Purifying Water Physiology with Silver Nanoparticles and Bi-Objective Design-for-Control for Improving the Pressure Management
Received: 02-Aug-2022 / Manuscript No. bcp-22-71849 / Editor assigned: 04-Aug-2022 / PreQC No. bcp-22-71849 / Reviewed: 10-Aug-2022 / QC No. bcp-22-71849 / Revised: 17-Aug-2022 / Manuscript No. bcp-22-71849 / Published Date: 24-Aug-2022 DOI: 10.4172/2168-9652.1000394
Introduction
Water is an important factor for plant growth as it helps to fulfill all the vital activities of plants. Water is essential for photosynthesis, respiration, absorption of minerals and nutrients, metabolism and even to maintain the soil temperature too. Beside this, water is also important in various other processes too, as it helps in the germination of seeds and in the process of transpiration etc. Water helps a plant by transporting nutrients through the roots. Nutrients are drawn from the soil and used by the plant [1]. Without enough water in the cells, the plants droop so water helps a plant stand. Water carries the dissolved sugar and other nutrients through the roots. Plants absorb water through their entire surface- roots, stems and leaves. However, the majority of water is absorbed by root hairs.
Physical Properties of Water
As per studies on global water covers about 73% of earth‘s surface and provides the most extensive medium for all aquatic life because of its unique properties from ecological point of view. Water occurs in in all three physical forms in the earth at moderate temperature. It is present in either in the form of fresh water or in saline water form in sea and salt lakes [2]. The fresh water of active ground water, glaciers and ice caps, rivers, lakes dams, streams, soil moisture etc. represents only 1.92% of the total water stock. But even from this small segment as much as 98.65% is shared between active ground water and ice on mountain tops and poles, lakes and rivers constitute only 0.98% and 0.004% fresh water stock respectively.(Figure 1)
Water Structure and Properties
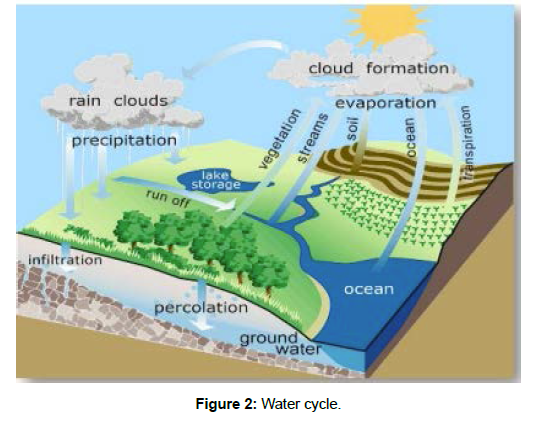
Water comprises over about 90% of the chemical content of many organisms and so we can say justifiably that water is the fluid of life but before this it is necessary to understand the different physiological processes related to the diffusion and absorption of water, and fundamental chemical and physical properties of water and its interaction with other substances [3]. Water participates in all metabolic reactions either directly or indirectly. Water is a remarkable compound with unique properties that results from its molecular configuration and hydrogen bonding. (Figure 2)
Water comprises over about 90% of the chemical content of many organisms and so we can say justifiably that water is the fluid of life but before this it is necessary to understand the different physiological processes related to the diffusion and absorption of water, and fundamental chemical and physical properties of water and itsinteraction with other substances. Water participates in all metabolic reactions either directly orindirectly. Water is a remarkable compound with unique properties that results from itsmolecular configuration and hydrogen bonding.
Molecular Structure of Water
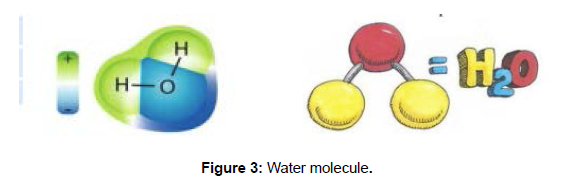
A single water molecule is composed of two hydrogen atomsbonded covalently to one side of an oxygen atom. Water absorbs large quantity of heat and tolerates other physical stresses without breakage of the bonds. Water is a polar inorganic compound at the room temperature at room temperature associates with each other because of the asymmetrical distribution hence due to these association i.e. adhesion or cohesion that are critical to the movement of water in soils and the translocation of water in plants. (Figure 3)
Diffusion
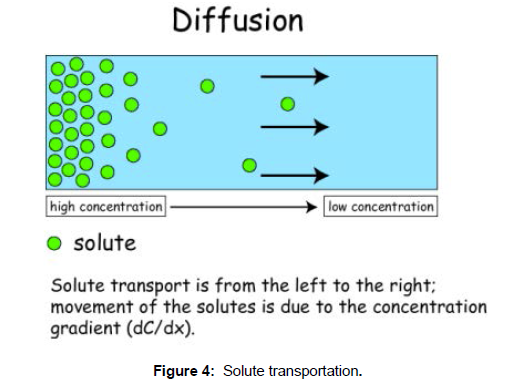
The diffusion means to spread; to flow out, to extend Diffusion can be simply defined as the movement of particles of matter due to their kinetic energy or the net movement from one point to another because of the random kinetic activities of molecules or ions is called diffusion [4,5]. Diffusion refers to the process by which molecules intermingle as a result of their kinetic energy of random motion. However, the direction of movement of diffused particles is form the region of higher concentration to the region of lower concentration till both the concentrations equalize. The molecules in the region of higher concentration contain more kinetic energy and that is why they allow fast movement [6]. The diffusion of particles still continues in both the directions though it is not detectable. Diffusion is random movement of molecules but has a net direction towards regions of lower concentration in order to reach equilibrium. Simple and passive diffusion occurs when small molecules pass through the lipid bilayer of a cell membrane. (Figure 4)
The gases diffuse through gases. Liquids and solids; liquids diffuses through gases, liquids and solids and likewise solids diffuses through gases, liquids and solids. In some casesthe rate of diffusion may be either very fast or very slow as per the condition. Hydrogen diffuses four times faster as far as oxygen and five times as fast as carbon dioxide, these rates are determined by the relative intensity of the gas.
Examples of diffusion are
Gas into liquid- Foam, Liquid intogas- Clouds, Solid into gas- Smoke Solid into solid- diffusion of copper into zinc and zinc into copper, although this process takes pretty long time, if the basis of two metals are kept one another.
Osmosis Phenomenon
To explain osmosis if two different solutions of different concentration are separated by a semi-permeable and will not allow or permit soluble molecules to pass through it. As per the lows of diffusion the movement of solvent molecules will be from the region of higher concentration to the region of lower concentration or from dilute solution to concentrated solutions [7,8]. The reason behind this is that the concentration of solvent molecules will lower in the concentrated solution and higher in the dilute solution. On the basis of concentration of solute molecules the solutions may be defined as hypertonic and hypotonic solutions.
Exosmosis
Osmosis towards the inside of a cell or vessel or the flow of a substance from an area of lesser concentration to one of greater concentration, while the movement of water molecules from outside to inside of a cell through osmosis is known as endosmosis. Or the process by which water molecules move out of the cell is called exosmosis. A solution is a mixture of two or more than two substance in which the concentration of the solute in solvent may be expressed as weight of solute per unit of solvent or in terms of molarity, normality or equivalent to this.
Water of Hydration
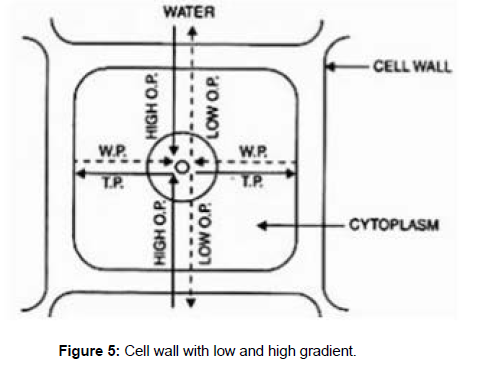
The water associated with the particles of hydrophilic solutes or colloids is known as water of hydration. An ideal molar solution at 0 0C has an osmotic pressure of 22.4 0 C atmosphere [9,10]. Osmotic pressure increases at higher temperature. Modern workers have substituted the term osmotic pressure by osmotic potential is numerically equal to the osmotic pressure but is negative in sign, which indicates decrease in pressure that occurs due to additionof the solute. When we add more solute the osmotic potential increases more negatively, but dilution of solution with the solvent decreases the value of the osmotic potential. In a solvent the value of osmotic potential is zero. Plant Cell- Asan Osmotic System: The first cell of the plant which absorbs water and acts like an osmotic system or as an Osmometer is the root hair. The big vacuolated plant cell is an osmotic system which acts as follows: When root hair comes in contact with a solution with higher concentration of water molecules than that of cell sap, than the process of osmosis begins. Firstly, the water or solution of external solutions (higher concentration) begins to enter inside the cell through osmosis (endosmosis). The movement of water takes place due to concentration differences between external and internal solutions in which plasma membrane acts as a semipermeable membrane. The wall of root hair works as a specialized wall and facilitates water absorption. The high efficiency of root hair as an absorbing organ is due to the fact that the soil particles are attached to its pectic substances and bringing the soil water very close to wall of the root hair. Besides the pectic substances, the root hairs also secrets carbonic acid capable to dissolving everything required by plants. (Figure 5)
Due to diffusion pressure deficit (DPD) water is absorbed by the root hair. The amount /qualityof water absorbed by the root hair is depends on deficit. If this deficit is greater, larger quantity of water will diffuse and greater amount of water will enter into the cell. The force per unit area of entrance of water is termed as suction pressure the potentiality of which depends upon DPD, the suction pressure that exists between the cell and environment. It can be said that-
DPD= Osmotic pressure- turgor pressure.
Entrance of water inside the cell affects it in two ways- i) it brings down the concentration of cell sap and ii) it starches the elastic wall of the cell. This entered water results swelling of cell wall and causes a pressure known as turgor pressure. Due to this, cell increases in volume but due to elastic nature of the wall, offers resistance to this force. This resistance is works in opposite direction to turgor pressure and known as wall pressure. The wall pressure is exerted by wall in order to restore normalcy in size. The turgor pressure is fully turgid. Water stops to enter or diffusion of water molecules in both the directions stops when the concentration of two solutions becomes equal, till the balance is fully stretched and thus entry of water is checked. At this stage cell is fully stretched and is said to be turgid. When osmotic pressure may exist the suction pressure will be zero in a turgid cell.
The entered water decreases simultaneously resulted in an increased turgor pressure and a decrease in osmotic pressure of cell sap. Due to this the concentration differences simultaneously and brings down the suction pressure. All forces are interrelated and work together.
The water present in the soil may be classified as follows-
(i) Gravitational Water: The water which reaches deeply into the soil after rains due to gravitational force is called gravitational water. This water is not available to plants and percolates downwards through large pores between the soil particles under the gravity and hence is called gravitational water. It reaches the low water table in the earth.
(ii) Capillary Water: The water which remains present in the intercellular spaces of soil particles. This water is available to plants, which fills the spaces between thenon- colloidal particles or it forms a thin film around the non- colloidal small particles of soil. This water is present in the capillaries of soil particles thus known as capillary water and one of the most important and significant form of water which is held by capillary forces and absorbed by the plants, or the water that is left in soil along with hygroscopic moisture and water vapours after the gravitational water has drained off is known as capillary water.
(iii) Hygroscopic Water: The water which is present around the soil particles in the form of thin vapour layer and held tightly by soil particles of colloidal complex due to adhesive force. This water is also non available water and cannot be drawn/absorbed by the plants.
(iv) Chemically Combined Water: The water which remains chemically bound to the soil particle is called chemically combined water. This water is also non- available to plants.
(v) Run- off Water: The water which run- off drown through slopes after rains is called running water or run- off water. This is also non- available to plants. (Figure 6)
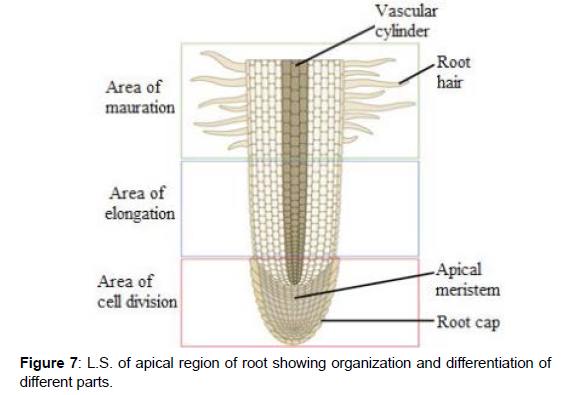
Wilting Co- Capacity: The percentage of water that remains in a soil when permanent wilting is attained is called wilting co- efficient or permanent wilting percentage. On the basis of above studies it is clear that plants have a specific relationship with water. Hence there is a strong plant water relationship which means how plants control the hydration of their cells, including the collection of water from the soil, its transport within the plants and its loss by evaporation from the leaves. This water status of plants is usually expressed as water potential, which has unit of pressure is always negative and in simple form is algebraic sum of the hydrostatic pressure and the osmotic pressure of water. Flow of water through plant and soil over microscopic distance is driven by gradients in hydrostatic pressure over microscopic distances (e.g. across semi-permeable membrane). It is driven by gradients in water potential. (Figure 7)
References
- Hartmann, Thomas From waste products to ecochemicals: fifty years research of plant secondary metabolism.Phytochemistry 2831-2846.
- Crozier, Alan, Clifford, Michael N, Ashihara, et al. (2007) Chapter 1. Phenols, Polyphenols and Tannins: An Overview.Plant Secondary Metabolites: Occurrence, Structure and Role in the Human Diet.Phenol chem 365-410.
- Wink, Michael (2010) Introduction: Biochemistry, Physiology and Ecological Functions of Secondary Metabolites .Annual Plant Reviews Volume 40: Biochemistry of Plant Secondary Metabolism,Second Edition 1-19.
- Fred R West, Jr Edward, MikaS Synthesis of Atropine by Isolated Roots and Root-Callus Cultures of Belladonna.Botanical Gazette 50-54.
- Venturi S, Donati FM, Venturi A, Venturi M (2000)Environmental Iodine Deficiency: A Challenge to the Evolution of Terrestrial Life.Thyroid 727-920.
- Amsler Mark (1986)The Languages of Creativity: Models, Problem-solving, Discourse. University of Delaware Press.
- Astbury WT (1961) Molecular Biology or Ultrastructural Biology?.Nature 190(4781): 11-24.
- Ben-Menahem Ari (2009) Historical Encyclopedia of Natural and Mathematical Sciences.Historical Encyclopedia of Natural and Mathematical Sciences by Ari Ben-Menahem Berlin Springer Springer 29-82.
- Burton Feldman (2001) The Nobel Prize: A History of Genius, Controversy, and Prestige.Arcade Publishing 12-30.
- Butler John M (2009)Fundamentals of Forensic DNA Typing. Academic Press 14-19.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: Li JW (2022) Purifying Water Physiology with Silver Nanoparticles and Bi-Objective Design-for-Control for Improving the Pressure Management. Biochem Physiol 11: 394. DOI: 10.4172/2168-9652.1000394
Copyright: 2022 Li JW. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 2169
- [From(publication date): 0-2022 - Nov 07, 2025]
- Breakdown by view type
- HTML page views: 1698
- PDF downloads: 471