busilvex. The quantification of both analytes was performed after cycle 1, cycle 2, and cycle 3 of freeze-thaw; the results have shown <7.9% variation between the three cycles for DMA and<4.8% for MMAMMA. Further experiments were performed to study the temperature effect on the stability of both compounds using spiked plasma with DMA and MMA at three different concentrations (10 µM, 250 µM and 1000 µM; n=6 for each concentration).The plasma was stored immediately at 4 and -20°C, and later extracted and analyzed after 24 hours. Post-preparation stability was considered by re-quantifying extracted samples after they had been left in room temperature for more than 24 hours. The stability results have shown a variation that is <6.8% for DMA and <14.1% for MMA (Table 2).
| DMA | MMA | ||||||
|---|---|---|---|---|---|---|---|
| Mean | STDV (µM) | CV% | Mean | STDV | CV% | ||
| (µM) | (µM) | (µM) | |||||
|
Inter-day Variation |
417.42 |
19.1 |
4.58 |
23.71 |
0.81 |
3.41 |
|
|
Intra-day Variation |
94.9 |
5.92 |
6.24 |
29.63 |
2.53 |
8.53 |
|
|
Freeze-Thaw Cycles |
371.86 |
29.33 |
7.89 |
23.27 |
1.11 |
4.77 |
|
|
DMA |
MMA |
||||||
|
Stability |
Concentration |
Mean (µM) |
STDV |
CV% |
Mean |
STDV |
CV% |
|
|
(µM) |
|
(µM) |
|
(µM) |
(µM) |
|
|
4°C |
10 |
11.21 |
0.39 |
3.52 |
11.69 |
0.23 |
2.01 |
|
|
250 |
247.89 |
8.7 |
3.51 |
231.35 |
12.13 |
5.24 |
|
|
1000 |
954.32 |
11.83 |
1.24 |
993.71 |
24.95 |
2.51 |
|
-20°C |
10 |
10.38 |
0.2 |
1.94 |
11.81 |
0.41 |
3.43 |
|
|
250 |
239.33 |
16.15 |
6.75 |
237.34 |
9.18 |
3.87 |
|
|
1000 |
989.65 |
37.07 |
3.75 |
972.77 |
43.73 |
4.5 |
|
Post-Preparation (RT) |
25 |
27.01 |
0.24 |
0.89 |
26.61 |
3.73 |
14.03 |
|
|
500 |
517.96 |
9.7 |
1.87 |
585.47 |
36.84 |
6.29 |
|
|
2000 |
1937.01 |
58.17 |
3 |
2044.33 |
87.83 |
4.3 |
*Inter- and intra-day variations along with the stability through freeze-thaw cycles (3 cycles) were studied in fresh unknown plasma sample from unknown patient
receiving the i.v. busulphan containing DMA. Results are showing CV <7.9% for DMA and <8.6 for MMA.
*Stability of DMA and MMA in different temperatures using spiked plasma with low, medium and high concentrations; CV <6.8% for DMA and <14.1% for MMA.
Table 2: Results of Precision (n=6) and Stability (n=6) of DMA and MMA in Human Plasma.
The extraction recovery was studied in spiked human plasma with DMA and MMA at low, medium and high concentrations (10µM, 250µM, and 1000µM) (n=6). The recovery of DMA ranged between 97-101%, while for the MMA the recovery was between 76 - 100% (Table 3).The variability observed for MMA recovery We agree most probably is due to the matrix effect since the extractions from plasma (calculated peak area MMA/peak are IS)were 0.11, 3.84 and 14.87 at the levels measured10, 500 and 2000 µM, respectively; compared to that extracted from aqueous solutions (0.20, 5.20 and 17.08) at the same concentration levels (n=3 each). The results have shown less than 10% variation between the three cycles for both DMA and MMA.
| DMA | MMA | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Concentration | Mean (µM) | STDV | CV% | Recovery | Mean | STDV | CV% | Recovery | |
| (µM) | (µM) | % | (µM) | (µM) | % | ||||
|
Recovery |
10 |
9.56 |
0.22 |
2.28 |
100.81 |
10.17 |
1.53 |
15.01 |
100 |
|
|
250 |
253.15 |
16.15 |
6.38 |
99.89 |
197.26 |
7.16 |
3.63 |
76.49 |
|
|
1000 |
1000.09 |
17.75 |
1.77 |
97.21 |
804.87 |
42.66 |
5.3 |
83.54 |
Table 3:Results of Recovery of DMA and MMA in Human Plasma (n=6).
Clinical application
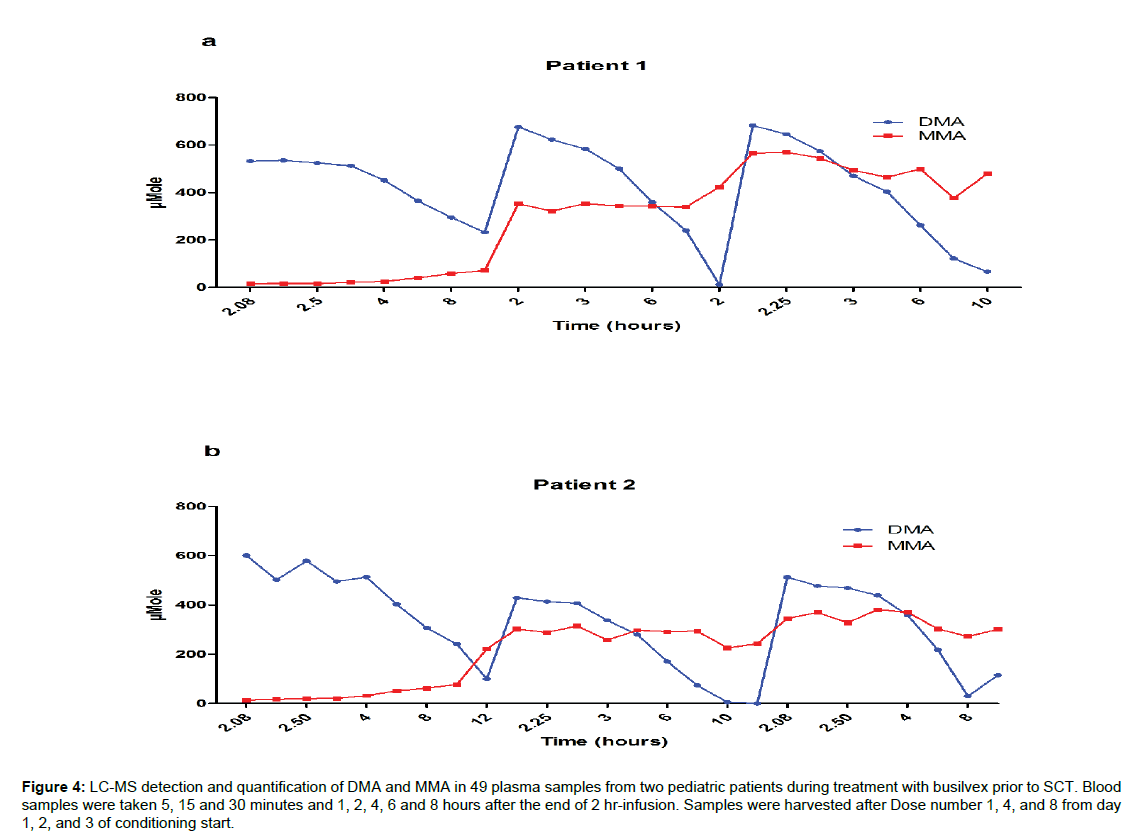
The samples used for the clinical application study were taken from the same samples used for the TDM of busilvex. Patients were administered a dose of busulphan of 1.8 mg/kg twice daily for four days. Blood samples were taken 5, 15 and 30 minutes and 1, 2, 4, 6, and 8 hours after the end of drug administration (2 hours infusion). We quantified both DMA and MMA in a total number of 49 samples after the administration of dose number 1, 4, and 8 of the drug (Figure 4). We were able tofollow simultaneously the kinetics, elimination and accumulation of DMA and MMA in all samples. The concentrations of the two compounds measured in these patients follow the same pattern; in other words, DMA has shown no accumulation throughout the period of treatment, which is in agreement with a previous study mentioned earlier [13]; MMA on the other hand showed clear accumulation during the 4 days treatment period.
Figure 4: LC-MS detection and quantification of DMA and MMA in 49 plasma samples from two pediatric patients during treatment with busilvex prior to SCT. Blood samples were taken 5, 15 and 30 minutes and 1, 2, 4, 6 and 8 hours after the end of 2 hr-infusion. Samples were harvested after Dose number 1, 4, and 8 from day 1, 2, and 3 of conditioning start.
Discussion
Methods have previously been developed for DMA quantification in biological matrices, with the first assay based on GC-MS for detection of DMA in whole blood in 1988 [14]. More recently, LC-MS for determination of DMA in plasma and GC with Nitrogen Phosphorous Detector for MMA in urine were established independently with respective LLOQ of 0.25 mg·L-1and 1 mg· L-1 [15,19]. There is an obvious exposure risk for workers in factories using DMA; accordingly, many studies have tried to correlate DMA exposure to the urine levels of its metabolite MMA [16,20]. In the present study, we observed that MMA is accumulated during the 4 days treatment to reach a maximum after three days,which indicates that the correlation between urine levels of MMA and plasma levels of DMA after single exposure time is not accurate enough to explore DMA toxicity. Moreover, in patients the high concentration of DMA in certain drug formulations (e.g.,busulphan) could promote possible toxicities when the treatment is extended over several days,which in turn causes an accumulation of MMA. On the other hand, the accumulation of MMA may lead to an induction/inhibition of enzymes involved in drug/DMA metabolism and hence alert treatment efficacy and/or cause drug interactions.
Conclusions
This is the first study for the determination and quantification of both DMA and MMA simultaneously in human plasma.
The described method is simple, robust and showed high sensitivity, accuracy and reproducibility.
The calibration curves for DMA and MMA are linear and covering wide range of concentrations for both the parent compound and its metabolite.
The present method will easily allow pharmacokinetic, toxicological and metabolic studies as well as other investigations in order to protect environment and public health from exposure to DMA.
Acknowledgements
The present project was supported by grant (CAN2014/795) from the Swedish Cancer Society.
References
- European Chemicals Agency (2012).
- Hassan M, Ljungman P, Bolme P, Ringdén O, Syrůcková Z, et al. (1994) Busulfan bioavailability. Blood84: 2144-2150.
- Andersson BS, Madden T, Tran HT, Hu WW, Blume KG, et al. (2000) Acute safety and pharmacokinetics of intravenous busulfan when used with oral busulfan and cyclophosphamide as pretransplantation conditioning therapy: a phase I study. Biology of Blood and Marrow Transplantation 6: 548-554.
- Bhagwatwar HP, Phadungpojna S, Chow DS, Andersson BS (1996)Formulation and stability of busulfan for intravenous administration in high-dose chemotherapy. Cancer Chemother Pharmacol 37: 401-408.
- Silvia M, Vincenzo L, Arturo M, Giovanni GP (1994) Microsomal metabolism of N,N-diethylacetamide and N,N-dimethylacetamide and their effects on drug-metabolizing enzymes of rat liver. Biochemical pharmacology 48: 717-726.
- Hundley SG, McCooey KT, Lieder PH, Hurtt ME, Kennedy GL (1994)Dimethylformamide pharmacokinetics following inhalation exposure in monkeys. Toxicology letters 73: 213-225.
- Kinney L, Burgess B, Stula E, Kennedy G (1993) Pharmaceutical composition, a method of preparing it and a method of treatment by use there of.Drug and chemical toxicology 16: 175-194.
- Malley LA, Slone TW, Makovec GT, Elliott GS, Kennedy GL(1995)Chronic toxicity/oncogenicity of dimethylacetamide in rats and mice following inhalation exposure. Toxicological Sciences 28: 80-93.
- Johannsen FR, Levinskas GJ, Schardein JL (1987) Teratogenic response of dimethylacetamide in rats. Toxicological Sciences 9: 550-556.
- Menegola E, Broccia ML, Prati M, Giavini E (1999)In vitro embryotoxicity study of n,n-dimethylacetamide and its main metabolite N-monomethylacetamide.Toxicology in vitro 13: 409-415.
- Weiss AJ, Jackson LG, Carabasi RA, Mancall EL, White JC (1962) A phase I study of dimethylacetamide. Cancer chemotherapy reports16: 477-485.
- Trame MN, Bartelink IH, Boos J, Boelens JJ (2013)Population pharmacokinetics of dimethylacetamide in children during standard and once-daily IV busulfan administration. Cancer Chemother Pharmacol 72: 1149-1155.
- Hempel G, Oechtering D, Lanvers-Kaminsky C, Klingebiel T, Vormoor J, et al. (2007)Cytotoxicity of dimethylacetamide and pharmacokinetics in children receiving intravenous busulfan.Journal of clinical oncology 25: 1772-1778.
- LindströmB, SjöbergP, FlobergLindströmS, SjöbergBP (1988) Gas chromatographic-mass spectrometric method for the determination of dimethylacetamide and metabolites in whole blood.Journal of Chromatography B: Biomedical Sciences and Applications 428: 156-159.
- Oechtering D, Boos J, Hempel G (2006) Monitoring of N,N-dimethylacetamide in children during i.v.-busulfan therapy by liquid chromatography-mass spectrometry. Journal of chromatography- B, Analytical technologies in the biomedical and life sciences 838: 129-134.
- Kawai T, Mizunuma K, Okada Y, Odachi T, Horiguchi S, et al.(1997) Monitoring of Occupational Exposure to 1-Butanol by diffusive sampling and Urinalysis.Journal of Occupational Health 39: 113-118.
- Polli JE, Abrahamsson BS, Lawrence XY, Amidon GL, Baldoni JM, et al. (2008) Summary workshop report: bioequivalence, biopharmaceutics classification system, and beyond. The AAPS journal 10: 373-379.
- Shah VP, Midha KK, Dighe S, McGilveray IJ, Skelly JP, et al. (1992) Analytical methods validation: bioavailability, bioequivalence and pharmacokinetic studies. Pharmaceutical Research 9: 588-592.
- Hong-fang T, Zheng R, Dan-hua LIU, Han W, Hai-bao Z, et al. (2012)The Analysis of urinary N-methylacetamide by GC-NPD with a direct injection. Chinese journal of industrial hygiene and occupational diseases 30: 386-388.
- Perbellini L, Princivalle A, Caivano M, MontagnaniR (2003) Biological monitoring of occupational exposure to N,N-dimethylacetamide with identification of a new metabolite.Occupational and environmental medicine 60: 746-751.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 13466
- [From(publication date):
August-2016 - Apr 05, 2025] - Breakdown by view type
- HTML page views : 12428
- PDF downloads : 1038