Research Article Open Access
Protective Effects of Quercetin and Ursodeoxycholic Acid on Hepatic Ischemia-Reperfusion Injury in Rats
| Fares EM Ali1, Amira M Abo-Youssef2, Basim AS Messiha2* and Ramadan AM Hemeda1 | |
| 1Department of Pharmacology, Faculty of Pharmacy, Al-Azhar University Assuit Branch, Egypt | |
| 2Department of Pharmacology, Faculty of Pharmacy, Beni Sueif University, Egypt | |
| Corresponding Author : | Basim A.S. Messiha Department of Pharmacology, Faculty of Pharmacy Beni Sueif University, Egypt Tel: (+2) 012-24459164 Fax: +2 082 2317958/+2 082 2319397 E-mail: drbasimanwar2006@yahoo.com |
| Received December 19, 2014; Accepted December 26, 2014; Published December 31, 2014 | |
| Citation: Ali FEM, Abo-Youssef AM, Messiha BAS, Hemeda RAM (2015) Protective Effects of Quercetin and Ursodeoxycholic Acid on Hepatic Ischemia-Reperfusion Injury in Rats. Clin Pharmacol Biopharm 3:128. doi:10.4172/2167-065X.1000128 | |
| Copyright: © 2014 Ali FEM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Clinical Pharmacology & Biopharmaceutics
Abstract
This study aims to evaluate the protective effects of Quercetin and Ursodeoxycholic acid (UDCA), as compared to standard agent N-acetylcysteine (NAC), on hepatic ischemia-reperfusion (IR)-induced injury in rats. Briefly, rats were divided into five groups, namely sham control, IR control, NAC, Quercetin and UDCA groups. Assessed biomarkers included serum alanine transaminase (ALT), aspartate transaminase (AST), alkaline phosphatase (ALP), lactate dehydrogenase (LDH) and total bilirubin (tBil) as hepatocyte integrity parameters, serum tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), cyclooxygenase-II (COX-II) and Lipooxygenase (LOX), and hepatic myeloperoxidase (MPO) and nitric oxide end products (NOx) as inflammatory biomarkers, hepatic malondialdhyde (MDA), glutathione reduced (GSH), catalase (CAT), superoxide dismutase (SOD) and glutathione-S-transferase (GST) as oxidative stress biomarkers, and finally hepatic adenosine triphosphate (ATP) as energy store biomarker. To confirm results of biochemical estimations, a histopathological study was conducted. Results showed that Quercetin and UDCA significantly reduced hepatocyte injury evidenced by significant reductions in serum ALT, AST, ALP, LDH, tBil, TNF-α, IL-6, COX-II and LOX levels, significant reductions in hepatic MPO, NOx and MDA levels, and significant elevations in hepatic GSH, CAT, SOD, GST and ATP levels. Quercetin effect was significantly better than UDCA effect on most parameters. Histopathological findings strongly supported results of biochemical estimations. In conclusion, Quercetin and UDCA, with Quercetin being better, can protect against hepatic IR injury in rats, at least through anti-oxidant, anti-inflammatory and energypreserving effects, and may be promising for further clinical trials.
| Keywords |
| Quercetin; Ursodeoxycholic acid; Ischemia; Oxidative stress; Inflammation; Energy depletion |
| Introduction |
| Ischemia reperfusion (IR) injury is a phenomenon whereby cellular damage in a hypoxic organ is accentuated following the restoration of oxygen delivery [1]. Hepatic IR injury is common in hypovolemic shock, hepatic trauma, liver surgery, myocardial infarction and cerebrovascular disease [2]. Pathophysiology of hepatic IR injury is associated with several inter-related oxidative and inflammatory pathways, including activation of Kupffer cells, infiltration of neutrophil, production of reactive oxygen species (ROS), increased levels of adhesion molecules and release of cytokines, mostly interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α), ultimately leading to hepatocyte injury and separation of sinusoidal endothelial cells [3]. |
| Quercetin is the most common flavonoid present in plant kingdom, being abundant in capers, lovage, fennel, onion, blueberry, apples, tea and others [4]. Quercetin shows a powerful antioxidant, antiinflammatory and anti-fibrotic properties [5]. Antioxidant activity of Quercetin is partly due to its direct ability of radical scavenging. It was also reported to inhibit TNF-α production and gene expression in a dose-dependent manner [6]. |
| Ursodeoxycholic acid (UDCA) is the therapeutic agent most widely used for the treatment of cholestatic hepatopathies [7]. However, a number of recent clinical and experimental data has shown the beneficial effects of UDCA in various non-cholestatic liver injuries [8,9]. This could be mediated by displacement of toxic bile acids from the bile acid pool in addition to choleretic, immunomodulatory and cytoprotective properties [10]. |
| N-acetyl cysteine (NAC) is the most clinically effective source of glutathione (GSH) and sulfhydryl groups, and is a direct scavenger of free radicals due to its ability to interact with ROS. It has also been reported to prevent apoptosis and increase cell survival by activating the extracellular signal regulated kinase pathway [11]. |
| Based on these findings, the present investigation aims to evaluate the possible protective effects of Quercetin and UDCA, as compared to the standard NAC, on hepatic injury induced by ischemia followed by reperfusion in a rat model. The protective effects of test agents were to be evaluated by assessment of serum levels of alanine transaminase (ALT), aspartate transaminase (AST), alkaline phosphatase (ALP), lactate dehydrogenase (LDH) and total bilirubin (tBil) as hepatocyte integrity markers, serum levels of TNF-α, IL-6, cyclooxygenase-II (COX-II) and Lipooxygenase (LOX), and hepatic levels of myeloperoxidase (MPO) and nitric oxide end products (NOx) as inflammatory biomarkers, hepatic contents of malondialdhyde (MDA), glutathione reduced (GSH), catalase (CAT), superoxide dismutase (SOD) and glutathione- S-transferase (GST) as oxidative stress biomarkers, and finally hepatic content of adenosine triphosphate (ATP) as energy store biomarker. A histopathological study was also performed to confirm results of biochemical estimations. |
| Materials and Methods |
| Material |
| Animals: Adult male Wistar albino rats weighing 200-250 g were obtained from the animal house of Faculty of Medicine, Assuit University, Egypt. The animals were housed in a conditioned atmosphere at a temperature of 25 ± 1°C and kept free on standard diet and tap water ad libitum. Animal housing and handling were conducted in accordance with the recommendations of the National Institutes of Health (NIH) Guide for Care and Use of Laboratory Animals (Publication No. 86-23, revised 1985). |
| Drugs, chemicals and reagent kits: Quercetin was purchased from Sigma-Aldrich chemical company (St Louis, MO, USA). UDCA and NAC were obtained from SEDICO (6th October, Giza, Egypt). Chemicals such as Hexadecayl trimethyl ammonium bromide, Dimethoxy benzidine, Thiobarbituric acid, GSH, Pyrogallol, N-(1- Naphthyl) ethylenediamine dihydrochloride and 5,5`-dithio-bis-(2- nitrobenzoic acid; DTNB) were purchased from Sigma-Aldrich (St. Louis, MO, USA). All other chemicals were obtained from local sources and were of analytical grade. Kits of ALT, AST and LDH were obtained from Bio System Company (Barcelona, Spain). ALP and tBil kits were obtained from Bio Diagnostic Company (Cairo, Egypt). TNF-α, IL- 6, COX-II and LOX ELISA kits were purchased from Glory Science Company (Del Rio, USA). ATP ELISA kit was purchased from Wkea Med Supplies Company (China). |
| Experimental design |
| Thirty rats were divided into five groups, each of six rats. Allocation of animals in their groups was completely random, where rats were numbered from 1-30 and divided into groups 1-5 in group orders using random digit table in a two-digit manner. Doses of test agents were determined in pilot trials guided with published literature illustrated below. Animal groups were treated as follows: |
| Group I: Received vehicle alone, exposed to sham-operation and served as sham control. |
| Group II: Received vehicles alone, exposed to hepatic IR and served as IR control. |
| Group III: Received NAC in a dose of 150 mg/kg/day, p.o., once daily for seven consecutive days [12], followed by hepatic IR on seventh day and served as standard treatment group. |
| Group IV: Received Quercetin in a dose of 50 mg/kg/day, p.o., once daily for seven consecutive days [13], followed by hepatic IR on seventh day. |
| Group V: Received UDCA in a dose of 60 mg/kg/day, p.o. once daily for seven consecutive days [14], followed by hepatic IR on seventh day. |
| On seventh day, animals were sacrificed and blood and liver samples were collected as discussed in the Sampling section. |
| Methods |
| Induction of hepatic IR injury: Rats were anaesthetized by ketamine (100 mg/kg) and xylazine (10 mg/kg) by intraperitoneal (i.p.) injection. The abdominal region was shaved with a safety razor and sterilized with povidone iodine solution. The rats in the sham control group and IR group were given sterile CMC 0.5% (4 ml/kg) as a vehicle. The rats in the IR group were exposed to total hepatic ischemia by clamping the hepatic artery, the portal vein, and the common bile duct (portal triad) with a micro-vascular clamp for 30 minutes. After 30 minutes of complete hepatic ischemia, the clamp was removed and the reperfusion was initiated for 30 minutes [15]. |
| Sampling: Soon after reperfusion, blood samples were collected through cardiac puncture technique. Serum was separated following centrifugation at 3500 rpm for 15 minutes at 4°C using a cooling centrifuge (Beckman model L3-50, USA) and stored at -20°C. Rats were then euthanized by cervical dislocation and livers were immediately dissected out. A portion of the liver tissue (obtained from median lobe) was homogenized in ice-cooled phosphate buffer saline using a homogenizer (Cole-Parmer instrument Company, USA) to obtain 10% homogenate. Aliquots of the liver homogenate were stored at 0-4°C prior to biochemical analysis. Another portion of the liver was preserved in 10% formalin solution in saline for histopathological examination. |
| Assessment of biochemical parameters: Serum ALT and AST activities were assayed by the method of Reitman and Frankel [16]. ALP activity was determined according to the method of Belfield and Goldberg [17]. Serum LDH was determined as previously described [18]. Serum tBil was determined according to the method of Walters and Gerarde [19]. Serum TNF-α was measured according to the method described by Wolters et al. [20]. Serum IL-6 was assayed according to the method of Chan and Perlstein [21]. Serum COX-II and LOX levels, and hepatic ATP content were measured by using ELISA kits according to manufacturing instructions based on the principle described by Van Weemen and Schuurs [22]. MPO was determined according to Manktelow and Meyer [23]. NOx was determined according to the method described by Montgomery and Dymock [24]. Hepatic MDA content was assayed according to methods of Mihara and Uchiyama [25]. Hepatic GSH content was measured according to the method of Ellman [26]. Antioxidant enzymes CAT and SOD were assayed according to Claiborne [27] and Marklund [28], respectively. GST was determined according to method described by Keen et al. [29]. Histopathological examination was performed according to the method described by Bancroft and Steven [30]. |
| Statistical analysis: Data were presented as mean ± SEM. Statistical analysis of the data was carried out using one way analysis of variance (ANOVA) followed by Tukey-Karmer multiple comparisons test for post hoc analysis. Statistical significance was acceptable to a level of p < 0.05. Data analysis was accomplished using the Statistical Package for Social Sciences (SPSS) software program (version 20). |
| Results |
| Effect of test agents on hepatocyte injury biomarkers |
| Serum ALT, AST, ALP, LDH and tBil levels were significantly increased in rats exposed to hepatic IR injury. Pretreatment of rats with NAC, Quercetin or UDCA significantly reduced serum levels of ALT, AST, ALP, LDH and serum tBil. Pretreatment with Quercetin showed a significantly higher hepatoprotective effect, while pretreatment with UDCA showed a lower hepatoprotective effect, when compared to NAC pretreatment regarding ALT and AST levels. Both agents were better than NAC regarding serum ALP, while NAC was better regarding serum LDH. Only UDCA was better than NAC regarding serum tBil (Table 1). |
| Effect of test agents on inflammatory biomarkers |
| Rats exposed to hepatic IR injury showed significantly higher serum TNF-α, IL-6, COX-II and LOX levels as well as tissue MPO and NOx levels compared to sham control rats. Alternatively, pretreatment with NAC, Quercetin or UDCA significantly corrected all these inflammatory markers by varying degrees. Quercetin was significantly better than NAC regarding TNF-α, COX-II, LOX and NOx levels. Alternatively, UDCA was significantly better than NAC concerning NOx, while NAC was better concerning MPO (Table 2). |
| Effect of test agents on oxidative stress biomarkers |
| Hepatic IR injury was associated with significant elevation of hepatic MDA production, coupled with significant reductions of hepatic GSH, CAT, SOD and GST contents. Pretreatment of rats with NAC, Quercetin or UDCA significantly corrected all markers of oxidative stress compared to IR control rats. In comparison to NAC pretreated group, Quercetin and UDCA caused more significant reduction in hepatic MDA which suggested that Quercetin and UDCA are stronger antioxidants than NAC. Regarding hepatic SOD and GST levels, Quercetin was better than NAC, while NAC was better than UDCA (Table 3). |
| Effect of test agents on energy store biomarkers |
| Significant depletion of ATP stores in IR rats compared to sham control rats was observed. Pretreatment with NAC, Quercetin or UDCA significantly restored hepatic ATP levels when compared to rats exposed to hepatic IR injury alone. Compared to NAC, Quercetin was significantly better than NAC regarding ATP restoration, while NAC was better than UDCA in this regard (Table 3). |
| Histopathological study |
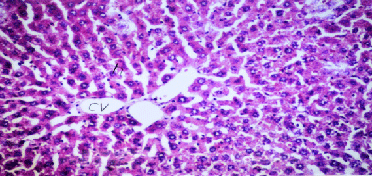
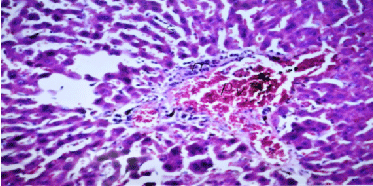
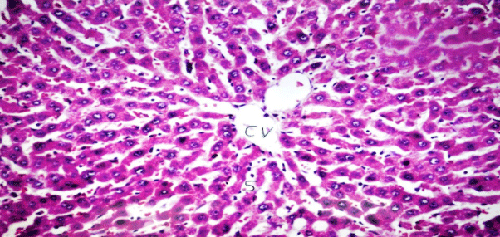
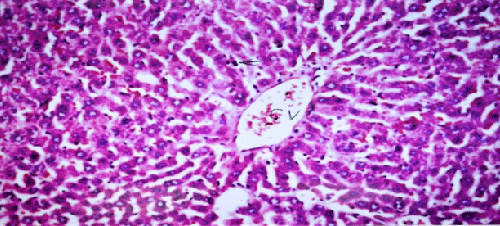
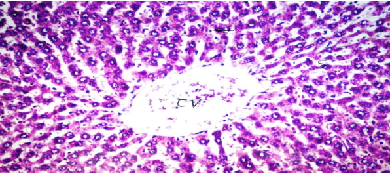
| Results of histopathological study showed loss of normal hepatic architecture with massive inflammatory infiltration in IR sections, coupled with restoration of hepatic architecture with reduction of inflammatory infiltration in all treatment groups (Figures 1-5). |
| Discussion |
| Hepatic IR is an important problem after liver transplantation and surgical resection. Since it is inevitable in most cases [31], it needs proper prophylactic intervention. We focused our analysis on the early phase of IR-induced liver damage, which is characterized by ROS formation following re-oxygenation, associated with the activation of pro-inflammatory mediators such as cytokines coupled with infiltration of inflammatory cells such as liver Kupffer cells, which are able to produce even more ROS [32,33]. |
| Serum ALT, AST, ALP, LDH and tBil are the most sensitive biochemical markers employed in the diagnosis of hepatic dysfunction [34]. Cytokines like TNF-α and IL-6 are released in inflammatory conditions [35,36], while COX-II and LOX enzymes participate strongly in progression of inflammation [37,38]. MPO is an indicator of inflammation as it reflects degree of inflammatory infiltration [39]. NOx is released from endothelium and other cells in inflammed tissues [40]. MDA is an end product of lipid peroxidation and reflects degree of oxidative stress [41]. Anti-oxidant peptides like GSH, and enzymes like CAT, SOD and GST, are depleted or suppressed in oxidative stress conditions [42]. Energy is stored in cells in the form of ATP, and hence ATP content in tissues reflects energy store [43,44]. That is, assessment of the aforementioned parameters estimates effects of test agents on hepatocyte injury, inflammation, oxidative stress and energy depletion. |
| The present investigation aims to elucidate the possible protective effects of two agents with promising backgrounds, namely Quercein and UDCA, on liver injury induced experimentally in rats by ischemia followed by reperfusion. |
| In our model, rats subjected to hepatic IR showed marked hepatocellular injury evidenced by serum ALT, AST, ALP, LDH and tBil elevations compared to sham control rats (Table 1), supported by histopathological lesions in liver sections obtained from IR control rats (Figures 1 and 2). These findings come in agreement with the results found in previous studies of hepatic IR injury in rats [45,46]. On the other hand, significant decreases of ALT, AST, ALP, LDH and tBil compared to IR control rats were observed (Table 1), and again confirmed by results of histopathological examination (Figures 3-5). These results suggest that Quercetin and UDCA are able to protect the membrane integrity and hepatocyte functionality against hepatic IR that induces leakage of marker enzymes into the circulation. In agreement, Quercetin and UDCA were reported to protect hepatocytes against noxious insults in different experimental models of liver injury, like chemical injury with polychlorinated biphenyls or with amoxicillin/ clavulanic acid combination [47,48]. |
| Results of our work showed a strong link between hepatic IR injury and inflammation, evidenced by significant elevations of all measured serum and tissue inflammatory markers, namely serum TNF-α, IL-6, COX-II and LOX, and tissue MPO and NOx, coupled with apparent histopathological inflammatory infiltration. In agreement, inflammatory cytokines in general, mostly TNF-α and IL-6, were reported to be up-regulated in IR injury and can initiate additional cellular inflammatory responses, affecting liver cell survival, ultimately causing organ injury [35,36]. In parallel, other important inflammatory enzymes like COX-II and LOX were reported to play an important role in initiation and activation of inflammatory cells such as Kupffer cells and neutrophils that initiate inflammatory and oxidative injury during hepatic IR [37,38]. Additionally, MPO level in tissues is known to be a direct marker of inflammatory infiltration as this enzyme is present in high amounts in inflammatory cells like neutrophils and macrophages [39]. Additionally, during ischemia, there is increased inducible nitric oxide synthase (iNOS) expression, yielding huge amounts of nitric oxide (NO) and its reaction products like peroxynitrite, which have been shown to be involved in oxidative and nitrosative stress that accompany inflammatory hepatic injury [40]. |
| According to our results, pretreatment with Quercetin or UDCA significantly corrected all measured inflammatory biomarkers, and corrected histopathological inflammatory infiltration by varying degrees (Table 2, Figures 2-5). In agreement, anti-inflammatory potentials of Quercetin and UDCA were reported in different animal models. Quercetin was reported to have anti-inflammatory effects in rats with non-alcoholic fatty liver or cholestatic injury [49,50]. Additionally, UDCA was shown to have anti-inflammatory potential on chemically-induced liver injury [48] and inflammatory bowel disease both in vivo and in vitro [51]. In disagreement, Quercetin was reported to downregulate COX-II transcription in human lymphocytes ex vivo but not in vivo [52]. |
| Our results showed also association of hepatic IR injury with oxidative stress, evidenced by elevated hepatic MDA level, coupled with suppressed hepatic GSH, CAT, SOD and GST levels (Table 3). In agreement, hepatic IR injury in experimental rats was associated with oxidative stress [53]. Inflammatory infiltration and release of inflammatory mediators was reported to be coupled with oxidative stress. Activation of kupffer cells and neutrophils causes endothelial and hepatocellular damage through release of ROS and proteases, which exacerbates the structural damage and functional impairment of the liver [1,54]. MDA is an important indicator of oxidative injury [41]. Additionally, levels of GSH and anti-oxidant enzymes like CAT, SOD and GST were reported to be suppressed in conditions of oxidative stress caused by hepatic IR injury in rats [42]. |
| In the present investigation, oxidative stress caused by hepatic IR injury was significantly corrected by Quercetin or UDCA pretreatments, evidenced by significant corrections of tissue MDA, GSH, CAT, SOD and GST levels (Table 3). In agreement, Quercetin was reported to alleviate oxidative stress in a rat model of hepatic IR injury [41]. Moreover, UDCA was recently reported to decrease oxidative stress in a rat model of bile duct obstruction [55]. |
| Concerning hepatocyte energy stores, hepatic ATP level was significantly depleted in IR rats (Table 3). In agreement, hepatic ATP depletion was reported to associate hepatic IR injury [56]. Mitochondrial damage has been recognized as an important component of IR-induced tissue injury. Deprivation of O2 and nutrient delivery during liver ischemia depletes cellular ATP and energy stores, leading to further injury. Meanwhile, ATP is consumed by ATPase pumps trying to correct ion overload [43,44]. |
| Quercetin and UDCA pretreatments significantly replenished the depleted ATP stores in IR rats in the present investigation (Table 3). In agreement, Quercetin was reported to protect mitochondrial membranes and improve ATP stores in isolated pancreatic cells through inhibition of cholecystokinin mitochondrial dysfunction [57]. In disagreement, UDCA was reported to cause ATP depletion in HepG2 cells in vitro [58]. Preservative effects of Quercetin and UDCA on hepatic ATP stores in the present study may be secondary to antioxidant and anti-inflammatory effects. |
| Conclusion |
| Quercetin and UDCA have protective effects on hepatic IR injury in experimental rats, mostly due to anti-inflammatory, anti-oxidant and energy-preserving potentials. Quercetin is a more powerful antioxidant, anti-inflammatory and energy-preserving agent compared to NAC, whereas UDCA has more or less the same power of NAC. These results are promising for further clinical trials on Quercetin and UDCA on clinical cases of inevitable hepatic IR injury in man. |
| Acknowledgements |
| Many thanksgivings should be directed to Dr. Amal Khorshid, Assistant Professor of Analytical Chemistry, Faculty of Pharmacy, Cairo University, for her continuous help and support, specially directing us to the suitable journal for publication. |
| References |
References
- Teoh NC, Farrell GC (2003) Hepatic ischemia reperfusion injury: pathogenic mechanisms and basis for hepatoprotection. J Gastroenterol Hepatol 18: 891-902.
- Liu CM, Sun YZ, Sun JM, Ma JQ, Cheng C (2012) Protective role of Quercetin against lead-induced inflammatory response in rat kidney through the ROS-mediated MAPKs and NF-κB pathway. Biochim Biophys Acta 1820: 1693-1703.
- Sahin T, Begeç Z, Toprak HI, Polat A, Vardi N, et al. (2013) The effects of dexmedetomidine on liver ischemia-reperfusion injury in rats. J Surg Res 183: 385-390.
- Tang Y, Tian H, Shi Y, Gao C, Xing M, et al. (2013) Quercetin suppressed CYP2E1-dependent ethanol hepatotoxicity via depleting heme pool and releasing CO. Phytomedicine 20: 699-704.
- Uzun FG, Kalender Y (2013) Chlorpyrifos induced hepatotoxic and hematologic changes in rats: the role of quercetin and catechin. Food Chem Toxicol 55: 549-556.
- Hirpara KV, Aggarwal P, Mukherjee AJ, Joshi N, Burman AC (2009) Quercetin and its derivatives: synthesis, pharmacological uses with special emphasis on anti-tumor properties and prodrug with enhanced bio-availability. Anticancer Agents Med Chem 9: 138-161.
- Feng M, Wang Q, Wang H, Guan W (2013) Tumor necrosis factor-alpha preconditioning attenuates liver ischemia/reperfusion injury through preserving sarco/endoplasmic reticulum calcium-ATPase function. J Surg Res 184: 1109-1113.
- Mohammed Saif M, Farid SF, Khaleel SA, Sabry NA, El-Sayed MH (2012) Hepatoprotective efficacy of ursodeoxycholic acid in pediatrics acute lymphoblastic leukemia. Pediatr Hematol Oncol 29: 627-632.
- Pirinççioglu M, Kizil G, Kizil M, Kanay Z, Ketani A (2014) The protective role of pomegranate juice against carbon tetrachloride-induced oxidative stress in rats. Toxicol Ind Health 30: 910-918.
- Alhumaidha KA, El-Awdan SA, El-Iraky WI, El-Denshary ES (2014) Protective effects of ursodeoxycholic acid on ceftriaxone-induced hepatic injury in rats. Bull Fac Pharm 52: 45-50.
- Zafarullah M, Li WQ, Sylvester J, Ahmad M (2003) Molecular mechanisms of N-acetylcysteine actions. Cell Mol Life Sci 60: 6-20.
- Demiralay R, Gürsan N, Ozbilim G, Erdogan G, Demirci E (2006) Comparison of the effects of erdosteine and N-acetylcysteine on apoptosis regulation in endotoxin-induced acute lung injury. J Appl Toxicol 26: 301-308.
- Heeba GH, Mahmoud ME (2014) Dual effects of quercetin in doxorubicin-induced nephrotoxicity in rats and its modulation of the cytotoxic activity of doxorubicin on human carcinoma cells. Environ Toxicol.
- Buko VU, Kuzmitskaya-Nikolaeva IA, Naruta EE, Lukivskaya OY, Kirko SN, et al. (2011) Ursodeoxycholic acid dose-dependently improves liver injury in rats fed a methionine- and choline-deficient diet. Hepatol Res 41: 647-659.
- Tokyol C, Yilmaz S, Kahraman A, Cakar H, Polat C (2006) The effects of desferrioxamine and quercetin on liver injury induced by hepatic ischaemia-reperfusion in rats. Acta Chir Belg 106: 68-72.
- Reitman S, Frankel S (1957) A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol 28: 56-63.
- Belfield A, Goldberg DM (1971) Normal ranges and diagnostic value of serum 5'nucleotidase and alkaline phosphatase activities in infancy. Arch Dis Child 46: 842-846.
- [No authors listed] (1970) Recommendations of the German Society for Clinical Chemistry. Standardization of methods for the determination of enzyme activities in biological fluids. Z Klin Chem Klin Biochem 8: 658-660.
- Walters MI, Gerarde HW (1970) An ultramicromethod for the determination of conjugated and total bilirubin in serum or plasma. Microchem J 15: 231-243.
- Wolters G, Kuijpers LP, Kaçaki J, Schuurs AH (1977) Enzyme-linked immunosorbent assay for hepatitis B surface antigen. J Infect Dis 136: S311-317.
- Chan DW, Perlstein MT (2012) Immunoassay: A practical guide. Academic Press, San Diego, USA.
- Van Weemen BK, Schuurs AH (1971) Immunoassay using antigen-enzyme conjugates. FEBS Lett 15: 232-236.
- Manktelow A, Meyer AA (1986) Lack of correlation between decreased chemotaxis and susceptibility to infection in burned rats. J Trauma 26: 143-148.
- Montgomery HAC, Dymock DF (1961) The determination of nitrite in water. Analyst 86: 414-416.
- Mihara M, Uchiyama M (1978) Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem 86: 271-278.
- Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82: 70-77.
- Claiborne A (1985) Catalase activity. In: Greenwald RA (Ed) CRC handbook of methods for oxygen radical research. CRC Press Inc, Boca Raton, Florida, USA 283-284.
- Marklund SL (1985) Superoxide dismutase isoenzymes in tissues and plasma from New Zealand black mice, nude mice and normal BALB/c mice. Mutat Res 148: 129-134.
- Keen JH, Habig WH, Jakoby WB (1976) Mechanism for the several activities of the glutathione S-transferases. J Biol Chem 251: 6183-6188.
- Bancroft GD, Steven A (1983) Theory and practice of histological technique. Churchil Livingstone Publications, London, UK.
- Kapoor S (2011) Hepatic ischaemia-reperfusion injury from bench to bedside (Br J Surg 2010; 97: 1461-1475). Br J Surg 98: 459.
- Walsh KB, Toledo AH, Rivera-Chavez FA, Lopez-Neblina F, Toledo-Pereyra LH (2009) Inflammatory mediators of liver ischemia-reperfusion injury. Exp Clin Transplant 7: 78-93.
- Rauen U, Viebahn R, Lauchart W, de Groot H (1994) The potential role of reactive oxygen species in liver ischemia/reperfusion injury following liver surgery. Hepatogastroenterology 41: 333-336.
- Amacher DE, Schomaker SJ, Aubrecht J (2013) Development of blood biomarkers for drug-induced liver injury: an evaluation of their potential for risk assessment and diagnostics. Mol Diagn Ther 17: 343-354.
- Marshall KM, He S, Zhong Z, Atkinson C, Tomlinson S3 (2014) Dissecting the complement pathway in hepatic injury and regeneration with a novel protective strategy. J Exp Med 211: 1793-1805.
- Lin CM, Lee JF, Chiang LL, Chen CF, Wang D, et al. (2012) The protective effect of curcumin on ischemia-reperfusion-induced liver injury. Transplant Proc 44: 974-977.
- Tolba RH, Fet N, Yonezawa K, Taura K, Nakajima A, et al. (2014) Role of preferential cyclooxygenase-2 inhibition by meloxicam in ischemia/reperfusion injury of the rat liver. Eur Surg Res 53: 11-24.
- Connor AJ, Chen LC, Joseph LB, Laskin JD, Laskin DL (2013) Distinct responses of lung and liver macrophages to acute endotoxemia: role of toll-like receptor 4. Exp Mol Pathol 94: 216-227.
- Qiao YL, Qian JM, Wang FR, Ma ZY, Wang QW (2014) Butyrate protects liver against ischemia reperfusion injury by inhibiting nuclear factor kappa B activation in Kupffer cells. J Surg Res 187: 653-659.
- Brenner C, Galluzzi L, Kepp O, Kroemer G (2013) Decoding cell death signals in liver inflammation. J Hepatol 59: 583-594.
- Su JF, Guo CJ, Wei JY, Yang JJ, Jiang YG, et al. (2003) Protection against hepatic ischemia-reperfusion injury in rats by oral pretreatment with quercetin. Biomed Environ Sci 16: 1-8.
- Tao X, Wan X, Xu Y, Xu L, Qi Y, et al. (2014) Dioscin attenuates hepatic ischemia-reperfusion injury in rats through inhibition of oxidative-nitrative stress, inflammation and apoptosis. Transplantation 98: 604-611.
- Nogueira MA, Coelho AM, Sampietre SN, Patzina RA, Pinheiro da Silva F, et al. (2014) Beneficial effects of adenosine triphosphate-sensitive K+ channel opener on liver ischemia/reperfusion injury. World J Gastroenterol 20: 15319-15326.
- Duenschede F, Erbes K, Kircher A, Westermann S, Schad A, et al. (2007) Protection from hepatic ischemia/reperfusion injury and improvement of liver regeneration by alpha-lipoic acid. Shock 27: 644-651.
- Savvanis S, Nastos C, Tasoulis MK, Papoutsidakis N, Demonakou M, et al. (2014) Sildenafil attenuates hepatocellular injury after liver ischemia reperfusion in rats: a preliminary study. Oxid Med Cell Longev 2014: 161942.
- Tanrikulu Y, Sahin M, Kismet K, Kilicoglu SS, Devrim E, et al. (2013) The protective effect of diosmin on hepatic ischemia reperfusion injury: an experimental study. Bosn J Basic Med Sci 13: 218-224.
- Rocha de Oliveira C, Ceolin J, Rocha de Oliveira R, Gonçalves Schemitt E, Raskopf Colares J, et al. (2014) Effects of quercetin on polychlorinated biphenyls-induced liver injury in rats. Nutr Hosp 29: 1141-1148.
- El-Sherbiny GA, Taye A, Abdel-Raheem IT (2009) Role of ursodeoxycholic acid in prevention of hepatotoxicity caused by amoxicillin-clavulanic acid in rats. Ann Hepatol 8: 134-140.
- Lin SY, Wang YY, Chen WY, Chuang YH, Pan PH, et al. (2014) Beneficial effect of quercetin on cholestatic liver injury. J Nutr Biochem 25: 1183-1195.
- Zhou DS, Liang ZQ, Qin Q, Zhang MH, Li SL (2013) Therapeutic efficacy and mechanisms of quercetin in a rat model of nonalcoholic fatty liver disease. Zhonghua Gan Zang Bing Za Zhi 21: 134-137.
- De AK, Sana S, Datta S, Mukherjee A (2014) Protective efficacy of ursodeoxycholic acid nanoparticles in animal model of inflammatory bowel disease. J Microencapsul 31: 725-737.
- de Pascual-Teresa S, Johnston KL, DuPont MS, O'Leary KA, Needs PW, et al. (2004) Quercetin metabolites downregulate cyclooxygenase-2 transcription in human lymphocytes ex vivo but not in vivo. J Nutr 134: 552-557.
- Adam AN (2014) Some mechanisms of the protective effect of ischemic preconditioning on rat liver ischemia-reperfusion injury. Int J Gen Med 7: 483-489.
- Mouzaoui S, Djerdjouri B, Makhezer N, Kroviarski Y, El-Benna J, et al. (2014) Tumor necrosis factor-a-induced colitis increases NADPH oxidase 1 expression, oxidative stress, and neutrophil recruitment in the colon: preventive effect of apocynin. Mediators Inflamm.
- Ale-Ebrahim M, Eidi A, Mortazavi P, Tavangar SM, Tehrani DM (2015) Hepatoprotective and antifibrotic effects of sodium molybdate in a rat model of bile duct ligation. J Trace Elem Med Biol 29: 242-248.
- Ibrahim MA, Abdel-Gaber SA, Amin EF, Ibrahim SA, Mohammed RK, et al. (2014) Molecular mechanisms contributing to the protective effect of levosimendan in liver ischemia-reperfusion injury. Eur J Pharmacol 741: 64-73.
- Weber H, Jonas L, Wakileh M, Krüger B (2014) Beneficial effect of the bioflavonoid quercetin on cholecystokinin-induced mitochondrial dysfunction in isolated rat pancreatic acinar cells. Can J Physiol Pharmacol 92: 215-225.
- Fickert P, Pollheimer MJ, Silbert D, Moustafa T, Halilbasic E, et al. (2013) Differential effects of norUDCA and UDCA in obstructive cholestasis in mice. J Hepatol 58: 1201-1208.
Tables and Figures at a glance
| Table 1 | Table 2 | Table 3 |
Figures at a glance
 |
 |
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 | Figure 5 |
Relevant Topics
- Applied Biopharmaceutics
- Biomarker Discovery
- Biopharmaceuticals Manufacturing and Industry
- Biopharmaceuticals Process Validation
- Biopharmaceutics and Drug Disposition
- Clinical Drug Trials
- Clinical Pharmacists
- Clinical Pharmacology
- Clinical Research Studies
- Clinical Trials Databases
- DMPK (Drug Metabolism and Pharmacokinetics)
- Medical Trails/ Drug Medical Trails
- Methods in Clinical Pharmacology
- Pharmacoeconomics
- Pharmacogenomics
- Pharmacokinetic-Pharmacodynamic (PK-PD) Modeling
- Precision Medicine
- Preclinical safety evaluation of biopharmaceuticals
- Psychopharmacology
Recommended Journals
Article Tools
Article Usage
- Total views: 14249
- [From(publication date):
February-2015 - Jan 31, 2025] - Breakdown by view type
- HTML page views : 9754
- PDF downloads : 4495
