Short Communication Open Access
Protein Enrichment of Opuntia Spp. Using Different Biotechnological Treatments
| Rodríguez-Hernández JL1,goncalves A2, Moraes-Rochag3, Nevárez gV1, Peralta MR1, Muñoz-Castellanos L1, Arevalo S1, Ayalag1, Carrillo- Campos J1 and Ballinas-CasarrubiasmL1* | |
| 1School of Chemistry, University of Chihuahua. Circuit No. 1, New University Campus, Chihuahua, Chih, C.P. 31125, PO 669 and 1542-C, Mexico | |
| 2Department of Biotechnology, School of Engineering of Lorena, Universidade de São Paulo, Cx. Postal 116, 12600-000 Lorena, SP, Brazil | |
| 3National Laboratory of Bioethanol Science and Technology do, PO Box 6170 Campinas, São Paulo 13083-970 Brazil. | |
| Corresponding Author : | Ballinas-CasarrubiasmL School of Chemistry, University of Chihuahua Circuit No. 1, New University Campus, Chihuahua, Chih, Mexico Tel: +526142366000 Fax: (614) -236-6000 E-mail: lourdes.ballinas@gmail.com. |
| Received: June 19, 2015 Accepted: August 21, 2015 Published: August 27, 2015 | |
| Citation: Rodríguez-Hernández JL, Goncalves A, Moraes-Rochag, Nevárez GV, Peralta MR, et al. (2015) Protein Enrichment of Opuntia Spp. Using Different Biotechnological Treatments. Adv Crop Sci Tech 3:186. doi:10.4172/2329-8863.1000186 | |
| Copyright: © 2015 Rodríguez-Hernández JL, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
| Related article at Pubmed, Scholar Google | |
Visit for more related articles at Advances in Crop Science and Technology
Abstract
Chihuahua desert possesses 60% of the Opuntia species (nopal) naturally grown in Mexico. These species are considered a reserve material due to its tolerance to adverse weather conditions, and some have been used as cattle forage regardless their low protein content. One of the traditional ways for protein enrichment is through yeast fermentation using S. cerevisae. In the present work, several routes have been assessed to augment cellulose bio availability during fermentation. The protein enrichment process, coupled with an additional thermal, enzymatic or fungal treatment in a semi-solid culture is evaluated. The yeast concentration was the constant parameter in the culture at 15% wet basis. Six different treatments were proposed considering :a. Thermal treatment; b. Fermentation using Phanerochaete chrysosporium A594; c. Novozyme 188 and Celluloclast thydrolysis; d. Fermentation with S. cerevisiae at 37° C for 48 h, 180 rpm. For each sample, lignin, cellulose and protein were measured. The results are as follows. Protein at original sample was of 0.042%, and its content (%) in the different treatment combinations was: a and d: 16.5; a,b and d:22.8; a,c and d:10.6; d:14.3; b and d: 25.9, c and d:8.75. Therefore, thermal processing did not contribute to the increase in protein content in the final product, neither was the enzymatic pre-treatment. Fungal solid state fermentation contributed significantly to the increment in protein content due, principally, to the lignin depletion in the fermentation media.
| Keywords |
| Nopal; Protein enrichment; Fermentation; Forage |
| Introduction |
| Opuntia spp is a traditional cactacea, which comprises more than 200 species reported in the world [1]. In Mexico, coexist 188 species and 78 of them are endemic [1,2].This genera has been used for many functional proposes such as cosmetics, a source of natural colorants and for animal and human feeding [2]. Recently, its usage as forage has been increased due to the severe drought periods accounting for the irregular distribution of the rain and water supply. These incidences have caused a reduction of pasture and common fodder deficiency [3]. The solution that has been applied is the usage of supplements and concentrates whose market prices are many times non affordable by the consumers [4]. |
| Cactacea genera have been used worldwide as a cheap alternative for cattle feeding, in particular Opuntia indica has successfully been used in Brazil, South Africa and Tunez [5-7]. Due to its physiological characteristics, it is of special importance in the arid and semiarid regions as a reserve material due to its tolerance to adverse weather conditions and it potential for providing minerals, water and carbohydrates. In spite of these advantages, Opuntia doesn’t possess the required protein content, especially for the milk producer’s cows [5,8]. One of the traditional ways for protein enrichment is trough yeast fermentation using S. cerevisaeas inoculum. Cell protein from microorganisms is one of the possible high quality sources that can be considered for this purpose. Besides, some bacteria fungi and yeasts possess a high growth rate and a beneficial metabolism for cattle [9]. Some yeasts, as S.cerevisae, could also provide some of the vitamins of the B complex. This alternative has been recently reported in the studies performed by Araujo and collaborators, reaching 465% of protein increment compared to the control [4].In a fermentation process, bio-availability of the substrate could be crucial for increasing the respective reaction yields. In the case of S. cerevisae, glucose is the principal substrate used, which comes from the cellulosic substrate. The glucose concentration affects the rate of its metabolism and the presence of lignin substrates could diminish the total efficiency performance. |
| In this regard, the present work provides information about several treatment alternatives to increase cellulose bio-availability of Opuntia cladodes. Also, for these different alternatives, thermal treatment is assessed and free glucose in the fermentation liquors is analyzed. The effect of fungal or enzymatic fermentation is considered as well. The process of protein enrichment of the Opuntia, using the Saccharomyces cerevisiae yeast, in a semi-solid culture to improve the nutritional value, is evaluated. Biopolymers distribution in the fermentation media are correlated to the maximum protein production. |
| Methodology |
| Determination of moisture |
| Cladodes of Opuntiaspp. from Milpa Alta, Mexico (20.65° latitude, -100.32° length)of 15-20 cm length were harvested on May 2008 and sent to Chihuahua. They were cooled at 4°C until the moisture analysis was carried out. Cladodes were crushed by triplicate, obtaining a final volume of 10 mL. The sample was subjected to 65°C for 48 h. Moisture content, based on dry matter (DM), was calculated using the mass weight differences. |
| Drying of nopal |
| The nopal was dehydrated in order to have better handling and storage. Subsequently, it was reconstituted by taking 5 g of dried cactus and adding 95 mL of distilled water, to homogenize the water activity for all samples. |
| Opuntia treatments |
| A full factorial design with two variables, one with two levels (thermal treatment) and one with three levels (non-hydrolysis, fungal hydrolysis and enzymatic hydrolysis) was performed for six different treatments (Table 1). All analyses were made by triplicate. To determine if there were significant differences among them, a statistical analysis was performed by ANOVA using Minitab software (Minitab© 15.1.1.0Inc. 2007). |
| Thermal treatment |
| The treatment method was based on the use of water vapor at high pressure and temperature. After heating, the decompression of the material occurred, causing the breakdown of lingo cellulosic structures. The cactus was placed in an autoclave (100 mL of reconstituted nopal) at 121°C and 1.05 atm of pressure, for different periods of time. Decompression was of 0.157 psi/s in 90s. |
| Fungal hydrolysis |
| Phanero chaetechrysosporium fungus A594 strain fresh culture provided by UAM-México was inoculated into PDA (Potato Dextrose Broth) and incubated for a period of 7 days at 30°C. The spores were harvested using Tween 80 0.01%. For 100 mL of sterile reconstituted nopal was shaken with an inoculum of 1 × 107 spores per gram, in dry weight, for 7 days at 180 rpm, 30°C. |
| Enzymatichydrolysis |
| The thermal-treated and non-treated Opuntia samples were enzymatically hydrolyzed by Celluclast 1.5 and Novozyme 188 enzymes (Novozymes Inc.). Tests were carried out with 100 mL of previously reconstituted nopal, adjusting the pH to 4.8 with a phosphate buffer. The activity of the enzyme Celluclast 1.5 L was of 75 FPU/g and for Novozyme 188 of 12.6 IU/g dry weight. The treatment time was 72 h at 50°C in an orbital shaker at 85 rpm. |
| Fermentation |
| Saccharomyces cerevisiae strain fresh culture (Nutrient Agar, 37°C, 24 h) was inoculated into TSA (Tryptone Soy Broth) and incubated for 24 h at 37°C. Yeast biomass was determined by turbidimetry (620 nm) to determine the appropriate volume of inoculum. Opuntia fermentation was set up in batch, in 50 mL Erlenmeyer flasks, with 100 mL of sterile cladode biomass (5 g DM) and inoculated with the yeast culture to achieve a 15% w/w inoculum. The flask was covered with a cotton plug and fermentation was done at 40°C for 48 h at 180 rpm. |
| Chemical composition of lignocellulosic materials |
| Acid hydrolysis was performed for chemical characterization of the solid fractions of each of the treatments made.2 g of previously filtered material were weighed and dried at 65 °C, and placed in a water bath at 45.0 °C. Sample digestion has been described elsewhere [10].Liquors were analyzed directly, without digestion. Both fractions were analyzed by hPLC. For soluble lignin analysis, UV-Vis spectrometry was used at 280 nm; insoluble lignin was quantified by gravimetry [10]. |
| Determination of carbohydrates and organic acids by hPLC |
| All samples digested were analyzed by high performance liquid chromatography (HPLC, Shimadzu CR 7A) using a column AminexhPX-87H (300 × 7.8 mm, Bio-Rad Laboratories Ltd), a mobile phase of H2SO4 0.005 mol L-1 with a flow of 0.6 mL min-1, at 45 °C. The compounds were analyzed using a refractive index detector Shimadzu RID 6A. Cellulose and hemicellulose were calculated as stated by Moraes-Rocha [10]. |
| Determination of proteins |
| Protein analysis was made by the Bradford method. The description of the assay can be found elsewhere [11]. Bradford reactive (100 mL) was made with 10 mg of Coomassie blueg-250, mixed with 10 mL of 88% phosphoric acid and 4.7 mL of absolute ethanol. Different dilutions of the sample were made (1:10, 1:20, 1:50) using 0.15 M of NaCl; 2 mL Bradford reagent was added and mixed. Calibration curves were prepared with dry bovine serum albumin-BSA (Sigma). |
| Results |
| Cladode is a part of the Opuntia plant that is frequently related to the flattened segment which replaces leaves for photosynthesis. It is also known as cactus stem. Cladode composition depends on edaphic factors at the cultivation site and season and age of the plant [3,12-14]. Initial characterization of the material was performed. Water quantity was measured as 94.82% in a dry matter basis (DM). Salim [15] found a 93% water content in Opuntiaficus Indica. In our results, Cellulose, hemicellulose and lignin (DM) are 28.15%, 2.82% and 12% respectively. According to Malainine [16], in 100 g of dry matter weight, there are 3.6 g of lignin, 21.6 g of cellulose, and 48 g of other polysaccharides. Peña- Valdivia et al. [2] have recently reported an extensive study on Opuntia spp. composition in different Mexican cultivars. Content of loosely bound hemicelluloses in “nopalitos” of the majority of cultivars was similar: 4-11% DM. On the other hand, tightly bound hemicelluloses correspond to 2.2-4.6% DM. The majority of the cultivars analyzed by them had intermediate cellulose contents (7.2-13.3% DM). Ben Thilja [17] reported mean values in DM of five Opuntiasp cladodes as follows: 11% cellulose, 8% hemicellulose and 3.9% lignin respectively. |
| Protein in fresh Opuntia analyzed was 0.34% DM. Salim reported a range of 1.4 a 7.61% of protein (DM), among different species and gebremariam et al. (2006), 8.3% DM [6]. Other authors [12-14] found 4-10 g of proteins in 100 g. Significant differences in protein are reported, nevertheless they are lower than other forage supplies, such as “alfalfa” which is of 20-28% DM [18]. |
| Thermal treatment |
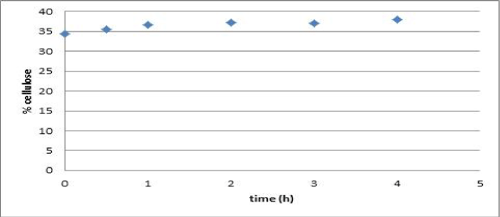
| Vapor explosion and decompression is a thermal treatment which has been used for lignocellulosic materials to increment cellulose availability [19]. Water penetrates and condenses into the material. When decompression occurs, water is cooled rapidly and the cellulose fiber rupture arises in the amorphous sections. For thermal treatment, no difference based on cellulose content in time, from 30 to 240 minutes, was noticed (Figure 1). Therefore, 30 minutes were fixed as the treatment time for further experiments. |
| Fungal and enzymatic fermentations |
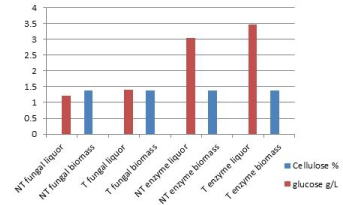
| The glucose and cellulose concentration were analyzed in the liquor and solid fraction respectively, for non-thermally treated and thermal treated cladodes (Figure 2). Cellulose remaining in all treatments into the solid fraction is almost equal. In the case of glucose, some differences are noticed. Thermal treatment always increases glucose compared to the non-thermal process. During fungal fermentation, glucose is liberated due to cellulose hydrolysis, such as it has been reported by other authors with Aspergillusniger [20]. |
| In the case of the enzymatic treatment, glucose increased from 8.32% to 30.45% and 34.75% in the liquors. Padron [21] reported enzymatic hydrolysis of Opuntiaboldinghi, where free glucose increased from 1-7%. In enzymatic fermentation, glucose is superior compared to fungal treatment. It is explained in terms of microorganism consumption during its metabolism. |
| Train treatments |
| Protein, cellulose, hemicellulose and lignin were evaluated for each treatment (Table 1). Results were evaluated statistically using the Fisher-Snedecortest (α=0.05, Fc=5.79). |
| Cellulose concentration varies among treatments performed (F>5.79) (Table 2). The minor values attained were for T3 and T5. These are originated by the action of the microorganisms which consume mainly glucose as their carbon source. hemicellulose concentration shows no significant differences among the treatments (F ≤ 5.79).Total Lignin concentration has been evaluated and there were significant differences (F>5.79). Maximum lignin in liquor was for T4 and the lowest for T5. Lower lignin content increases digestibility of biomass for animal feed [9]. Regarding the protein content, the analysis also shows significant differences. T5 is the treatment with the highest concentration, with an increment of 26%. Cladodes without treatment present 0.34% DM. Araujo [4] reported the bioconversion of cactus pear by yeast in solid medium at different inoculum level (5-15%). There, a maximum PI of 465% was reached in 48 h. This report corresponds to a 25.48% DM, which is similar to our best result, using non-thermal pretreatment, fungal and yeast fermentation during 9 days. In the work of Oliveira et al, using Aspergillusniger, protein increases to 12.80% DM in 120 h [20].Diaz-Plasencia reported the fermentation of Opuntiaspp with Kluyveromyces Lactis. Crude protein increases from 9.35 to 19.36% in 48 h [22]. |
| Conclusion |
| Thermal treatment does not benefit the yeast fermentation. In spite of a high glucose concentration into the liquor for the enzymatic process, protein enrichment was better for fungal treatment. Lignin depletion by the fungi is a strong factor to give better protein yields during yeast fermentation. Biological treatment with white rot fungi and yeast fermentation increases protein content to 26% DM. |
| Acknowledgements |
| The authors appreciate the funds of CONACYT-FOMIX project no.CHIH- 2009-C02-127570. The authors are in debt with Dr. Ma. Elena Fuentes Montero forher careful and time consuming revision of the present manuscript. |
| References |
References
- value="1" id="Reference_Titile_Link">Reyes-Agüero JA, Aguirre JR, Valiente-Banuet A (2006) Reproductive biology of Opuntia: A review. J Arid Environ 64: 549-585.
- value="2" id="Reference_Titile_Link">Peña-Valdivia CB, Trejo C, Arroyo-Peña BV, Sánchez-Urdaneta AB, Balois-Morales R (2012) Diversity of unavailable polysaccharides and dietary fiber in domesticated nopalito and cactus pear fruit (Opuntiaspp).Chem and Biodivers 9:1599-1610.
- value="3" id="Reference_Titile_Link">Stintzing FC, Carle R (2005) Cactus stems (Opuntia spp.): A review on their chemistry, technology, and uses. MolNutr Food Res49:175-194.
- value="4" id="Reference_Titile_Link">Araujo LF, Nunes-Medeiros A, Perazzo-Neto A, de Sousa ConradoOliveira L, Honorato da Silva FL (2005) Protein enrichment of Cactus pear (Opuntiaficus-indica Mill) using Saccharomyces cerevisae in solid state fermentation. Braz arch BiolTechn48: 161-168.
- value="5" id="Reference_Titile_Link">Mendez-Llorente F, Ramírez-Lozano RG, Aguilera-Soto JI, Arechiga-Flores CF (2008) Performance and nutrient digestion of lambs fed incremental levels of wild cactus (Opuntialeucotricia). Conference on International Research on Food Security, Natural Resource Management and Rural Development: University of Hohenheim.
- value="6" id="Reference_Titile_Link">Gebremariam T, Melaku S, Yami A (2006) Effect of different levels of cactus (Opuntiaficus-indica) inclusion on feed intake, digestibility and body weight gain in tef (Eragrostistef) straw-based feeding of sheep. Anim Feed SciTechnol131: 43-52.
- value="7" id="Reference_Titile_Link">Tegegne F, Kijora C, Peters KJ (2007) Study on the optimal level of cactus pear (Opuntiaficus-indica) supplementation to sheep and its contribution as source of water. Small Ruminant Res 49: 175-194.
- value="8" id="Reference_Titile_Link">Vasta A, Nudda A, Cannas A, Lanza M, Priolo A (2008) Alternative feed resources and their effects on the quality of meat and milk from small ruminants. Anim Feed SciTechnol 147: 223-246.
- value="9" id="Reference_Titile_Link">Saritha M, Arora A, Lata (2012) Biological pretreatment of lignocellulosic substrates for enhanced delignification and enzymatic digestibility. Indian J Microbiol52:122-130.
- value="10" id="Reference_Titile_Link">Rocha GJM, Martin C, Soares IB, Souto-Maior AM, Baudel HMet al(2011) Dilute mixed-acid pretreatment of sugarcane bagasse for ethanol production. Biomass Bioenerg35: 663-670.
- value="11" id="Reference_Titile_Link">Lucarini AC, Kilikian BV (1999) Comparative study of Lowry and Bradford methods: interfering substances. Biotechnol Tech 13: 149-154.
- value="12" id="Reference_Titile_Link">Yassen M, Barringer Y, Splittstoesser WE (1996) A note on the uses of Opuntia spp. in Central/North America. J Arid Environ 32: 347-353.
- value="13" id="Reference_Titile_Link">Mizrahi Y, Nerd A, Nobel PS (1997) Cacti as crops. Hort Rev 18: 291-320.
- value="14" id="Reference_Titile_Link">Batista AM, Mustafa AF, McAllister T, Wang Y, Soita H et al (2003) Effects of variety on chemical composition, in situ nutrient disappearance and in vitro gas production of spineless cacti. J Sci Food Agric 83: 440-445.
- value="15" id="Reference_Titile_Link">Salim N,Abdelwahe C, Rabah C, Ahcene B (2009) Chemical composition of Opuntiaficus-indica (L) fruit. Afr J Biotechnol 8: 1623-1624.
- value="16" id="Reference_Titile_Link">Melanine ME, Dufresne A, Dupeyre D, Vignon MR, Mahrouz M (2003) First evidence of weddelite crystallites in Opuntiaficus-indica parenchyma. Z NaturforshBiosci 58c: 812-816.
- value="17" id="Reference_Titile_Link">Ben-Thlija A (1987) Nutritional value of several Opuntia species. Master ThesisOregonStateUniversity, Corvallis/USA.
- value="18" id="Reference_Titile_Link">Faner C (2007) La pastura de alfalfa comoFuente de alimentaciónparacerdos en crecimiento y terminación. Boletín AACP.
- value="19" id="Reference_Titile_Link">Ballesteros I, Negro MJ, Oliva JM, Cabañas A, Manzanares P et al.(2006)Ethanol production from steam-explosion pretreated wheat Straw. ApplBiochem Biotech 129: 496-508.
- value="20" id="Reference_Titile_Link">Oliveira MA (2001)Production of fungal protein by solid substrate fermentation of Cactus cereus peruvianus and Opuntiaficus-indica. Quim Nova24:307-310.
- value="21" id="Reference_Titile_Link">Padron-Pereira CA, Moreno-Alvarez MJ, Medina-Martinez CA, Garcia Pantaleon DM (2009) Obtention of enzimatically hydrolyzed product from Cactus (Opuntiaboldinghii Britton and Rose) cladodes whole flour. Pak J Nutr 8: 459-468.
- value="22" id="Reference_Titile_Link">Diaz Plasencia D, Rodríguez-Muela C, Mancilas-Flores P,Ruíz-Holguin N, Mena-Munguía Fet al(2012) In vitro fermentation of forage prickly pear caCtus with yeast inoculum of Kluyveromyceslactis from Apple waste.REDVET13: 1-11.
Tables and Figures at a glance
| Table 1 | Table 2 |
Figures at a glance
 |
 |
| Figure 1 | Figure 2 |
Relevant Topics
- Agricultural science
- Agronomy
- Climate impact on crops
- Crop Productivity
- Crop Sciences
- Crop Technology
- Field Crops Research
- Hybrid Seed Technology
- Irrigation Technology
- Organic Cover Crops
- Organic Crops
- Pest Management
- Plant Genetics
- Plant Breeding
- Plant Nutrition
- Seed Production
- Seed Science and Technology
- Soil Fertility
- Weed Control
Recommended Journals
Article Tools
Article Usage
- Total views: 14587
- [From(publication date):
November-2015 - Jul 11, 2025] - Breakdown by view type
- HTML page views : 9937
- PDF downloads : 4650
Peer Reviewed Journals
Make the best use of Scientific Research and information from our 700 + peer reviewed, Open Access Journals
