Pseudomonas anguilliseptica Strain-A1 Degradation of Polycyclic Aromatic Hydrocarbons in Soil Microcosms: Focus on Detoxification Activity and Free Water-Soluble Protein Extracts Kinetics and Efficiency
Received: 01-Nov-2017 / Accepted Date: 09-Nov-2017 / Published Date: 13-Nov-2017 DOI: 10.4172/2155-6199.1000418
Abstract
Pseudomans anguilliseptica-A1 strain, isolated in an urban area, improved the efficiency of a microbial consortium, composed of Bacillaceae, Staphylococcacea, Xantomonadaceae and Enterbacteriaceae, whose ability to degrade five Polycyclic Aromatic Hydrocarbons (PAHs) among the priority pollutants was previously ascertained. Six soil microcosms were prepared with a slurry (60% soil, 40% water) artificially contaminated with anthracene (0.4 mg g-1), phenanthrene (0.2 mg g-1), naphthalene (0.2 mg g-1), pyrene (mg g-1) and benzo(a)pyrene (0.1 mg g-1) and opportunely aerated for two months. PAHs were monthly quantified by inverse phase High Performance Liquid Chromatography (HPLC), coupled with UV-Vis spectrophotometry and spectrofluorimetry. Acute toxicity assays vs Dapnia magna and Lepidium sativum, and chronic essays vs Ceriodaphniadubia were monthly performed. Our results showed a 100% degradation for naphthalene, 99.14% for anthracene, 99.23% for phenanthrene, 86% for pyrene and 72.5% for benzo[a]pyrene after two months of treatment. A sterile P. anguilliseptica-A1 lysate in Na-K buffer added with each of the chosen PAHs (53%, wtPAHs/volsusp), operated at 30°C the oxidative degradation of naphthalene, pyrene, benzo(a)pyrene and anthracene in a few hours, while the phenanthrene enzyme degradation process took about 15 h. The GC-MS analysis revealed interesting metabolite structures such as 2-hydroxynaphthalene, 9,10-phenanthrenedione, 2,2’ diphenic acid and methyl 4-hydroxybenzoate. The direct utilization of enzymes/microbial extracts from P. anguilliseptica-A1 could present specific advantages such as availability and a fast PAHs degradation time in bioremediation processes.
Keywords: Soil bioremediation; Pseudomonas anquilliseptica-A1; Polycyclic Aromatic Hydrocarbons; Enzymatic remediation; Ecotoxicology
Introduction
Polycyclic Aromatic Hydrocarbons (PAHs) are organic compounds produced by an incomplete combustion of organic matter due to both natural occurrences (e.g., forest and rangeland fires and volcanic eruptions) and anthropogenic activities (e.g., the burning of fossil fuels and coal tar, oil pipes leaking). Currently, pollution by PAHs represents a serious risk for both the environment and human health as many of these compounds (and their conversion products) are believed to have carcinogenic and/or mutagenic effects [1,2]. Moreover, they are characterized by a high persistence in the environment and a consequent accumulation in food webs [3]. PAHs are not easily degradable under natural conditions. Nevertheless, microbial activity can be effective in the PAH-degradation process [4]. In fact, bacteria can utilize PAHs as their sole source of carbon and energy in solid media [5] as PAH-degrading microorganisms can have one or more broad-specificity enzymes for the PAH metabolism. Among other bacteria, Pseudomonadaceaeare able to use a large number of organic compounds as unique carbon and energy sources [6,7]. This versatility allows them to be present in many environments, with a high potential in the bioremediation processes. In the environment, such processes can take advantage of the synergy between different organisms. Phytoremediation, for example, can combine the action of plants and the microbial communities that they support within the rhizosphere, representing a promising technique for the remediation of land and waterways contaminated with hydrocarbons, including PAHs. [8].Besides bacteria, fungi are also good candidates in bioremediation processes because of their relevant ability to degrade PAHs. White-rot fungi, for example, can reach organic compounds penetrating contaminated soil and producing extracellular enzymes, such characteristics giving fungi a significant advantage over bacteria [9]. However, even in the case of white rot fungi, the PAH degradation process remains extremely long, requiring from many days to over a month. New strategies, more efficient than bioremediation, are therefore being developed. One of these consists in the direct use of enzymes extracted from plants or bacteria in remediation processes. This approach could solve certain issues related to the limited growth of plants/microorganisms due to PAH toxicity and to the low bioavailability of such complex compounds. The potential utilization of free enzymes could have other advantages, such as the lack of any need for special environmental conditions, such as CO2, H2O, carbon sources, lighting, micronutrients and macronutrients, which, on the contrary, are essential for bacteria or plant proliferation. Dioxygenase, peroxidase, dehydrogenase and catalase enzymes are able to catalyze the oxidation of PAHs, for example, is well known in the case of the Ring Hydroxylating Dioxygenase (RHD) enzyme [10]. With the advance in biochemical techniques, the extraction and purification of enzymes from microorganisms is more affordable than in the past. In this study, we investigated the effect of the addition of two Pseudomonas anguilliseptica strains to the remediation and detoxification activity of soil contaminated by five PAHs, considered to be priority pollutants, in a specialized microbial consortium composed of Bacillaceae, Enterobacteriaceae, Staphilococcaceae and Xanthomonadaceae, previously tested in an in-batch experiment [3]. We have also investigated the degradation activity of a P.anguilliseptica lysate vs naphthalene, phenanthrene, pyrene, benzo(a)pyrene and anthracene, with a particular focus on PAHs metabolite and degradation kinetics in order to obtain information about the enzymes involved in the upper degradation pathway and the possible utilization of free enzymes/microbial lysates for water/soil remediation.
Materials and Methods
Isolation of P. anguilliseptica
P. anguilliseptica, like the other strains used in this study, was isolated from soot samples taken from a site characterized by intense urban traffic [3,11]. Soot suspension in NaCl 0.9% solution was diluted, plated on Pseudomonas Agar Base with CFC supplement (PAB-CFC, Oxoid) and incubated at 30°C for 72 hours. Colonies were opportunely isolated on Nutrient Agar (NA, Oxoid). DNA was then extracted, opportunely amplified by PCR and analysed for nuclear ribosomal Internal Transcribed Spacer (ITS) region. Sequences were processed with Vector NTI software (Invitrogen) and similarity search was performed using FASTA algorithm against the EMBL-EBI database. The analysis revealed a 100% similarity with P. anguilliseptica and 99.6% with P. fluorescens.
P. anguilliseptica PAHs in-vitro degradation activity
The ability of P.anquilliseptica to degrade naphthalene, anthracene, phenanthrene, pyrene, and benzo[a]pyrene was assessed by an in vitro degradation test. Briefly, a Mineral Salt Medium (MSM) was prepared using a Winogradsky Mineral Solution (WMS) whose composition was (per litre): Na2HPO4, 2.0 g; KH2PO4, 1.0 g; (NH4)2SO4, 0.4 g; MgSO4,7H2O, 0.4 g; and a Trace-Element Solution (TES), 2.0 ml. The composition of the TES was as follows (per litre): Al(OH)3, 0.10 g; SnCl2 2H2O, 0.5 g; KI, 0.05 g; LiCl, 0.05 g; MnSO4 H2O,0.80 g; H3BO3, 0.50 g; ZnSO4 7H2O, 0.10 g; CoCl2 6H2O, 0.10 g; NiSO4 6H2O, 0.10 g; BaCl2, 0.05 g; and (NH4)6 Mo7O2 4H2O, 0.05 g. The medium was added with Bacteriological Agar (Oxoid) at a concentration of 3.5% and autoclaved. The final pH was 7.2. The MSM was used to prepare, in each petri dish (10 cm in diameter), two layers: the upper layer contained each of the chosen PAHs as the unique carbon source dissolved in acetone (8 mgPAHs ml-1), while the lower layer contained a vitamin stock solution to support bacterial growth and cicloheximide to discourage fungal growth. The PAHs, as well all the other salts, were of analytical grade (Sigma-Aldrich, St. Louis, USA). A negative control composed of an MSM double layer with acetone but without any PAHs was prepared. The ability of microorganisms to degrade PAHs was confirmed by their growth and, in some cases, by the presence of clearing zones around the colonies on the plates after 4 weeks at 22°C. All the tests were performed in duplicate.
Microcosms remediation experiments
3 Kg of soil was sampled in a forest and called Uncontaminated Soil (UCS). The UCS was sieved at 2 mm, dried at 120°C for 2 h and added with naphthalene (0.4 mg g-1), anthracene, phenanthrene, pyrene (0.2 mg g-1respectively), and benzo[a]pyrene (0.1 mg g-1), previously dissolved in acetone. This contaminated soil (CS) was kept in the dark for a month in order to allow PAHs to absorb on the soil particles and to avoid photodegradation. The final PAH concentration was 1.1 g kg-1 of soil, from 10 to 40 fold higher than the Critical Threshold Concentration (CTC) for industrial sites (0.01-0.05 mg g-1) of United States Environmental Protection Agency (2002). The bioreactor was composed of 6 batches (1 liter each in volume) and set up using a bioslurry (40% wsoil/V) prepared with the CS and WMS. Then, a microbial consortium composed of Stenotrophomonas sp., Alcaligenes faecalis, Achromobacter spp., Xanthomonas spp., Staphylococcus succinus, Bacillus mojavensis, Enterobacter aerogenes and Arthrobactermisorens, used in a previous survey (Nastro et al, 2014), was added to P.anguilliseptica and, then, to the slurry. The obtained final microbial concentration was 107-108 UFC gsoil-1. The microcosms were incubated at 21°C, opportunely oxygenated and stirred in the absence of light for two months. A UCS slurry was inoculated with the microbial consortium and used as a negative control. Additionally, a slurry with just CS (no microorganisms added) was used as a positive control. Samples of the bioslurry were taken after one (BS1M) and two months (BS2M) for chemical, microbiological and ecotoxicological analyses. The chemical and ecotoxicological results were compared to the findings of our previous study [3] in which Pseudomonas mendocinaand a Pseudomonas spp. were the only Pseudomonas species present.
Microbiological analyses
Microbiological analyses were performed after one and two months of bioremediation treatment to quantify the total microbial load according to standard procedures. The slurry samples were centrifuged at 1400g for 10 minutes, maintaining a temperature of 5°C. Then, 10g of the pellet were suspended in 90 ml of Na-K phosphate buffer and tenfold serially diluted with Na-K phosphate buffer (0.9%). 100 ml of the microbial suspension, at different dilutions, was spread on Nutrient Agar (NA) to quantify the total microbial load during the treatment. All the plates were incubated in aerobic conditions at 30±2°C for 48 hrs. The colonies grown on NA were further isolated and stuck on a Pseudomonas Agar Base (PAB)+CFC supplement, and on MacConckey Agar (MCA), Baird Parker Agar (BPA), Mannitol Egg Yolk Polymixine (MYP) Agar selective media. All media used were by Oxoid (OXOID, Basingstoke, UK).
Ecotoxicological Analyses
UCS, CS, BS1M and BS2M samples were centrifuged (10 min at 1400g). The pellet was eluted (1:4 w/v) in breeding water, stirred for 60 min and then decanted for 24 h. The liquid phase (elutriate) was used for all the ecotoxicological (APHA, AWWA, WEF, 1995), acute, chronic and phytotoxicity assays. The acute toxicity tests vs D.magna and C.dubia were in accordance with the United States Environmental Protection Agency guidelines [12].The assays were performed under illumination (300 lux, photoperiod of 16 h) with an oxygen concentration not below 40%, pH ranging from 7.00 to 7.50 and an environmental temperature of 20±1°C. After 24 h and 48 h of exposure, the immobility rate of 24 h newborns was observed. Immobilization rates over 80% were indicative of a high acute toxicity.
Chronic toxicity assays vs C. dubia
C. dubia newborns, less than 24 hours old, were used to perform the chronic toxicity assays on undiluted and diluted samples. The test was performed in triplicate under constant illumination (300 lux), and at a temperature of 25°C and pH of 7.5±0.2. The overall duration of this assay was 144 h [13]. Immobilization rates over 80% were indicative of a high acute toxicity. The chronic tests were considered acceptable if the survival of the control organisms was of at least 80% and if at least 60% of the surviving control adults produced three broods of offspring in 7 ± 1 days [14].
Phytotoxicity assays
The phytotoxicity tests were performed in accordance with Zucconi F, et al.,[15]. Lepidium sativum seed germination and root length were measured after 72 h of contact with elutriates at 25 °C ±2 (cress seeds) in the absence of light. Distilled water was used as a control. The seed germination was ascertained in terms of a minimum root length of 5 mm. In this test, the germination index (GI) after a 72h period of exposure was calculated as shown by the equation 1 [16] :
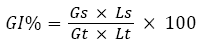
where: Gs=the average number of germinated seeds in the sample,
Gt=the average number of seeds germinated in the blanks,
Ls=the average root length of the sample and
Lt=the average root length of the blank
A GI of less than 40% indicates a high toxicity, a value ranging from 40% to 60% a moderate toxicity (with a delay in the growth) while values higher than 60% the absence of any negative effect.
Chemical analysis
The PAH extraction was carried out according to Derudi et al. [17]. One gram of the soil was treated with 10 mL of an acetonitrile:acetone mixture (3:2), stirred for 3 h and then sonicated for 45 min. The samples were refrigerated at -22°C for 24 h. Thereafter, the supernatant was filtered with a 0.22 μm pore glass fiber membrane, previously rinsed with acetonitrile. The quantitative analysis was performed by inverse phase (HPLC), with a C18 column. PAHs were detected by both UV-Vis spectrophotometry and spectrofluorimetry (HPLC UF Shimadzu Prominence) [18].
P.anguilliseptica lysate degradation assays
In order to investigate the dynamics of the PAHs degradation in P.anguilliseptica, we tested the ability of the cell lysate to degrade naphthalene, phenanthrene, pyrene, benzo(a)pyrene and anthracene at 30°C after 0, 5 and 24 hours. Then, two 1.25 OD546 microbial suspensions in Na-K phosphate buffer (50 mM; pH 7.3) were prepared starting from fresh culture on Mueller Hinton Agar (OXOID, Basingstoke, UK). The suspensions were shaken by a Vortex for 2 minutes and then centrifuged at 8900 g for 15min, maintaining a temperature of 5°C. After being re-suspended in 10 ml of Na-K phosphate buffer, the pellets were centrifuged again, according to the previous experimental scheme, before being frozen at -20°C for one night. The microbial lysis was obtained by applying for two days cycles of heat shock (from -20°C to 30°C) and cycles of sonication with impulse wave form (90 W) for 1 minute (with 20 seconds of stop) at 20°C (S 10H ELMA Sonic, FISHER). Microbial lysis was ascertained by the observation at light microscopy using a 1000x magnification (ZEISS MICROSCOPE). Cellular debris was removed by centrifugation at 10220 g for 20min at 5°C. The lysate supernatant was sterilized by microfiltration using membrane filters with a pore size of 0.22µm (Millipore, Sartorius) to remove the bacteria and fines. The protein content of the lysate was measured by the UV absorbance method [19]. The incubation of the lysate obtained with each PAH chosen was performed in multiwell plates in order to avoid volatilization and to reduce the dead space on the incubation mixture. In the case of the Gas Chromotography - Mass Spectrometry (GC-MS) analyses, the incubation of the water-soluble protein extracts with each PAH was performed as follows [20]. PAH were previously dissolved in 2-methoxyethanol to a final concentration of 20mg/ml, subsequently 250ml corresponding to 5.0 mg of each PAH were added to a mixture of the Pseudomonas extracts (10 μg) and sodium phosphate buffer (10 mM, pH 7.0), to a final volume of 0.5 mL at a temperature of 25°C to a final PAH’s concentration of 1,0 × 104 ppm. In order to avoid volatilization and reduce the dead space on the incubation mixture, the incubation was performed in sealed 850μL, total volume, vials in 675μL of the usable volume. All the incubation and other relevant analyses were carried out in triplicate. The control incubation experiments, carried out by only using phosphate buffer (10mM, PH:7.0) or the sole boiled protein extract, showed that no PAH degradation had taken place. In addition, these experiments also allowed us to ensure that the volatilization of PAHs was not appreciable during the whole process. In order to check the rate of transformation of the PAHs, 50 μL of the incubation mixture were taken at intervals of 15-20 min, readily placed in ice to quickly stop the enzymatic reaction, and extracted with ethyl acetate (450 μL) in a sealed vial. The samples were centrifuged at 10,000 rpm by a high-speed centrifuge (Hermle–Z32HK), the aqueous phases were discarded and the organic supernatants analyzed by GC-MS and thin layer chromatography (TLC). The analytical TLC (silica gel 60 F254, 5 × 10 cm, 0.25 mm thickness; E. Merck Darmstadt, Germany) was developed using n-hexane/ethyl acetate (8:2 v/v) and butanol-acetic acid-water (BAW) (8:1:1) respectively for the naphthalene, pyrene, benzo(a)pyrene, anthracene and phenanthrene degradation products, and the spots were detected by UV light irradiation (254 nm).
Gas chromatography-mass spectrometry (GC-MS) analysis of PAH degradation products
The ethyl acetate extracts were analyzed by GC-MS [21] on an Agilent Technologies unit mod 6850-Series II, equipped with an auto sampler G45134 and an Agilent capillary column (DB-5 type, 0.18 mm ID, film 0.18 μm, length 20 m), using the Agilent Mass Selective Detector mod 5973 as the detector. Helium was used as the carrier gas at a flow rate of 13.8 mL/min. The split ratio applied was 10:1. The injector temperature was 270°C. The gradient applied was as follows: an isotherm of 2 min at 60°C, a first ramp from 60 to 250°C for 20 min (9.5°C min-1), followed by a second ramp from 250 to 300°C for 10 min (10°C min-1); the temperature was then maintained at 300°C for 5 min. The Nist Mass Spectral Program Version 2.0 was used as the software for the interpretation of the mass spectra.
Standard curves
Standard curves were elaborated for all the PAHs investigated, quantifying them by GC-MS analysis. The gas chromatographic analysis of the PAH standards was performed in the conditions previously described for the PAH incubation extracts. The peak area was plotted against the PAH concentration to obtain a linear relationship. In particular, the following values were obtained. Phenanthrene r^2: 0.9973 (y=-9.7738 × +110.86); Naphthalene r^2: 0.9965 (y=-11.048 × +108.71); Benzo(a)pyrene r^2: 0.9937 (y=-7,9167 × +109.75); Pyrene r^2: 0.9917 (y = -9.5714 × +112.57); and Anthracene r^2: 0.9967 (y=-8.1429 × +106.14). The limit of detection by GC-MS was 1 pmole per injection. All the analyses were carried out in triplicate; a confidential level of 95% and a coverage factor K=2 were applied.
PAHs degradation products
The incubation mixture was extracted with ethyl acetate twice (2 × 2 mL) and the combined organic phases were taken to dryness by a stream of oxygen-free nitrogen. The residue, dissolved in 0.5 mL of ethyl acetate, was subjected to HPLC (HPLC, Jasco PU-2089 equipped with an UV/Vis detector plus-2075), using a silica gel column (Luna, Phenomenex, 250 × 4.60 mm, 5 μm, 50 μL /injection). Increasing amounts of methanol in dichloromethane (linear gradient from 0% to 20%, v/v, in 60 min, flow rate 0.5 mL min-1, wavelength 254 nm) were used as the eluent. The isolated products were taken to dryness by an oxygen-free nitrogen stream and the structures determined by GC-MS. The Nist Mass Spectral Program Version 2.0 was used as the software for the interpretation of the mass chromatograms.
GC-MS analysis
Mass spectrometer using the electrospray ionization (ESI) technique in the positive mode. 1. 1–naphtalenol, m/z: 144.1699 (M+H+), 2. 9,10–phenanthrenequinone m/z: 208.2121 (M+H+), 3. 2,2’- diphenic acid, m/z 242.2268 (M+H+) and 4. Methyl p-hydroxybenzoate m/z 152.1490 (M+H+).
Results and Discussion
Microbiological Results
The growth on the double layer mineral medium proved the ability of all strains to use naphthalene, anthracene, phenanthrene, pyrene, benzo[a]pyrene as the sole source of carbon and energy.
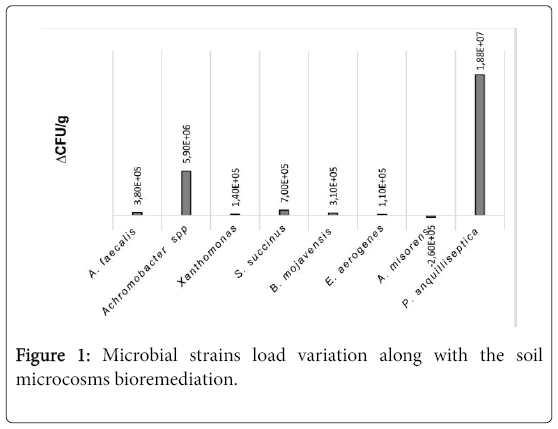
In some cases, clear degradation halos were detected [3]. Microbiological analyses of slurries after one and two months revealed a significant increase of P. anguilliseptica followed by Achromobacter spp., S.succinus and A.faecalis. A.misorens, instead, decreased (Figure 1). The overall increment of most part of microbial strains revealed a positive interaction among them, with the sole exception of A.misorens. Further investigations are needed to clarify the interactions among the different microbial species and the role played by each of them in the remediation process.
Ecotoxicological Results
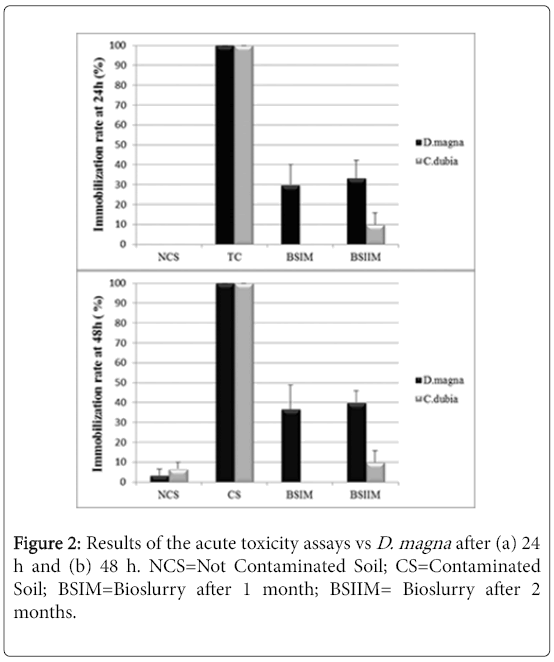
The UCS, used as the negative control, showed no acute toxicity vs Daphnia magna and Ceriodaphniadubia, while the CS showed the maximum toxicity after 24 hours (100% of immobilization) in both assays. A high chronic toxicity was observed in the CS but not in the UCS sample. As for the acute test vs Ceriodaphniadubia, the environmental toxicity of CS slurry decreased after one month of treatment (Figure 2).
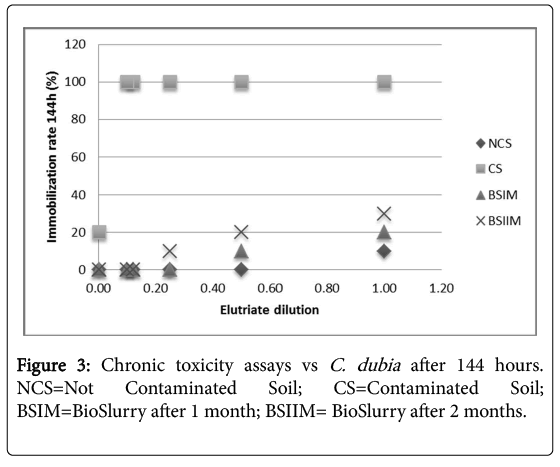
Figure 2 shows a significant decrease of sample toxicity in consequence of the bioremediation process after 2 months, in Daphnia magna and Ceriodaphniadubia, unlike what observed in absence of P. anguilliseptica [3], the chronic toxicity assay showed a dramatic decrease of toxicity in consequence of the in-batch treatment (Figure 3). The phytotoxicity tests vs L. sativum confirmed a detoxification activity in consequence of the bioremediation process the germination index was 45% in the CS, increasing after one month of treatment to 110% in the BSIM and to 122% in the BSIIM, whereas the germination index of the UCS was 61%. The phytotoxicity significantly decreased in the presence of P.anguilliseptica: in our previous study the germination index was 38.7% in the CS and 57.7% in the BSIM, whereas the germination index of the UCS was 60.5% [3].
Chemical Results
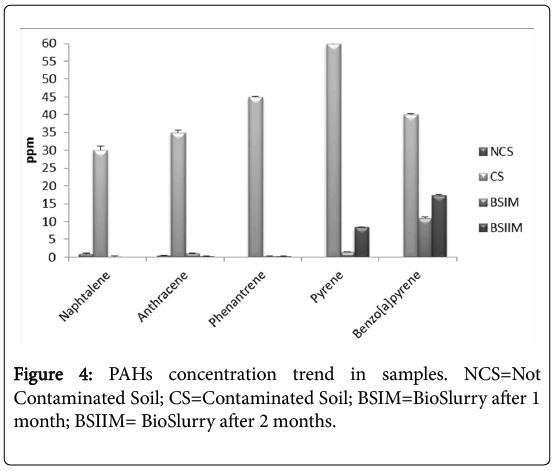
The HPLC analysis showed a high degradation rate of all PAHs chosen, even of pyrene and benzo(a)pyrene, despite their complex structure (Figure 4). The PAH degradation rates, during the bioremediation process, were 100%, 99.14%, 99.23% 86% and 72.5% for naphthalene, anthracene phenanthrene, pyrene and benzo[a]pyrene, respectively. If compared to our previous results, the consortium degradation activity increased by more than 30% [3].
Lysate testing and enzymatic kinetics
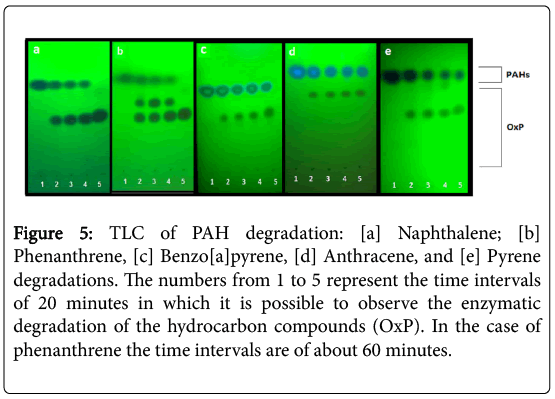
The TLC analysis of the P.anguilliseptica lysate-PAH mixtures allowed us to rapidly control the reaction progress (Figure 1, left). In all cases, the formation of a more polar product at Rf ca. 0.6-0.8 was observed. The disappearance of the starting product can be appreciated; in the case of the phenanthrene the formation of two degradation products is clearly visible in comparison with the other processes (Figure 5).
Figure 5: TLC of PAH degradation: [a] Naphthalene; [b] Phenanthrene, [c] Benzo[a]pyrene, [d] Anthracene, and [e] Pyrene degradations. The numbers from 1 to 5 represent the time intervals of 20 minutes in which it is possible to observe the enzymatic degradation of the hydrocarbon compounds (OxP). In the case of phenanthrene the time intervals are of about 60 minutes.
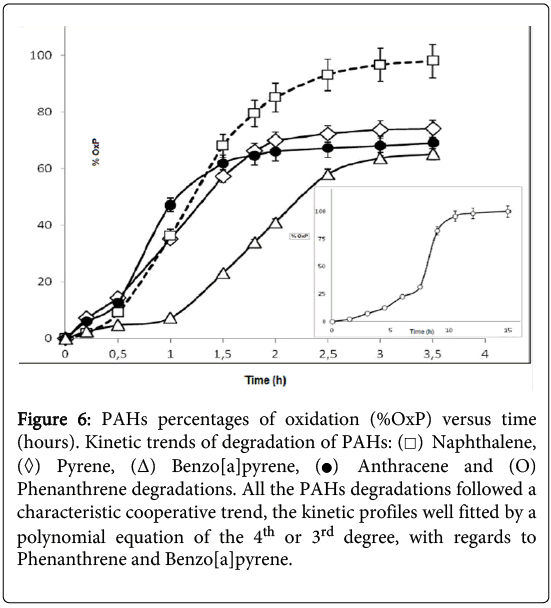
It was observed that the consumption of naphthalene was complete after about 210 minutes (Figure 6) but, in the case of the phenanthrene degradation, the kinetic profile appeared more complex and required a longer time (about 15 hours). The GC-MS kinetic analysis of the water-soluble protein extracts from P.anguilliseptica, incubated with PHAs, revealed some interesting features. In particular, as observed by TLC, in the case of naphthalene the oxidation proceeded quickly, and an almost complete disappearance of the starting product was observed after about 200 minutes (Figure 6). Instead, the phenanthrene oxidation reaction process was slower, and only after about 15 hours was the oxidation reaction considered completed. It is not surprising that the PAH degradation rate is related to the number of the aromatic rings and to the geometry of these molecules. Further information on the enzymatic kinetics was obtained by analyzing the data with the Polynomial Regression Data Fit (P. Lutus – 2013 software). In all cases the plotting of ln[S] versus time indicates a polynomial regression of the 4th degree. In particular, the following correlation coefficients were found: for naphthalene, a correlation coefficient (r^2) of 0.9991 (SE: 1.27), for anthracene (r^2) of 0.9885 (SE: 3.23), and for pyrene and benzo(a)pyrene, (r^2) of 0.9982 (SE: 1.31) and (r^2) of 0.9950 (SE: 1.95), respectively. For the phenanthrene kinetics, the best fitting of the enzymatic kinetics, there is a polynomial regression of the 3rd degree with a correlation coefficient (r^2) of 0.996 (SE: 2.35). Furthermore, for anthracene, naphthalene, pyrene and benzo(a)pyrene the T1/2, namely the time at which the degradations of the polycyclic aromatic compounds correspond to 50%, was 57, 75, 84 and 108 hours, respectively. Therefore, the enzymatic degradation of all these PAHs shows a cooperative trend (Figure 3), more evident in the case of the phenanthrene kinetics profile. In this latter case, it is interesting to note that the T1/2 was reached after about 8 hours. It can be hypothesized that in the case of the phenanthrene degradation, the formation of an intermediate, the 9,10-phenanthrenequinone, had a limiting effect on the degradation rate. The possibility that the oxidation products obtained from the PAHs could arise from the P.anguilliseptica extracts themselves could be discounted following control experiments in which it was shown that PAH degradation does not occur when the enzymatic proteins are inactivated by boiling the crude aqueous extracts (110°C for 5 minutes).
Figure 6: PAHs percentages of oxidation (%OxP) versus time (hours). Kinetic trends of degradation of PAHs: 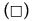 Naphthalene,
Naphthalene, 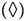 Pyrene, (Δ) Benzo[a]pyrene,
Pyrene, (Δ) Benzo[a]pyrene,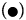 Anthracene and (O) Phenanthrene degradations. All the PAHs degradations followed a characteristic cooperative trend, the kinetic profiles well fitted by a polynomial equation of the 4th or 3rd degree, with regards to Phenanthrene and Benzo[a]pyrene.
Anthracene and (O) Phenanthrene degradations. All the PAHs degradations followed a characteristic cooperative trend, the kinetic profiles well fitted by a polynomial equation of the 4th or 3rd degree, with regards to Phenanthrene and Benzo[a]pyrene.
PAH degradation and metabolites
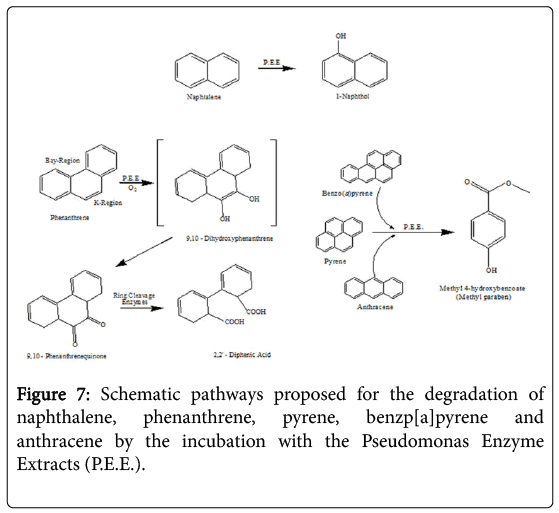
The incubation in vitro of naphthalene with the P. anquilliseptica enzyme extracts led to the rapid formation of 1-naphthol, as the main degradation product (Figure 7). It is interesting to note the strong analogy to the result reported for the fungus and mammalian metabolisms in which the naphthalene oxidation, via a cytochrome P-450, produces naphthalene 1,2-oxide, this arene oxide undergoing rearrangement to form 1-naphthol via the NIH shift mechanism [22].
Phenanthrene degradation was longer and more complex, with the formation of an intermediate, the 9,10-phenanthrenequinone, successively oxidized to 2,2'-diphenic acid. Such oxidative degradation has strong similarities with that observed for Rhizobacterium ensifermeliloti [23]. and for the Mycobaterium sp. Strain PYR-1. However, the use of enzymatic extracts from P. anguilliseptica resulted in a faster degradation of this PAH, namely of about 15 hours, in comparison with that of Rhizobacterium ensifermeliloti and the Mycobacterium sp. Strain PYR-1, which required about 10 and 3 days respectively.
In the case of benzo[a]pyrene, anthracene and pyrene, the enzymatic incubations showed the formation of the same final product, 3-Hydroxybenzoic acid methyl ester, more commonly known as Methyl paraben. However, the kinetics of these PAHs showed different profiles and the T1/2 values, previously reported, show that the enzymatic degradations are certainly in correlation with the geometry of the molecule.
Degradative pathways
The degradation of PAHs using free enzyme extracts from P.anguilliseptica presents interesting similarities to and differences from other metabolic pathways. In particular, there is the example of the degradation of phenanthrene, which is different from that of the microsomal cytochrome P-450 while it is extremely similar to the ligninolytic processes of Phanerochaetechrysosporium and Rhizobacterium ensifermeliloti [23].
These two processes lead to the formation of the first 9,10-Quinone (PQ), whose subsequent oxidation and cutting give 2,2'-Diphenic acid (DPA), this latter compound being easily mineralizable by other organisms. It is important to note that the degradation of phenanthrene by the free enzymes from the P. anguilliseptica strain proceeds similarly to the process reported for mammals and for the white rot fungus Pleurotusostreatus. Very likely, cytochrome P-450 monooxygenase system plays a role in the initial oxidation of the phenanthrene, which begins in its “K-Region” [24].
Differently from the degradation of phenanthrene, in the case of naphthalene we did not observe any similarities with fungi or with bacteria but instead a similarity with mammals, namely the cytochrome P-450 monooxygenases, with the formation of a first compound, the 1,2-oxide, and then the spontaneous transformation into 1-Naphthol via an NIH shift mechanism. However, it is also very interesting to note that this degradation pathway is common in fungi such as Agrocypeaegerita, which secretes a peroxidase that can regioselectively hydroxylate the naphthalene, producing 1-naphtol and traces of 2-naphtol (ratio 36: 1) [25].
In the case of benzopyrene, anthracene and pyrene, their enzymatic degradation results in the formation of the same final compound, methyl 4-hydroxybenzoate, commonly known as Methyl paraben. This degradation product is not present in the usual bacterial degradation pathways because it is a well-known pharmacologically active compound due to its potent antimicrobial and antifungal activity already exercised at extremely low concentrations from 10-3 to 10-5 mg ml-1 against multiple bacterial strains such as S. aureus, E. coli, C. albicans and A. niger [26]. It is very likely that, in the course of PAHs microbial degradation powerful molecules, such as methyl paraben, can be produced that would result in an inhibition of the microbial proliferation. Therefore, the use of enzymatic extracts allows us thus to avoid these degradative inhibitions enabling faster degradations of these polycyclic aromatic compounds. In contrast to the findings which have been reported in fungi, mammals and many bacteria, it is necessary to emphasize that in all the cases of degradation listed above, free enzymes from P.anguilliseptica crude extracts take only a few hours to realize the degradation. In the case of fungi, bacteria and mammals this process requires days, weeks or even months.
Conclusions
We have presented the results of in-batch bioremediation experiments on CS using a microbial consortium isolated from an urban environment added to a P.anguilliseptica strain from the same sampling site. The PAHs removal, as well as the detoxification of the polluted soil, was enhanced in the presence of the P.anguilliseptica strain, with a significant decrease in chronic toxicity and phytotoxicity. The PAHs removal increased by 30% when P. anguilliseptica was present. Microbiological analyses of the slurry after two months of treatment revealed a significant increase of P.anguilliseptica, Arthrobactermisorensand Staphilococcussuccinus, probably in consequence of a synergistic activity among these three strains. The enzymatic activity of a P.anguilliseptica sterile lysate vs phenanthrene, naphthalene, benzo(a)pyrene, pyrene and anthracene gave very interesting and promising results. To the best of our knowledge, all degradation processes using free enzyme extracts were faster in comparison to those observed in whole bacteria and fungi. A new efficient remediation approach using cell-free enzymes derived from Pseudomonas anquilliseptica to degrade some representative PAHs could avoid certain problems, such as the low bioavailability associated with the use of the whole plant, microbial cells that have shown many limitations of growth in very polluted soils and specific nutrient requirements. The direct utilization of enzymes/microbial extracts could present specific advantages such as availability (a high biomass of Pseudomonas spp. is easy to obtain) and a fast PAH degradation time, particularly useful in the remediation of contaminated soils or waste waters.
Acknowledgments
The authors are grateful to Prof. Luciano Mayol for their useful discussions, Dr. Marco Annetta and Dr Olga De Castro for some chemical and biomolecular analyses.
References
- Huang XD, El-Alawi Y, Gurska J, Glick BR, Greenberg BM (2005) A Multi-Process Phytoremediation System for Decontamination of Persistent Total Petroleum Hydrocarbons (TPHs) from Soils. Microchemical Journal 81: 139-147.
- Shimada T (2006) Xenobiotic-Metabolizing Enzymes Involved in Activation and Detoxification of Carcinogenic Polycyclic Aromatic Hydrocarbons. Drug MetabPharmacokinet 21: 257-276.
- Nastro RA, Suglia A, Toscanesi M, Trifuoggi M, Guida M, et al. (2014) Bioremediation Process Efficiency for Polycyclic Aromatic Hydrocarbons Through Ecotoxicological Tests. International Journal of Performability Engineering 10: 411-418.
- Cerniglia CE, Heitkamp MA (1989) Microbial degradation of polycyclic aromatic hydrocarbons in the aquatic environment. In: Metabolism of Polycyclic Aromatic Hydrocarbons in the Aquatic Environment. CRC Press, Boca Raton, Florida, USA pp: 42-64.  Â
- Kaushik CP, Haritash AK (2006) Polycyclic aromatic hydrocarbons (PAHs) and environmental health, Our Earth 3.
- Garcia-Valdes E, Czar E, Rotger R, Lalucat L, Ursing J (1988) New naphthalene-degrading marine Pseudomonas strains. Appl Environ Microbiol 54: 2478-2485
- Obradors N, Aguilar J (1991) Efficient biodegradation of high-molecular-weight polyethylene glycols by pure cultures of Pseudomonas stutzeri. Appl Environ Microbiol 57: 2383-2388.
- Denys S, Rollin C, Guillot F, Baroudi H (2006) In-Situ Phytoremediation of PAHs Contaminated Soils Following a Bioremediation treatment. Water, Air, & Soil Pollution: Focus 6: 299-315.
- Pointing SB (2001) Feasibility of bioremediation by white-rot fungi. ApplMicrobiolBiotechnol 57: 20-33
- Butler CS, Mason JR (1996) Structure-function Analysis of the Bacterial Aromatic Ring-hydroxylating Dioxygenases. Advances in Microbial Physiology 38: 47-84.
- Gambino E, Nastro RA, Trifuoggi M, Guida M (2015) Pseudomonas anguilliseptica enhances the efficiency of a microbial consortium in the detoxification and remediation of a soil contaminated by Polyciclic Aromatic Hydrocarbon (PAHs). VI International Conference on Environmental, Industrial and Applied Microbiology -BioMicroWorld2015, Barcelona (Spain).
- USEPA (2002) Methods for Measuring the Acute Toxicity of Effluents and Receiving Waters to Freshwater and Marine Organisms. Fifth Edition.
- USEPA (1994) Short-term methods for estimating the chronic toxicity of effluents and receiving water to freshwater organisms. Third Edition.
- Viganò L (1996) Metodo per la valutazionedellatossicità acuta con Ceriodaphniadubia. NotiziariodeiMetodiAnalitici IRSA, pp: 9-19.
- Zucconi F, Monaco A, Forte M, Bertoldi M (1985) Phytotoxins during the stabilization of organic matter. In: Composting of agricultural and other wastes. Elsevier Applied Science, New York, USA, Pp: 73-88.
- Hoekstra AY, Hung PQ (2002) Virtual water trade: A quantification of virtual water flows between nations in relation to international crop trade. DELFT, Value of Water Research Report Series No.11, UNESCO-IHE.
- Derudi M, Nano G, Rota R (2004) ProcessiBioslurry per la Degradazione di IdrocarburiPolicicliciAromatici e Fenoli in SuoliContaminati. Atti del 22 Convegno Nazionale, Palermo, Italy, pp: 290-294.
- Bogardt AH, Hemmingsen BB (1992) Enumeration of Phenanthrene-Degrading Bacteria by an Overlayer Technique and its Use in Evaluation of Petroleum-Contaminated Sites. Applied and Environmental Microbiology 58: 2579-2582.
- Stoscheck CM (1990) Quantitation of Protein. Methods in Enzymology 182: 50-69.
- Barone R, De Biasi MG, Piccialli V, De Napoli L, Oliviero G, et al. (2016) Degradation of some representative polycyclic aromatic hydrocarbons by the water-soluble protein extracts from Zea mays L. cv PR32-B10. Chemosphere 160: 258-265.
- Santos FJ, Galceran MT (2002) The application of gas chromatography to environmental analysis. Trends in Analytical Chemistry 21: 672-685.
- Usha V (2000) Metabolism of Polycyclic Aromatic Hydrocarbons in the Aquatic Environment. CRC Press, Boca Raton, Florida, USA, Pp: 48-50.
- Muratova A, Pozdnayakova N, Makarov O, Turkovskaya O (2014) Degradation of phenanthrene by the Rhizobacterium Ensifermeliloti. Biodegradation 25: 787-795.
- Bezalel L, Hadar Y, Cerniglia CE (1997) Enzymatic mechanisms involved in phenanthrene degradation by the white rot fungus Pleurotusostreatus. Applied and Environmental Microbiology 63: 2495-2501.
- Ullrich R, Hofrichter M (2005) The haloperoxidase of the agaric fungus Agrocybeaegerita hydroxylates toluene and naphthalene. FEBS Letters 579: 6247-6250.
- Zang S, Lian B (2009) Synergistic degradation of 2-naphthol by Fusarium proliferatum and Bacillus subtilis in wastewater. Journal of Hazardous Materials166: 33-38.
Citation: Barone R, Nastro RA, Gambino E, Toscanesi M, Picciall G, et al. (2017) Pseudomonas anguilliseptica Strain-A1 Degradation of Polycyclic Aromatic Hydrocarbons in Soil Microcosms: Focus on Detoxification Activity and Free Water-Soluble Protein Extracts Kinetics and Efficiency. J Bioremediat Biodegrad 8: 418. DOI: 10.4172/2155-6199.1000418
Copyright: © 2017 Barone R, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 7835
- [From(publication date): 0-2017 - Apr 16, 2025]
- Breakdown by view type
- HTML page views: 6446
- PDF downloads: 1389