Quality of Life and Exercise Performance in COPD- is there a Link?
Received: 15-Feb-2022 / Manuscript No. jcpr-22-54445 / Editor assigned: 17-Feb-2022 / PreQC No. jcpr-22-54445(PQ) / Reviewed: 03-Mar-2022 / QC No. jcpr-22-54445 / Revised: 08-Mar-2022 / Manuscript No. jcpr-22-54445(R) / Published Date: 15-Mar-2022 DOI: 10.4172/jcpr.1000158
Abstract
Background: Chronic obstructive pulmonary disease (COPD) is a debilitating disease. The severity of airflow limitation alone is not strongly correlated with quality of life (QoL) and, therefore, exercise testing is employed to measure functional performance objectively, which is generally believed to correlate with QoL.
Objectives: Understand the relation between exercise capacity and quality of life.
Methods: Prospective study. Stable COPD patients were consecutively recruited. We analysed demographic, clinical, functional and exercise performance data. Patients answered symptoms and quality of life questionnaires - modified Medical Research Council (mMRC) dyspnoea scale, COPD Assessment Test (CAT), COPD Clinical Questionnaire (CCQ).
Results: 124 subjects - 13% female, mean age 66 ± 9years. Most patients were GOLD B or D (36% and 33%). Median FEV1 was 47%, (38-65%). Forty subjects (32%) had mMRC ≥ 2, CAT was ≥ 10 in 35% and CCQ ≥ 1.5 in 40% of patients. FEV 1 and hyperinflation, measured by IC/TLC, correlated with mMRC and CCQ but not with CAT. DLCO correlated better with mMRC and CCQ than CAT. The median peak VO2, in CPET, was 15.5 mL/min/kg (13.6-17.8 mL/min/kg). The 6MWD median was 479 meters (404-510 m), with median ΔSpO2 5% (3-9%). Patients with mMRC ≥ 2 had worse performance in both 6MWT and CPET - lower 6MWD, peak workload and peak VO2 and higher ΔSpO2 and ΔBorg. The 6MWD, peak workload and peak VO2 also correlated with quality of life (CAT and CCQ). Higher mMRC and CCQ were associated with dynamic hyperinflation, but not CAT.
Conclusion: Symptoms and QoL can estimate exercise performance.
Keywords
QoL; PRO; 6-min walk test; 6MWD; CPET; Peak VO2
Introduction
Exertional dyspnoea and reduced exercise capacity are typical features of chronic obstructive pulmonary disease (COPD) [1]. Lung function parameters evaluated at rest cannot accurately predict exercise capacity, thus exercise testing is a precious tool to evaluate these patients [2].
Dyspnoea is the most common exercise-limiting symptom in COPD [3, 4] and a better explanatory factor for exercise inactivity than FEV1 [5]. Dyspnea correlates with patient’s performance during the 6-minute walk test (6MWT) [6] better than FEV1 [7]. Nonetheless, characterizing COPD only according to breathlessness does not achieve a full vision of the disease and the patient`s daily limitations as it is recognized that COPD has multiple symptomatic effects on health-related quality of life (HRQoL) [8]. Several patient-reported outcome (PRO) measures are used to assess HRQoL [9-12]. The severity of airflow limitation alone is not strongly correlated with HRQoL in COPD patients and exercise testing is believed to better assess functional performance and overall HRQoL [13, 14]. Nonetheless, the extent to which laboratory and fieldbased exercise test correlate with PROs in not fully understood. Since 2011, Global Initiative for Chronic Obstructive Lung Disease strategy (GOLD) adopted St. George’s Respiratory Questionnaire (SGRQ) [15] as a new assessment tool for COPD patients. SGRQ is the most widely used and valid tool to evaluate the health status of COPD patients [9] but it is composed of 50 items with 76 weighted responses which may be difficult to use in clinical practice. The COPD Assessment Test (CAT) was developed to minimize the complexity of SGRQ, and it is associated with clinically important variables in COPD patients – dyspnoea measured by mMRC, exacerbations and lung function [11]. The clinical COPD Questionnaire (CCQ) is a simple questionnaire; it is easy to apply in daily routine and correlates well with SGRQ, CAT and lung function [12, 16-18]. CCQ also seems to be a good instrument for assessing health status and predict mortality in COPD patients [19]. GOLD recommends a comprehensive approach to adequately manage symptoms in COPD and proposes the mMRC, CAT and CCQ as interchangeable instruments for evaluation health status [17, 20].
The easiest and most used exercise test in COPD is the 6MWT [21]. It provides important data such as the walked distance and desaturation during the test and it is a crucial parameter of the BODE index [22]. The variation in the distance covered during the 6MWT correlates with changes in spirometry [23] and it is also a better predictor of mortality than FEV1 [24, 25]. Still, the cardiopulmonary exercise test (CPET) is considered the gold standard in these patients, due to its ability to determine the level of exercise limitation and its causes [26]. CPET indexes, such as peak oxygen uptake (VO2 peak), ventilatory equivalents for carbon dioxide production (VE/VCO2) and arterial oxygen saturation (SpO2) provide important functional information and are better prognostic predictors than lung function measurements obtained at rest [27-29].
In clinical practice, exercise testing is not always available or is not used by clinicians and the severity of symptoms or QoL impairment should be a stimulus for further investigation. Only limited evidence is available to support an association between exercise test outcomes and PRO in patients with COPD [30]. The limited data available for CPET suggests that it may be more closely associated with the SGRQ and other HRQoL outcomes than the 6MWT [30-32], but more studies are needed. The aim of the present study is, therefore, as primary outcome to investigate if breathlessness, measured by mMRC, and respiratory symptoms and QoL, measured by CAT and CCQ, can predict maximal exercise capacity assessed by CPET and 6MWT, using a large cohort of COPD patients across all severity stages and the scope of this association.
Material and Methods
Study design and participants
Single-centre, prospective studies to evaluate the impact of PRO in exercise capacity in COPD patients. We included patients with stable COPD defined by GOLD. Patients were selected consecutively after reference to the lung function department for respiratory function assessment between October 2018 and March 2019, if they could complete exercise tests (Cyclergometer – incremental protocol and 6MWT) and QoL questionnaires, irrespectively of their age, smoking history and GOLD stage. All bronchodilators and corticosteroid medication were allowed in the study. Patients were classified as nonexacerbators if they did not have moderate to severe exacerbations in the past 12 months.
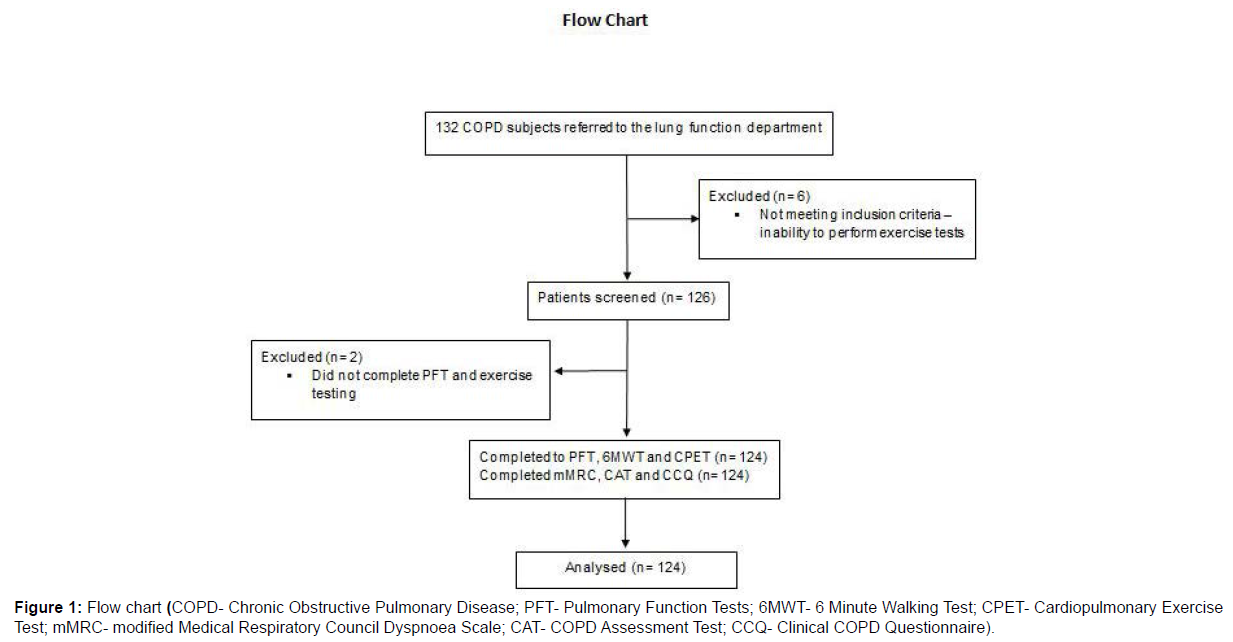
Exclusion criteria included: Diagnosis of asthma; Severe emphysema requiring endobronchial interventions within 6 months prior to screening; Pregnancy; History of myocardial infarction within 6 months; Life-threatening cardiac arrhythmia; Diagnosis of thyrotoxicosis; Known active tuberculosis. A total of 8 participants were excluded (132 subjects screened): 6 subjects had physical limitations that impaired the ability to perform exercise tests and 2 subjects missed the scheduled exams (Figure 1).
Procedure
Ethical approval for this study was obtained from the Ethics Committee (CHVNG – 199/2018). The study was conducted according to the ethical principles of the Declaration of Helsinki. The purpose of the study was explained to prospective participants and informed consent form was obtained before any assessment was performed.
Data were collected by the authors and respiratory function tests were performed by the Pulmonary Function Testing Laboratory cardiopulmonary technicians. Sociodemographic characteristics and medications prescribed were recorded and anthropometric characteristics were measured. CAT, CCQ and mMRC dyspnoea scale were collected before respiratory testing. In assessment of COPD, An mMRC value<2, a CAT value<10 or a CCQ value<1.5 suggest stable health status [1].
Patients performed pulmonary function testing following ATS/ ERS guidelines [33] including routine post-bronchodilator spirometry [34] and lung volumes measurement with plethysmography [35]. The 6MWT followed the ATS recommendations [36-37]. Participants were encouraged to resume walking as soon as possible but they were allowed to stop during the test if their symptoms became intolerable. A single operator monitored and recorded the 6MWD simultaneously. The distance walked in the test was reported in meters and as a percentage of predicted value using reference equations previously developed for healthy population [38]. Oxygen desaturation and dyspnoea and leg discomfort measured by BORG scale in the beginning and end of the test [39] were recorded. Desaturation was defined as ≥ 4% reduction between arterial oxygen saturation measured by Pulse oximetry preand post-test (ΔSpO2 ≥ 4%) and post-test SpO2<90% [24,40]. Use of oxygen during the test was standardised, if required (patients with long term oxygen therapy (LTOT) completed the test with oxygen). Due to practical reasons (time and staff constrains), each patient performed only one 6MWT. Incremental CPET followed ATS/ACCP standards [41]. Patients were subjected to symptom-limited incremental exercise with progressively increasing work rate until fatigue was reached (increment selected to maintain exercise for 8–10 min). We performed breath-by-breath monitoring of cardiopulmonary variables [Minute ventilation (VE), Pulmonary O2 uptake (VpO2), Pulmonary CO2 output (VCO2), Heart Rate (HR)]. Dynamic hyperinflation was assessed by measuring IC repetitively during CPET as patients were required to take a deep inspiration, after normal expiration, at predetermined intervals of 2 min [42]. Ventilatory limitation at peak exercise was defined by VE/maximum voluntary ventilation (MVV) above 85% [43,44].
Statistical analysis
Data are presented as frequency (%) and mean ± SD or median and interquartile range. The normality of the data distribution was checked by the Kolmogorov–Smirnov test. Unpaired t-test, Mann- Whitney test and Chi square test were used for comparisons when appropriate. Relationships between variables were assessed by Pearson correlation coefficient (r) or by Spearman's rank correlation coefficient (rho), depending on the data distribution. A statistical analysis was undertaken using SPSS 28.0. p<0.05 was considered significant.
Results
Main clinical and functional characteristics of the 124 study participants included in the study are presented in (Table 1). Our cohort was mostly male (87%) with a mean age of 66 years (±9). Only 6% of our patients were non-smokers. Most patients were GOLD B or D (36% and 33%, respectively) and had moderate or severe airflow limitation (median FEV1 was 47% of predicted, interquartile range (IQR) 38-65%). Static hyperinflation (TLC>120%) was present in 43% of patients. Forty-nine patients (40%) were enrolled in pulmonary rehabilitation (PR) programs. Forty patients (32%) had mMRC ≥ 2, CAT was ≥10 in 35% of our cohort and CCQ ≥1.5 in 40% of patients (Table 1).
| Sex - female, n (%) | 16(13) | Exacerbator, n (%) | 54(44) |
|---|---|---|---|
| Age (years-old) | 66 (±9)* | Respiratory Rehabilitation, n (%) | 49(40) |
| BMI (Kg/m2) | 27 (±4)* | LTOT, n (%) | 8(7) |
| Smoking history | OSA, n (%) | 13(11) | |
| Current smokers, n (%) | 37(30) | Heart disease, n (%) | 21(17) |
| Former smokers, n (%) | 79(64) | mMRC, median (P25-P75) | 1 (1-2) # |
| GOLD stage n (%) | mMRC ≥2, n (%) | 40 (32) | |
| A | 26(21) | CAT, median (P25-P75) | 8 (5-12) # |
| B | 44(35) | CAT ≥10, n (%) | 43 (35) |
| C | 13(11) | CCQ, median (P25-P75) | 1.2 (0.6-2.0) # |
| D | 41(33) | CCQ ≥1.5 n (%) | 52 (42) |
| Data presented as mean and standard deviation* or median and quartiles# and n(%) for qualitative variables abbreviations | |||
| BMI: Body Mass Index; GOLD: Global Initiative for Chronic Obstructive Lung Disease; LTOT: Long Term oxygen Therapy; OSA: Obstructive Sleep Apnoea; mMRC: modified Medical Respiratory Council Dyspnoea Scale; CAT: COPD assessment test; CCQ: Clinical COPD Questionnaire. | |||
Table 1: Baseline characteristics of study participants.
In our group of patients, PR was associated with less symptoms measured by lower mMRC<2 (p=0.02) and improved QoL with CCQ<1.5 (p<0.01). CAT was not associated with PR. Non-exacerbators had less symptoms and lower QoL, according to all questionnaires (p<0.01). Having heart diseases (6 patients had cardiac arrythmias, 2 had valvular disease, 3 had ischemic heart disease and 10 had heart failure) or sleep apnoea did not influence the presence of symptoms or patient´s QoL (p>0.05).
We found a strong correlation between CAT and CCQ. mMRC correlated moderately with both CCQ and CAT. DLCO correlated with both symptoms (mMRC) and QoL (CCQ and CAT) but the correlation with CAT was weak. FEV 1 and hyperinflation, measured by IC/TLC, correlated with mMRC and CCQ but not with CAT (Table 2a and 2b).
The median workload in CPET was 64 watts (46-88 W) with median peak VO2 15.5 mL/min/kg (13.6-17.8 mL/min/kg), corresponding to 65% predicted (56-74%) Table 3. Median peak VE was 40 L/min (31-49 L/min) and 71% of patients had ventilatory limitation in cyclergometry (76% presented with dynamic hyperinflation during the exam). In 6MWD, median distance was 479 meters (404-510 m), with median ΔSpO2 5% (3-9%) and ΔBorg dyspnoea 2 (0-3).
The mMRC scale correlated with exercise performance assessed by both tests as well as ΔSpO2 and ΔBorg Table 2a and 2b. In fact, patients with mMRC≥2 had significantly worse performance in both 6MWT and CPET, namely lower 6MWD, peak workload and peak VO2. ΔSpO2 was significantly higher in both tests in patients with mMRC≥2 (Table 3).
| CAT | mMRC | CCQ | FEV1 (L) | FEV1 (%) | IC/TLC | DLCO (%) | |
|---|---|---|---|---|---|---|---|
| CAT | 1 | .515** | .819** | -0.175 | -0.127 | -0.137 | -.208* |
| mMRC | .515** | 1 | .592** | -.180* | -0.143 | -.184* | -.330** |
| CCQ | .819** | .592** | 1 | -.235** | -0.161 | -.224* | -.289** |
| 6MWT | CPET | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Distance (m) | ∆spO2 (%) | ∆Borg dyspnea | ∆Borg fatigue | Peak W (watts) | Peak VO2 (%) | Peak VO2 (ml/min/kg) | ∆spO2 (%) | Peak O2/HR | Peak HR (%) | Peak VE (L/min) | |
| CAT | -.248** | -0,055 | .345** | .332** | -.294** | -.179* | -0,147 | -0,084 | -0,096 | -.344** | -.181* |
| mMRC | -.370** | .217* | .512** | .276** | -.422** | -.443** | -.382** | .238** | -.315** | -.339** | -.260** |
| CCQ | -.353** | 0,027 | .428** | .352** | -.432** | -.287** | -.293** | -0,011 | -0,153 | -.444** | -.262** |
| (Significant correlations on bold, * p<0.05, ** p<0.01) | |||||||||||
| Abbreviations: mMRC: modified medical respiratory council dyspnoea scale; CAT: COPD assessment test; CCQ: Clinical COPD Questionnaire; FEV1: Forced Expiratory Flow in 1sec; IC: inspiratory capacity; TLC: total lung capacity; DLCO: lung diffusion capacity for carbon monoxide; L: liters, %:percentage; 6MWT: 6-minute walk test; 6MWD: 6-minute walk distance; SpO2: pulse oxygen saturation; CPET: Cardiopulmonary exercise test; peak VO2: peak oxygen uptake; peak W: peak workload; AT: Anaerobic threshold; VE/VCO2: minute ventilation/carbon dioxide production; peak O2/HR: peak oxygen pulse; VE: minute ventilation; MVV: maximal voluntary ventilation; HR: heart rate; bpm: beats per minute; W: watt; L: liters, % pred: percentage predicted; ml: millilitre; min: minute; n: number. | |||||||||||
Table 2(a and b): Correlations between PRO, Lung function and exercise tests parameters.
The 6MWD, peak workload and peak VO2 were also lower in more symptomatic patients measured by both CAT and CCQ but ΔSpO2 was not different between groups with both questionnaires (Tables 2a, 2b and 3).
ΔBorg dyspnoea and ΔBorg fatigue during 6MWT were higher if patients were more symptomatic, using all questionnaires (Tables 2a, 2b and 3).
Higher mMRC and CCQ were associated with dynamic hyperinflation, but not CAT (Table 3).
| All patients | mMRC | CAT | CCQ | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| mMRC<2 | mMRC≥2 | p-value | CAT<10 | CAT≥10 | p-value | CCQ<1.5 | CCQ≥1.5 | p-value | ||
| Lung Function | ||||||||||
| FEV1 (%) | 47 (38-65) | 59 (38-69) | 43 (31-50) | 0.01 | 46 (38-68) | 47 (39-63) | 0.84 | 51 (38-59) | 40 (34-51) | <0.01 |
| IC (%) | 88 (73-105) | 89 (75-107) | 86 (64-94) | 0.08 | 89 (73-105) | 86 (74-106) | 0.89 | 90 (78-109) | 85 (69-94) | 0.03 |
| TLC (%) | 116 (106-130) | 114 (106-124) | 122 (106-135) | 0.07 | 116 (106-128) | 116 (106-134) | 0.31 | 113 (105-125) | 121 (110-134) | 0.04 |
| IC/TLC | 0.32 (0.26-0.39) | 0.34 (0.28-0.39) | 0.28 (0.22-0.35) | <0.01 | 0.33 (0.26-0.39) | 0.31 (0.26-0.39) | 0.51 | 0.34 (0.29-0.40) | 0.28 (0.23-0.35) | <0.01 |
| RV (%) | 161 (137-194) | 154 (135-183) | 173 (134-217) | <0.01 | 155 (132-192) | 168 (142-195) | 0.08 | 151 (126-159) | 174 (148-213) | <0.01 |
| DLCO (%) | 57 (46-73) | 63 (51-77) | 51 (36-59) | <0.01 | 58 (48-76) | 55 (42-69) | 0.12 | 61 (50-77) | 53 (40-68) | <0.01 |
| 6MWT | ||||||||||
| 6MWD (meters) | 479 (404-510) | 488 (431-524) | 412 (332-480) | <0.01 | 480 (423-519) | 430 (372-498) | <0.01 | 581 (519-581) | 424 (373-489) | <0.01 |
| ∆SpO2 (%) | 5 (3-9) | 5 (2-8) | 8 (4-11) | <0.01 | 5 (3-10) | 5 (3-8) | 0.65 | 3 (2-3) | 6 (4-9) | 0.29 |
| Supplemental oxygen (n/%) | 8/7 | 3/4 | 5 /13 | 0.06 | 5/2 | 3/7 | 0.86 | 0 | 4(8 | 0.64 |
| ∆Borg dyspnoea | 2(0-3) | 1 (0-2) | 3 (2-5) | <0.01 | 1 (0-2) | 2 (1-4) | <0.01 | 1 (0-2) | 3 (1-4) | <0.01 |
| ∆Borg fatigue | 1 (0-3) | 1 (0-2) | 3 (0-4) | <0.01 | 1 (0-3) | 2 (1-4) | <0.01 | 1 (0-2) | 2 (0-4) | <0.01 |
| CPET | ||||||||||
| Peak Workload | 64 (46-88) | 71 (53-95) | 47 (28-65) | <0.01 | 65 (49-92) | 56 (35-75) | <0.01 | 73 (55-92) | 50 (28-67) | <0.01 |
| Peak VO2 (mL/min) | 1101 (889-1311) | 1194 (1100-1411) | 928 (792-1102) | <0.01 | 1160 (927-1372) | 1030 (809-1205) | 0.01 | 1112 (987-1411) | 993 (800-1154) | <0.01 |
| Peak VO2 (mL/min/kg) | 15.5 (13.6-17.8) | 16.5 (14.9-18.7) | 13.2 (11.4-15.0) | <0.01 | 16.1 (14.1-18.2) | 15.5 (12.6-17-3) | 0.34 | 16.5 (14.6-18.6) | 13.8 (12.5-16.6) | 0.01 |
| Peak VO2 (%) | 65 (56-74) | 67 (61-78) | 54 (48-64) | <0.01 | 65 (59-72) | 61 (52-71) | 0.10 | 66 (60-78) | 60 (50-67) | <0.01 |
| ∆SpO2 (%) | 2 (1-6) | 1 (0-4) | 4 (1-9) | 0.01 | 2 (1-6) | 2 (0-6) | 0.37 | 2 (1-5) | 3 (0-7) | 0.75 |
| AT (%peakVO2) | 47 (42-54) | 49 (43-55) | 43 (37-48) | 0.09 | 47 (43-55) | 47 (40-53) | 0.66 | 47 (43-54) | 48 (40-54) | 0.90 |
| VE/VCO2@AT | 37 (34-42) | 37 (33-41) | 39 (34-44) | <0.01 | 37 (34-42) | 38 (34-43) | 0.64 | 37 (34-42) | 38 (34-43) | 0.40 |
| Peak O2/HR (mL/bpm) | 9.2 (7.7-10.7) | 9.7 (8.2-11.3) | 8.2 (7.0-9.7) | <0.01 | 9.3 (7.9-10.3) | 9.0 (7.2-10.1) | 0.37 | 9.3 (8.0-11.2) | 8.9 (7.1-10.1) | 0.17 |
| Peak HR (%pred) | 79 (71-86) | 82 (75-87) | 74 (68-81) | <0.01 | 81 (75-87) | 74 (68-82) | <0.01 | 83 (76-88) | 73 (66-80) | <0.01 |
| Peak VE (L/min) | 40 (31-49) | 43 (34-53) | 34 (28-39) | <0.01 | 41 (32-52) | 39 (30-45) | 0.15 | 43 (35-53) | 34 (28-42) | <0.01 |
| Peak VE (%MVV) | 89 (71-102) | 891 (73-102) | 91 (70-103) | 0.89 | 90 (73-103) | 87 (69-100) | 0.35 | 88 (71-100) | 91 (77-104) | 0.33 |
| Dynamic hyperinflation (n/%) | 94 /76 | 58 /69 | 36/90 | 0.01 | 59/48 | 35/28 | 0.29 | 51/41 | 43/35 | 0.02 |
| Number of patients (n/%) | 124/100 | 84/68 | 40/32 | 81/65 | 43/35 | 74/60 | 50/40 | |||
| Data presented as median and quartiles and n/% for qualitative variables; p<0.05 in bold. | ||||||||||
| Abbreviations: 6MWT: 6-minute walk test; 6MWD: 6-minute walk distance; %: percentage; SpO2: pulse oxygen saturation; CPET: Cardiopulmonary exercise test; peak VO2: peak oxygen uptake; AT: Anaerobic threshold; VE/VCO2: minute ventilation/carbon dioxide production; peak O2/HR: peak oxygen pulse; VE: minute ventilation; MVV: maximal voluntary ventilation; HR: heart rate; bpm: beats per minute; W: watt; L: liters, % pred: percentage predicted; ml: millilitre; min: minute; n: number. | ||||||||||
Table 3: Lungs function and exercise characteristics of study participants according to mMRC, CAT and CCQ.
Discussion
Our study found a significative relation between exercise capacity in COPD patients GOLD A-D using 6MWT and CPET and PRO, using mMRC, CAT and CCQ questionnaires. The CAT questionnaire and mMRC scale were previously identified has predictors of maximum exercise capacity in CPET (maximum VO2 and maximum workload) [31, 32] but 6MWT performance was not evaluated in these studies. Higher mMRC and CCQ were associated with dynamic hyperinflation, but not CAT. High dyspnoea scores had already been associated with high minute ventilation (VE) and dynamic hyperinflation during exercise [32] but we did not find previous studies using CCQ, which a novelty of our findings.
Previously, Durr S et al. found a correlation between 6MWD and CAT [45] and Liu W et al. reported a correlation between both SGRQ score and MRC scale and the 6MWD, with lower MRC and SGRQ scores when patients walked >350 m [46]. To our knowledge there is no data available using the CCQ questionnaire to estimate exercise capacity in COPD with both CPET and 6MWT.
The ΔBorg dyspnoea and the ΔBorg fatigue during 6MWT were higher if patients were more symptomatic, using all questionnaires, which is in accordance with previous studies that analysed mMRC and CAT [31, 32]. The ΔSpO2 measured during 6MWT and CPET was significantly higher in more dyspnoeic patients with (mMRC ≥ 2), but the CAT and CCQ did not correlate with desaturation in both exercise tests. Crisafulli E et al. had not been able to establish a relation between desaturation during CPET and mMRC [32].
PR is known to improve exercise capacity [42] and it was previously known that the CCQ, SGRQ, CRQ and CAT all significantly improved after PR [47-49] but in our group of patients, PR was associated with less symptoms (mMRC<2) and improved QoL measured by CCQ (CCQ<1.5) but not with CAT score, suggesting that CCQ may be a better choice to evaluate COPD patient’s response to rehabilitation, despite the good correlation between them. CAT is recommended in clinical practice to follow patients under rehabilitation [18, 50] and our contradictory results may be related to the inclusion of GOLD A COPD patients in our cohort, who are less symptomatic, have no indication for PR and usually are not included in PR studies. We need further studies comparing both questionnaires.
Non-exacerbators had less symptoms and lower QoL, measured with the three questionnaires (p<0.01), as expected from previous literature [11, 16].
FEV 1 and hyperinflation, measured by IC/TLC, correlated with mMRC and CCQ but not with CAT. In fact, no difference was found for FEV1 and hyperinflation between patients with CAT<10 and CAT ≥ 10. Similarly, DLCO correlated with both symptoms (mMRC) and QoL (CCQ and CAT) but the correlation with CAT was weak and no difference was found when we compared patients with CAT<10 and patients with CAT ≥ 10. Previous studies had found weak to moderate correlations between FEV1 and both CCQ and CAT [12] and this difference from our cohort may be related to the broad inclusion of COPD patients of all severity stages. Hyperinflation and DLCO were previously associated with mMRC in one observational study [32] and IC (at rest and at exercise peak) correlated with both CAT and mMRC in another recent study [31]. Further investigation and sample enlargement may help to clarify the association between QoL and lung function, specifically in different GOLD stages.
We found a strong correlation between CAT and CCQ, consolidating the idea that these are good COPD specific health related QoL instruments, easier to use than SGRQ [16, 18, 49]. mMRC correlated moderately with both CCQ and CAT, also confirming previous findings [11, 12].
One limitation of our results is the lack of a duplicate 6MWT, but a second test is currently mainly recommended for pharmacological and non-pharmacological impact evaluation over time [36]. Also, 89% of our patients had previously performed the test, which reduces the potential impact of a lack of the learning effect.
The current study was based on a large set consisting of patients with clinically stable COPD in GOLD stages A-D and it represents a real-world setting, since we included non-smokers and smokers or former smokers, patients with heart diseases and both patients with and without PR. The sample size, the wide spectrum of disease severity, the analysis of several variables related to exercise response including dynamic hyperinflation and the use of mMRC, CAT and CCQ simultaneously are clear strengths of our study. To our knowledge, this is the first prospective study to consider the relation between several COPD HRQoL scales and exercise capacity measured with both field and laboratory studies. Our study was an observational study and, therefore, we cannot establish the contributing factors for exercise limitation. Further longitudinal studies evaluating maximum exercise capacity in COPD patients with varying degrees of dyspnoea and QoL over time may add valuable information.
Conclusion
Our study highlights the value of PRO, measured with the mMRC, CAT and CCQ questionnaires scales in the assessment of the daily living activity in patients with COPD and confirms that dyspnoea and QoL are associated with maximum exercise capacity was measured by 6MWT and CPET in this population. Along with mMRC and CAT, we state that the CCQ questionnaire is an important and frequently overlooked tool to estimate exercise capacity in COPD patients. Our findings suggest that both mMRC and CCQ correlate better with lung function and response to PR than CAT, contrarily to some previously available data which needs to be further evaluated and clarified in future investigations.
Acknowledgement
We would like to thank all the personnel from the lab for their professional assistance with data collection.
References
- GOLD (2021) Global Initiative for Chronic Obstructive Lung Disease.
- Kohli P, Pinto-Plata V, Divo M, Malhotra A, Harris RS, et al. (2015) Functional capacity, health status, and inflammatory biomarker profile in a cohort of patients with chronic obstructive pulmonary disease. J Cardiopulm Rehabil Prev 35: 348-355.
- O'Donnell DE, McGuire M, Samis L, Webb KA (1995) The impact of exercise reconditioning on breathlessness in severe chronic airflow limitation. Am J Respir Crit Care Med 152: 2005-2013.
- O’Donnell DE, Webb KA (1993) Exertional breathlessness in patients with chronic airflow limitation. The role of lung hyperinflation. Am Rev Respir Dis 148: 1351-1357.
- Kerti M, Balogh Z, Kelemen K, Varga JT (2018) The relationship between exercise capacity and different functional markers in pulmonary rehabilitation for COPD. Int J Chron Obstruct Pulmon Dis 13: 717-724.
- Marin JM, Carrizo SJ, Gascon M, Sanchez A, Gallego B, Celli BR (2001) Inspiratory capacity, dynamic hyperinflation, breathlessness, and exercise performance during the 6-minute-walk test in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 163: 1395-1399.
- Nishimura K, Izumi T, Tsukino M, Oga T (2002) Dyspnea Is a Better Predictor of 5-Year Survival than Airway Obstruction in Patients with COPD. Chest 121: 1434-1440.
- Jones PW (2001) Health status measurement in chronic obstructive pulmonary disease. Thorax 56: 880-887.
- Jones PW, Quirk FH, Baveystock CM, Littlejohns P (1992) A self-complete measure of health status for chronic airflow limitation. The St. George's Respiratory Questionnaire. Am Rev Respir Dis 145: 1321-1327.
- Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Kline Leidy N (2009) Development and first validation of the COPD Assessment Test. Eur Respir J 34: 648-654.
- Kelly JL, Bamsey O, Smith C, Lord VM, Shrikrishna D, Jones PW, et al. (2012) Health status assessment in routine clinical practice: the chronic obstructive pulmonary disease assessment test score in outpatients. Respiration 84: 193-199.
- Zhou Z, Zhou A, Zhao Y, Chen P (2017) Evaluating the Clinical COPD Questionnaire: A systematic review. Respirology 22: 251-262.
- Jones P, Miravitlles M, van der Molen T, Kulich K. Beyond FEV(1) in COPD: a review of patient-reported outcomes and their measurement. Int J Chron Obstruct Pulmon Dis 7: 697-709.
- Calverley PM (2006) Dynamic hyperinflation: is it worth measuring? Proc Am Thorac Soc 3:239-244.
- Vestbo J, Hurd SS, Agustí AG, Jones PW, Vogelmeier C, et al. (2013) Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 187: 347-365.
- Sundh J, Stallberg B, Lisspers K, Kampe M, Janson C, et al. (2016) Comparison of the COPD Assessment Test (CAT) and the Clinical COPD Questionnaire (CCQ) in a Clinical Population. Copd 13: 57-65.
- Jo YS, Park S, Kim DK, Yoo CG, Lee CH (2018) The cutoff point of clinical chronic obstructive pulmonary disease questionnaire for more symptomatic patients. BMC Pulm Med 18: 38.
- Ringbaek T, Martinez G, Lange P (2012) A comparison of the assessment of quality of life with CAT, CCQ, and SGRQ in COPD patients participating in pulmonary rehabilitation. Copd 9: 12-15.
- Sundh J, Janson C, Lisspers K, Montgomery S, Ställberg B (2012) Clinical COPD Questionnaire score (CCQ) and mortality. Int J Chron Obstruct Pulmon Dis 7: 833-842.
- https://www.coursehero.com/file/32447692/GOLD-2018-v60-FINAL-revised-20-Nov-WMS-Copy-2pdf/
- Singh SJ, Puhan MA, Andrianopoulos V, Hernandes NA, Mitchell KE, et al. (2014) An official systematic review of the European Respiratory Society/American Thoracic Society: measurement properties of field walking tests in chronic respiratory disease. Eur Respir J 44: 1447-1478.
- Celli BR, Cote CG, Marin JM, Casanova C, Montes de Oca M, et al. (2004) The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med 350: 1005-1012.
- Awotidebe TO, Awopeju OF, Bisiriyu LA, Ativie RN, Oke KI, et al. (2017) Relationships between respiratory parameters, exercise capacity and psychosocial factors in people with chronic obstructive pulmonary disease. Ann Phys Rehabil Med 60: 387-392.
- Casanova C, Cote C, Marin JM, Pinto-Plata V, de Torres JP, et al. (2008) Distance and oxygen desaturation during the 6-min walk test as predictors of long-term mortality in patients with COPD. Chest 134: 746-752.
- Pinto-Plata VM, Cote C, Cabral H, Taylor J, Celli BR (2004) The 6-min walk distance: change over time and value as a predictor of survival in severe COPD. Eur Respir J 23: 28-33.
- Ferrazza AM, Martolini D, Valli G, Palange P (2009) Cardiopulmonary Exercise Testing in the Functional and Prognostic Evaluation of Patients with Pulmonary Diseases. Respiration 77: 3-17.
- Cote CG, Pinto-Plata V, Kasprzyk K, Dordelly LJ, Celli BR (2007) The 6-min walk distance, peak oxygen uptake, and mortality in COPD. Chest 132: 1778-1785.
- Fotheringham I, Meakin G, Punekar YS, Riley JH, Cockle SM, et al. (2015) Comparison of laboratory- and field-based exercise tests for COPD: a systematic review. Int J Chron Obstruct Pulmon Dis 10: 625-643.
- Cooper CB (2006) The connection between chronic obstructive pulmonary disease symptoms and hyperinflation and its impact on exercise and function. Am J Med 119: 21-31.
- Punekar YS, Riley JH, Lloyd E, Driessen M, Singh SJ (2017) Systematic review of the association between exercise tests and patient-reported outcomes in patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis 12: 2487-2506.
- Pisi R, Aiello M, Calzetta L, Frizzelli A, Tzani P, et al. (2021) The COPD assessment test and the modified Medical Research Council scale are not equivalent when related to the maximal exercise capacity in COPD patients. Pulmonol.
- Crisafulli E, Aiello M, Tzani P, Ielpo A, Longo C, et al. (2019) A High Degree of Dyspnea Is Associated With Poor Maximum Exercise Capacity in Subjects With COPD With the Same Severity of Air-Flow Obstruction. Respir Care 64: 390-397.
- Culver BH, Graham BL, Coates AL, Wanger J, Berry CE, et al. (2017) Recommendations for a Standardized Pulmonary Function Report. An Official American Thoracic Society Technical Statement. Am J Respir Crit Care Med 196: 1463-1472.
- Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, et al. (2005) Standardisation of spirometry. Eur Respir J 26: 319-338.
- Stocks J, Godfrey S, Beardsmore C, Bar-Yishay E, Castile R (2001) Plethysmographic measurements of lung volume and airway resistance. ERS/ATS Task Force on Standards for Infant Respiratory Function Testing. European Respiratory Society/ American Thoracic Society. Eur Respir J 17: 302-312.
- Holland AE, Spruit MA, Troosters T, Puhan MA, Pepin V, et al. (2014) An official European Respiratory Society/American Thoracic Society technical standard: field walking tests in chronic respiratory disease. Eur Respir J 44: 1428-1446.
- https://www.atsjournals.org/doi/full/10.1164/rccm.19310erratum
- Oliveira MJ, Marçôa R, Moutinho J, Oliveira P, Ladeira I, et al. (2019) Reference equations for the 6-minute walk distance in healthy Portuguese subjects 18-70 years old. Pulmonol 25: 83-89.
- Borg GA (1982) Psychophysical bases of perceived exertion. Med Sci Sports Exerc 14: 377-381.
- Wedzicha JA (1999) Domiciliary oxygen therapy services: clinical guidelines and advice for prescribers. Summary of a report of the Royal College of Physicians. J R Coll Physicians Lond 33: 445-447.
- Weisman IM (2003) Erratum: ATS/ACCP statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med 167: 1451-1452.
- Puente-Maestu L, Palange P, Casaburi R, Laveneziana P, Maltais F, et al. (2016) Use of exercise testing in the evaluation of interventional efficacy: an official ERS statement. Eur Respir J 47: 429-460.
- Sue DY, Hansen JE (1984) Normal values in adults during exercise testing. Clin Chest Med 5: 89-98.
- Palange P, Ward SA, Carlsen KH, Casaburi R, Gallagher CG, et al. (2007) Recommendations on the use of exercise testing in clinical practice. Eur Respir J 29: 185-209.
- Durr S, Zogg S, Miedinger D, Steveling EH, Maier S, et al. (2014) Daily physical activity, functional capacity and quality of life in patients with COPD. COPD 11: 689-696.
- Liu W, Liu Y, Li X (2021) Impact of Exercise Capacity Upon Respiratory Functions, Perception of Dyspnea, and Quality of Life in Patients with Chronic Obstructive Pulmonary Disease. Int J Chron Obstruct Pulmon Dis 16: 1529-1534.
- Kon SSC, Dilaver D, Mittal M, Nolan CM, Clark AL, et al. (2014) The Clinical COPD Questionnaire: response to pulmonary rehabilitation and minimal clinically important difference. Thorax 69: 793-798.
- Pangeni R, Mohan A, Guleria R, Khilnani GC, Madan K, et al. (2017) Effects of Pulmonary Rehabilitation (PR) on Exercise Capacity (EC) and Quality of Life (QOL) in Indian Patients with Severe COPD. Eur Respir J
- Kon SS, Dilaver D, Mittal M, Nolan CM, Clark AL, et al. (2014) The Clinical COPD Questionnaire: response to pulmonary rehabilitation and minimal clinically important difference. Thorax 69:793-798.
- Dodd JW, Hogg L, Nolan J, Jefford H, Grant A, et al. (2011) The COPD assessment test (CAT): response to pulmonary rehabilitation. A multicentre, prospective study. Thorax 66: 425-429.
Indexed at , Google Scholar , Crossref
Indexed at , Google Scholar , Crossref
Indexed at , Google Scholar , Crossref
Indexed at , Google Scholar , Crossref
Indexed at , Google Scholar , Crossref
Indexed at , Google Scholar , Crossref
Indexed at , Google Scholar , Crossref
Indexed at , Google Scholar , Crossref
Indexed at , Google Scholar , Crossref
Indexed at , Google Scholar , Crossref
Indexed at , Google Scholar , Crossref
Indexed at , Google Scholar , Crossref
Indexed at , Google Scholar , Crossref
Indexed at , Google Scholar , Crossref
Indexed at , Google Scholar , Crossref
Indexed at , Google Scholar , Crossref
Indexed at , Google Scholar , Crossref
Indexed at , Google Scholar , Crossref
Indexed at, Google Scholar , Crossref
Indexed at , Google Scholar , Crossref
Indexed at , Google Scholar , Crossref
Indexed at , Google Scholar , Crossref
Indexed at , Google Scholar , Crossref
Indexed at , Google Scholar , Crossref
Indexed at , Google Scholar , Crossref
Indexed at , Google Scholar , Crossref
Indexed at , Google Scholar , Crossref
Indexed at , Google Scholar , Crossref
Indexed at , Google Scholar , Crossref
Indexed at , Google Scholar , Crossref
Indexed at , Google Scholar , Crossref
Indexed at , Google Scholar , Crossref
Indexed at , Google Scholar , Crossref
Indexed at , Google Scholar , Crossref
Citation: Ladeira IT, Oliveira PN, Campos LC, Lima RJ, Guimarães MS (2022) Quality of Life and Exercise Performance in COPD – is there a Link? J Card Pulm Rehabi 6: 158. DOI: 10.4172/jcpr.1000158
Copyright: © 2022 Ladeira IT, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 2936
- [From(publication date): 0-2022 - Mar 31, 2025]
- Breakdown by view type
- HTML page views: 2398
- PDF downloads: 538