Putative Status of Actively Operative Performance Attributes as Determinants of Minimal Platform Oncogenesis in C-Myc Amplification
Received: 13-Apr-2016 / Accepted Date: 19-May-2016 / Published Date: 30-May-2016 DOI: 10.4172/2472-0429.1000108
Abstract
Delivery and effective modulation of intrinsically operative c-Myc gene amplification is a referential series of system pathways that appear to condition minimal platform utilization of dimensions that characterizes the outcomes as hyper-proliferation and increased growth rate, on the one hand, and of enhanced apoptotic activity, on the other. A small numerical genetic requirement for oncogenesis appears sufficient in the case of some neoplasms such as human prostatic carcinoma. Thus, targeting c-Myc appears a highly promising endeavor to induce therapeutic regression of neoplasms. On the other hand, loss of dependence on c-Myc of certain subsets of tumors and recurrence after partial regression contrast with the arrest of some neoplasms that have been investigated in certain animal models. In terms that implicate the delivery of transforming potentiality of this amplified master-oncogene, it is significant to view potentiality of the already established increased proliferative rate within contexts of modulated sensitivity for the instability of the genome due to subsequent tumor progression. Accumulation of multiple genetic lesions, as seen in advanced clinically detected tumors, would attest to an autocrine and paracrine series of lesions that include increased apoptosis of neighboring normal cells, and also to the angiogenesis that re-characterizes the malignant transformation process as essentially metastasizing attributes of systemic spread.
Keywords: Autocrine; Paracrine; Prostatic carcinoma; Neoplasms
127126Introduction
The combinatorial complex-action of amplified c-Myc in inducing both increased proliferation and also apoptosis of target cells implicates a derivative dysfunctional relationship of intrinsic tumor suppressor function within the dysfunctional mediating actions of a master oncogene. Increased proline biosynthesis in carcinogenesis by MYC, links the reprogramming of glucose, glutamine and pyridine nucleotides [1]. In such manner, promotional effects of increased cell proliferation must be interpreted within the substantial dysregulation and loss of terminal differentiation of these induced cells. Microarray gene expression profiling in osteosarcoma has revealed relationships of this tumor type and of recurrent medulloblastoma with c-Myc [2,3].
Realization of essential dynamics of turnover of proliferating cells appears to promote the emergence both of “escapers” of such proliferative clones of cells and also of “dormant” cells, that is, cells that are non-proliferative but still capable, in future, to resume increased proliferative activity. Nuclear factor, erythroid 2-like 2 (NFE2L2)- associated molecular signature is a robust prognostic gene signature independent of MYC level and lung cancer stage [4].
Dimensions of a minimal platform for the induction and progression of transformation per se would implicate the potentiality for further evolutionary pathways through dysregulation and enhanced activity of abnormal c-Myc homeostasis. Micro-R675 appears involved in epigenetic regulation of histones for gene expression during hepatocarcinogenesis including that of c-Myc [5].
Derivative dysfunctionality
Derivative genetic instability and also instability of the c-Myc gene/ protein per se would call into question the roles of such oncogene and oncoprotein within the global dynamic turnover of this modulator of cell transformation. Epidermal Growth Factor Receptor (EGFR) and c- MYC are essential in pancreatic carcinogenesis, and EGFR signaling activates the oncogenic and pro-proliferative transcription factor c- MYC [6]. Conceptual minimal platforms involving only two oncogenes would indicate a host of other inactive and also activated oncogenes that are less active in specific groups of affected cells. PAX2, an essential transcription factor for development, can act as either a classical oncogene or tumor suppressor gene as seen in ovarian epithelial neoplasms [7].
Inherent apoptotic activity
The inherent pro-apoptotic actions of c-Myc amplification lead to the realization of transient states of susceptibility as indicated also by instances of increased apoptotic rate in cells such as osteosarcoma that had previously undergone transient suppression of expression of the c- Myc gene. MYC disrupts the molecular clock in cancer cells, affecting the link between oncogenesis, circadian rhythms, and metabolism [8]. It is in terms of an ongoing idealization of the contexts and cell-type characterizations of action of the amplified c-Myc that ongoing processes of malignant transformation act as dimensional derivatives also of cell attachment and of cell-to-cell communication. The expression of pluripotency transcription factors is responsible for stemness properties, and indeed cancer stem cells are involved in the initiation, progressive metastatic spread, recurrence, and even resistance to chemo- and radio-therapy in patients with lung cancer [9]. Furthermore, induced increases in apoptotic cell death of such induced transforming cells would perhaps be viewed as interpretative consequences of such specific cell types linked to certain micro-environmentally conditioned milieu. The c-Myc family is implicated both in brain development and in oncogenesis of embryonal neural tumors, including poor prognosis and aggressive biologic behavior [10].
Incremental proliferative rates appear to operate within an essential conditioning restriction of differentiation potential of the induced groups of cells. Suppression of bromodomain 4 (an epigenetic regulator) inhibits human hepatocellular carcinoma through repression of MYC and enhancement of BIM expression [11]. Bromodomain 4 mediates the positive bidirectional loop between MYC and AP4 [12]. In such manner, the emergence of dormant cells is a result of clonal proliferation that is affected profoundly by such measured influences as epigenetic context of the amplified c-Myc gene. Diffuse Large B-Cell lymphoma with c-Myc rearrangement carries a poor prognosis [13]. Double-hit B-cell lymphoma is characterized by concurrent translocations of MYC and BCL2, BCL6, or other genes and does not always have a poor prognosis [14].
Amplification and protein stability
The stability of the c-Myc-protein is also probably abnormally regulated in the presence of excessive presence of such protein. Expression of c-Myc is downregulated, with enhanced p53 expression with combination treatment of docetaxel and piperlongumine in triple-negative breast cancer [15]. The induction of increased apoptosis of such transforming cells is a realization of a minimal platform for oncogenesis that arises as dimensions of action of operative pathogenic pathways of amplification of the c-Myc gene and of the dynamics of over-expression, subsequent to such gene amplification.
The “super-competitor” roles of increased c-Myc protein in such cells would necessarily implicate apoptosis of neighboring normal cells in terms of such phenomena as increased utilization of growth factors and other agonists that the normal cells are deprived of. It is within the strict frameworks of an ongoing transformation process of both global and specific dimensions that the phenomenon of super-competitor roles of increased c-Myc expression should be interpreted. Anti-MYC IgG might have prognostic value in the early diagnosis of lung cancer [16].
The evolutionary roles of a master oncogene such as c-Myc would appear to inherently arise as also an active agent of suppressor action within actively induced increases of proliferative cell activity. Increased c-Myc protein is an attribute of the dynamics of the amplification process itself in a manner that would strictly categorize in dimensional terms, the onset of both increased cell proliferation and increased apoptotic activity. Immuno-reactive nuclear c-Myc was noted in cases of angiosarcoma arising in a chronic expanding hematoma [17].
Modulatory Action in Tumor Evolution
Action modulation is apparent within the contexts of evolution and creation of the amplified c-Myc gene that collaborates with the ability for increased expression of influences that parallel the relative induction of the intrinsic and extrinsic pathways of apoptosis. The inter-activity between the specific intrinsic and extrinsic apoptotic pathways cooperatively potentiates the end-result consequences of both released cytochrome c within the cytoplasm and the ligation of cell-death receptors at the cell membrane, for example by ligation of Fas ligand and the Tumor Necrosis Factor-alpha.
Hence, there would emerge a concerted transformation of the functional induction/activation of the intrinsic and extrinsic apoptotic pathways that comes to re-define essential attributes of the parent malignant transformation process of the induced cells carrying amplified c-Myc gene expression. microRNA-132 is a novel contributor to B cell development and can inhibit cancer development in B cells expressing the c-Myc oncogene [18]. It is particularly with reference to such phenomenon that the putative model of minimal platform oncogenesis is a possible alternative to a global malignant change inherently operative in genomic instability.
Performance attribute modulating status
Performance attributes substitute in many instances for a promotional reactivation of cell proliferation that inherently suppresses or actively induces loss of differentiation status of the hyper-proliferating cells. Recognition of specific oncogene rearrangements may be prognostically valuable to guide therapy in patients with double-hit lymphomas [19]. It is within the essential context of substantial cooperative increases of apoptosis of cells that are increasingly proliferating that such loss of differentiation status should be interpreted. There is upregulation of c-Myc, and this may be a downstream event of NF-kappaB activation in immortalized nasopharyngeal epithelial cells [20].
It is important to emphasize that c-Myc can potentially affect the expression of up to 10% of all genes within the cell genome. Systemic analysis of endometrial cancer-associated Hub proteins revealed also interaction with c-Myc [21]. The potential roles of such a master-gene would incrementally induce a state of amplification that enhances a small number of essential genetic lesions (minimal platform) for ongoing oncogenesis and global genomic instability.
Incremental platform requirement in oncogenesis is an inherent attribute in ongoing genesis of the amplification status of a master-oncogene such as c-Myc that progresses towards further global induction of genetic damage. In this connection the acetylation and de-acetylation of the chromatin would further evidence the emerging consequences of the gene amplification process itself. Gene amplification is a likely substantial candidate in the active acetylation of specific nucleosomes that participate actively in the realization of such phenomena with increased gene expression. Previously unpredictable roles for c-Myc in gene expression regulation may constitute new potential targets for therapy in glioblastoma cells, particularly in the modification of chromatin structure [22].
The direct and also indirect consequences of c-Myc gene amplification can best be interpreted as a series of sequential transformations of genomic expression and repression as produced, maintained and further augmented by performance dynamics. The specific metabolic features of pancreatic cancer stem cells are dependent on non-suppression of c-Myc and resistance can be prevented/reversed for metformin by genetic/pharmacologic inhibition of c-Myc [23].
Re-conditioning of the transformation of cells
There would emerge a conditioning process that is phenomenally integral to actual ongoing performance dynamics that inherently re-characterizing the proliferative profiles of the intrinsic malignant transformation process. There is upregulation of transcription factors such as c-Myc, AP4 and Gata-3 in the gastric carcinoma induced by Helicobacter pylori [24]. In human cancers Beta-catenin accumulates in the nucleus and activates mRNA transcription of cyclin D1 and c-Myc [25].
Potentiality for further transformation
The identification of a transforming potentiality as further translated in terms of evolving performance dynamics involves establishment of malignancy as inherent to transforming agonist actions. The further compound pairing of the enhanced cooperation of intrinsic and extrinsic apoptotic pathways would include re-conditioning of clonally proliferating cells on the one hand and the creation of pools of both sleepers and dormant cells.
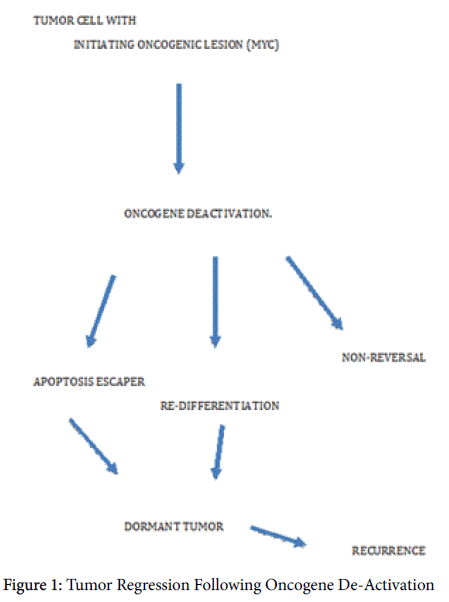
In this connection, also, the super-competitor status of cells possessing increased c-Myc protein expression is putative substrate for potential progression of the transforming and metastasizing oncogenic process. The N-myc downstream regulated gene 1 is associated with advanced hepatocellular carcinoma, and catenin degradation is prevented by direct interaction of this gene protein with glycogen synthase kinase 3beta and the orphan nuclear receptor Nur77 [26] (Figure 1).
Dimensions of oncogenesis
Promoter activation of the c-Myc gene, its hetero-dimerization with Max, its subsequent stabilization and amplification, its specific subl-ocalization within the nucleus or nucleolus, its ejection from the nucleus, and its myriad induced effects in terms of stabilization and also susceptibility to degradation by the proteosomal mechanisms would inherently parallel the instability of the malignant process and behavior of transformed cells; these may be reflected in angiogenesis and subsequent metastasization of the resulting malignant transformed clones of cells.
The dominant status of such transformed cells with regard to normal neighboring cells appears to call into operation a myriad of autocrine and paracrine phenomena that transfer the increased apoptosis potentialities of an amplified c-Myc gene expression to neighboring normal cells within the region and organ of the transforming clones of cells. Integrins alphavbeta5 and alphavbeta6 are necessary for invasion by squamous cell carcinoma; integrins in fact play essential roles in epithelial cell adhesion, proliferation, wound healing and cancer; focal adhesion-independent integrin alphav regulation of FAK and c-Myc is necessary for 3D skin formation and tumor invasion [27]. Potentiality for active attributes of transforming cells proves a transforming phenomenon in further inducing establishment of clonally metastasizing malignant cells.
In this regard, a plethora of features, both pathobiologic and also histologic and immunohistochemical, prove dimensionally targeted to perform the actual dynamics of a malignant phenotype arising as dynamics of cells undergoing malignant transformation.
Concluding Remarks
The establishment of the amplified status of the c-Myc gene, and of its increased expression potentialities, is inherently linked to the actively conditioning and conditioned potentialities of performance attributes; these are subsequently reflected in an inherent sensitization for apoptosis, and in increased apoptosis of neighboring normal cells. The angiogenesis that is induced locally reflects in some measure the integral autocrine and paracrine attributes of cells that hyper-proliferate and are hyper-sensitive to apoptosis.
It is further to realization of such processes as performing attributes of proliferation that compound inability of such cells to differentiate would arise as re-localization of the c-Myc protein within specific cellular sub-compartments and, in such manner, potentiate further the amplifying expression of the c-Myc gene. Modulation of contextual epigenetic influence, of increased cell growth and of division of the cellular DNA, may operate within the settings of abnormal cell cycle dynamics and apoptosis.
References
- Liu W, Hancock CN, Fischer JW, Harman M, Phang JM. (2015) Proline biosynthesis augments tumor cell growth and aerobic glycolysis: involvement in pyridine nucleotides Sci Rep 5: 17206
- Zhao L, Zhang J, Tan H, Wang W, Liu Y, et al. (2015) Gene function analysis in osteosarcoma based on microarray gene expression profiling. Int J Clin Exp Med 8: 10401-10410.
- Xu J, Margol AS, Shukla A, Ren X, Finlay JL, et al. (2015) Disseminated medulloblastoma in a child with germline BRCA2 6174delT mutation and without Fanconi anemia. Front Oncol 5: 191
- Qian Z, Zhou T, Gurguis CI, Xu X, Wen Q, et al. (2015) Nuclear factor, erythroid 2-like 2-associated molecular signature predicts lung cancer survival. Sci Rep 5: 16889.
- Li H, Li J, Jia S, Wu M, An J, et al. (2015) miR675 upregulates long noncoding RNA H19 through activating EGR1 in human liver cancera. Oncotarget 6: 31958-31984
- Diersch S, Wirth M, Schneewels C, Jors S, Geisler F, et al. (2015) Kras G12D induces EGFR-MYC cross signaling in murine primary pancreatic ductal epithelial cells. Oncogene
- Al-Hujaily EM, Tang Y, Yao DS, Carmona E, Garson K, et al. (2015) Divergent Roles of PAX2 in the Etiology and Progression of Ovarian Cancer. Cancer Prev Res (Phila) 8: 1163-1173.
- Lau AN, Vander Heiden MG (2015) Stopping the Clock with MYC. Mol Cell 60: 511-513.
- Saawek S, Szmyt K, Fularz M, Dziudzia J, Boruczkowski M, et al. (2016) Pluripotency transcription factors in lung cancer-a review. Tumour Biol 37: 4241-4249.
- Shalaby T, Grotzer MA (2016) MYC as Therapeutic Target for Embryonal Tumors: Potential and Challenges. Curr Cancer Drug Targets 16: 2-21.
- Li GQ, Guo WZ, Zhang Y, Seng JJ, Zhang HP, et al. (2015) Suppression of BRD4 inhibits human hepatocellular carcinoma by repressing MYC and enhancing BIM expression. Oncotarget 7: 2462-2474
- Choi SK, Hong SH, Kim HS, Shin CY, Nam SW, et al. (2016) JQ1, an inhibitor of the epigenetic reader BRD4, suppresses the bidirectional MYC-AP4 axis via multiple mechanisms. Oncol Rep 35: 1186-1194.
- Bergman CC, Dartigues PC, Baia M, Briere J, Delarue R, et al. (2015) MYC-IG rearrangements are negative predictors of survival in DLBCL patients treated with immunochemotherapy: a GELA/LYSA study. Blood 126: 2466-2474.
- Ye Q, Xu-Monette ZY, Tzankov A, Deng L, Wang X, et al. (2015) Prognostic impact of concurrent MYC and BCL6 rearrangements and expression in de novo diffuse large B-cell lymphoma. Oncotarget 7: 2401-2416
- Patel K, Chowdhury N, Doddapaneni R, Boakye CH, Godugu C, et al. (2015) Piperlongumine for Enhancing Oral Bioavailability and Cytotoxicity of Docetaxel in Triple-Negative Breast Cancer. J Pharm Sci 104: 4417-4426.
- Ye L, Wang W, Chen C, Meng Q, Yu Y (2015) Study of circulating IgG antibodies to BIRC5 and MYC in non-small cell lung cancer. FEBS Open Bio 5: 809-812.
- Lon CEB, Riddle ND, Lackman RD, Evenski AJ, Brooks JS (2015) Angiosarcoma Arising in Chronic Expanding Hematoma: Five Cases of an Underrecognized Association. Am J Surg Pathol 39: 1540-1547.
- Mehta A, Mann M, Zhao JL, Marinov GK, Majumdar D, et al. (2015) The microRNA-212/132 cluster regulates B cell development by targeting Sox4. J Exp Med 212: 1679-1692.
- Landsburg DJ, Petrich AM, Abramson JS, Sohani AR, Press O, et al. (2016) Impact of oncogene rearrangement patterns on outcomes in patients with double-hit non-Hodgkin lymphoma. Cancer 122: 559-564.
- Zhu DD, Zhang J, Deng W, Yip YL, Lung HL, et al. (2016) Significance of NF-κB activation in immortalization of nasopharyngeal epithelial cells. Int J Cancer 138: 1175-1185.
- Gao H, Zhang Z (2015) Systematic Analysis of Endometrial Cancer-Associated Hub Proteins Based on Text Mining. Biomed Res Int 2015: 615825.
- Mongiardi MP, Savino M, Bartoli L, Beji S, Nanni S, et al. (2015) Myc and Omomyc functionally associate with the Protein Arginine Methyltransferase 5 (PRMT5) in glioblastoma cells. Sci Rep 5: 15494.
- Sancho P, Burgos-Ramos E, Tavera A, Bou Kheir T, Jagust P, Schoenhals M et al. (2015) MYC/PGC-1alpha balance determines the metabolic phenotype and plasticity of pancreatic cancer stem cells. Cell Metab 22: 590-605
- Hu TZ, Huang LH, Xu CX, Liu XM, Wang Y, et al. (2015) Expressional profiles of transcription factors in the progression of Helicobacter pylori-associated gastric carcinoma based on protein/DNA array analysis. Med Oncol 32: 265
- Shi J, Wu WJ, Hu G, Yu X, Yu GS, et al. (2016) Regulation of β-catenin transcription activity by leupaxin in hepatocellular carcinoma. Tumour Biol 37: 2313-2320.
- Lu WJ, Chua MS, Wei W, So SK (2015) NDRG1 promotes growth of hepatocellular carcinoma cells by directly interacting with GSK-3β and Nur77 to prevent β-catenin degradation. Oncotarget 6: 29847-29859.
- Duperret EK, Dahal A, Ridky TW (2015) Focal-adhesion-independent integrin-αv regulation of FAK and c-Myc is necessary for 3D skin formation and tumor invasion. J Cell Sci 128: 3997-4013.
Citation: Agius LM (2016) Putative Status of Actively Operative Performance Attributes as Determinants of Minimal Platform Oncogenesis in C-Myc Amplification. Adv Cancer Prev 1: 108. DOI: 10.4172/2472-0429.1000108
Copyright: © 2016 Agius LM. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Conferences
42nd Global Conference on Nursing Care & Patient Safety
Toronto, CanadaRecommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 11864
- [From(publication date): 5-2016 - Apr 02, 2025]
- Breakdown by view type
- HTML page views: 10961
- PDF downloads: 903