Research Article Open Access
Protease, Amylase and Lactase Enzyme Stability in Gastroval® Capsules after Incubation at Acidic pH and Elevated Temperature
Edvinas Paliulis, Vaida Paketuryte and Daumantas Matulis*Department of Biothermodynamics and Drug Design, Institute of Biotechnology, Life Sciences Center, Vilnius University, Saulėtekio, Vilnius, Lithuania
- Corresponding Author:
- Daumantas Matulis
Department of Biothermodynamics and Drug Design
Institute of Biotechnology
Life Sciences Center, Saulėtekio 7, Vilnius, Lithuania
Tel: 0037052234364
E-mail: daumantas.matulis@bti.vu.lt, matulis@ibt.lt
Received date: November 11, 2016; Accepted date: November 14, 2016; Published date: December 19, 2016
Citation: Paliulis E, Paketuryte V, Matulis D (2016) Protease, Amylase and Lactase Enzyme Stability in Gastroval® Capsules after Incubation at Acidic pH and Elevated Temperature. Biochem Physiol 5:211. doi:10.4172/2168-9652.1000211
Copyright: © 2016, Paliulis E, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Biochemistry & Physiology: Open Access
Abstract
The stability of plant-derived enzymes present in digestion-alleviating food supplement capsules Gastroval® was studied by determining the survival of enzymatic activity of proteases, α-amylases, and lactases (β-galactosidase) after an exposure to acidic pH and elevated temperature. Most of the enzymatic activity after the prolonged (2 h) exposure to acidic conditions (pH 2.0) and elevated temperature (50°C) remained unchanged or slightly reduced, thus indicating that the enzymes would pass the stomach without losing their entire enzymatic activity and efficiently alleviate food digestion in the intestine.
Keywords
Protease; Amylase; Lactase; β-galactosidase; Gastroval®; Enzyme activity
Introduction
Enzymes are important in the digestion of foods for efficient nutrient absorption in the small intestine. If the digestive system is unable to function properly, various symptoms can surface from simple occasional stomach ache, acid indigestion or flatulence to more serious chronic conditions such as food allergies or digestive disease. The regular use of digestive enzyme supplements, coupled with digestive health strategies, can alleviate major symptoms, improve energy levels, help maintain proper weight and allow individuals to consume a wider variety of foods. Bioactive proteins and peptides may be widely used in food [1]. Supplemental enzymes may be vitally important for people with diseases such as cystic fibrosis [2,3]. However, dietary supplements may also be hepatotoxic and it is important to investigate their possible toxicity [4-6].
Supplemental enzymes are usually well tolerated and non-toxic, but they sometimes may not survive the complicated environment of human digestive tract. After the food is swallowed it enters the upper portion of the stomach which has a pH of 4.0-6.5. This is where "predigestion" occurs while the lower portion of the stomach is secreting hydrochloric acid (HCl) and pepsin until it reaches a pH of 1.5-4.0. After the food mixes with these juices it then enters the duodenum (small intestine) where the pH changes to 7.0-8.5. This is where 90% of the nutrient absorption takes place in the body while the waste products are passed out through the colon (pH 4.0-7.0).
There is significant correlation between protein biophysical stability and their enzymatic activity. However, enzymes tend to be not too stable since enzymatic activity may be compromised if they are conformationally too robust [7]. Engineering of proteins with improved stability patterns is an important subject in biotechnology.
The main objective of the present study was to determine whether the enzymatic activity of proteases [8-11], α-amylases [12-19] and lactases (β-galactosidase) [20,21], the main constituents of plantderived Gastroval® Capsules used as a source of supplemental digestion enzymes, remains after a prolonged incubation at reduced pH or elevated temperature. The activity was measured at standard pH and temperature, after the exposure to acidic stress pH and elevated temperature thus mimicking the travel of a swallowed tablet through the human digestive tract.
Materials and Methods
Gastroval® Capsules were obtained from a local pharmacy (Vilnius, Lithuania) and used without further treatment. One tablet contains approximately 75 mg of enzymes derived from the fungus Aspergillus, 25 mg of yellow gentian (Gentiana lutea) root powder, 25 mg of common dandelion (Taraxacum officinale) root powder and 25 mg of milk thistle (Silybum marianum) seed dry extract. Tablet material (250 mg) was carefully suspended in 5 ml of distilled water by mixing for 30 min to allow soluble material to fully dissolve. Insoluble debris was removed by centrifugation at low speed. Soluble fraction was used as a stock solution of enzymes.
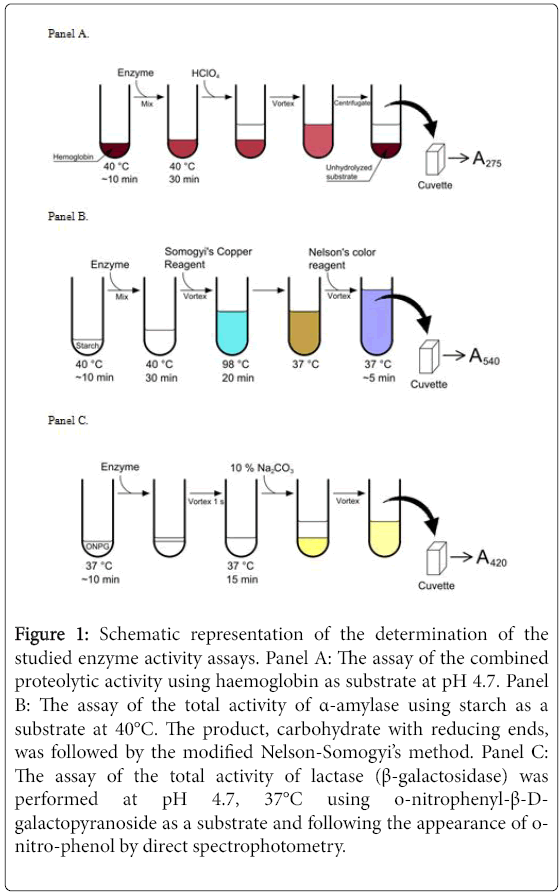
Enzymatic activity of protease, α-amylase and lactase (β- galactosidase) was determined according to the standard procedures of United States Pharmacopoeia. The schematic representation of the determination of the enzymatic activity is shown in Figure 1. The tablet is expected to contain numerous proteases, amylases and lactases that are likely to possess different enzymatic activity and different stability in acid and at elevated temperatures. The enzymatic assays determined the total enzymatic activity of each group of enzymes.
Figure 1: Schematic representation of the determination of the studied enzyme activity assays. Panel A: The assay of the combined proteolytic activity using haemoglobin as substrate at pH 4.7. Panel B: The assay of the total activity of α-amylase using starch as a substrate at 40°C. The product, carbohydrate with reducing ends, was followed by the modified Nelson-Somogyi’s method. Panel C: The assay of the total activity of lactase (β-galactosidase) was performed at pH 4.7, 37°C using o-nitrophenyl-β-Dgalactopyranoside as a substrate and following the appearance of onitro-phenol by direct spectrophotometry.
Determination of protease enzymatic activity
The proteolytic activity determination was based on the enzymatic hydrolysis of haemoglobin substrate at pH 4.7 and 40°C. Haemoglobin substrate solution contained 20 mg/ml haemoglobin, 12 mM sodium acetate, 12 mM acetic acid, 12 mM NaCl, pH 4.7 and was stored up to 1 week at 4°C. For the assay, the reaction was started by adding 0.1 ml of investigated enzyme solution to 0.7 ml haemoglobin substrate solution, preheated to 40°C in temperature-controlled water bath. Reaction was stopped by adding 0.7 ml of 1 M HClO4 at exact time points (0, 10, 20 and primarily 30 min) that precipitated the unhydrolyzed substrate which was removed by centrifugation for 2 min at 6000 rpm in an ependorffs centrifuge used for 1.5 ml ependorffs tubes.
The quantity of dissolved amino acids and peptides, the products of haemoglobin proteolysis, was determined spectrophotometrically at 275 nm. One unit of proteolytic activity is defined as such amount of protease that produces hydrolyte where A275=0.0084 (equivalent to 1.1 μg of tyrosine (Tyr) in 0.006 M HCl. Tyrosine extinction coefficient in acid is ∆©275=1431 M-1 cm-1).
Determination of α-amylase enzymatic activity
The activity of α-amylase was determined using the modified Nelson-Somogyi’s procedure that follows the appearance of reducing carbohydrates upon hydrolysis of carbohydrate polymers. Amylase activity represents an increase of reducing sugar ends upon dextrinizing enzyme action on starch at pH 5.0 and 40°C where α- amylase hydrolyses the 1-4 bonds but does not affect the 1-6 bonds. Determination of reducing sugars was based on the absorbance at 540 nm of a colored complex between a copper oxidized sugar and arsenomolybdate.
Preparation of reagents
Nelson’s Color Reagent was prepared by dissolving 5 g of ammonium molybdate tetrahydrate in water and adding 4 ml of concentrated sulphuric acid. Further, 0.6 g of sodium arsenate heptahydrate dissolved in 5 ml of water was added to the ammonium molybdate solution and diluted to 100 ml with water. Somogyi’s Copper Reagent contained the components A and B in the ratio of 25:1 and was prepared fresh daily. Component A consisted of 5 g of anhydrous sodium carbonate, 5 g potassium sodium tartrate tetrahydrate, 4 g anhydrous sodium sulphate and 4 g sodium bicarbonate diluted to 200 ml with water. Component B was prepared by dissolving 7.5 g copper (II) sulphate pentahydrate in 50 ml water and adding one drop of concentrated sulphuric acid.
Glucose was used as a standard carbohydrate for the calibration curve. Starch substrate was prepared by dispersing 1.0 g of starch in 50 ml of water, and slowly boiled the water until the starch gelatinized. 200 μl of 0.1 mg/ml enzyme solution and 200 μl of substrate solution (or 400 μl of glucose solution at various dilutions for the calibration curve) were added to a series of 1.5 ml ependorffs tubes and incubated for 0, 10, 20 and primarily 30 min at 40°C. To stop the enzymatic reaction 400 μl of Somogyi‘s Copper Reagent was added to each tube, vortexed and incubated at 98°C for 20 min in a thermostat. Then the tubes were cooled in a thermostat to room temperature and 400 μl of Nelson’s color reagent was added with brief vortex and placed at 37°C for 5 min. The absorbance was read at 540 nm with a spectrophotometer. One dextrinizing unit of α-amylase is defined as the quantity of α-amylase that will dextrinize soluble starch in the presence of an excess of β-amylase at the rate of 1 g/h at 40°C.
Determination of lactase (β-galactosidase) enzymatic activity
The activity determination of lactase was based on a 15 min hydrolysis of an o-nitrophenyl-β-D-galactopyranoside substrate at pH 4.5 and 37°C to release o-nitrophenol that is detected by measuring the absorbance at 420 nm using a spectrophotometer. Substrate (onitrophenyl- β-D-galactopyranoside) was prepared by transferring 37.0 mg of o-nitrophenyl-β-D-galactopyranoside to 10 ml of 0.1 M sodium acetate buffer, pH 4.5. The solution is usable for 2 h after making. For the enzymatic assay, 100 μl of 0.10 mg/ml enzyme solution (or water for the calibration curve) was added to a series of Ependorffs tubes. Then 400 μl of substrate solution was pipetted to each tube, mixed briefly and incubated in a thermostat at 37°C for 15 min. Then 500 μl of 10% sodium carbonate (Na2CO3) solution was added to each tube to stop the reaction. The samples were then diluted 5 times by adding distilled water and the absorbance was determined at 420 nm using a spectrophotometer. One lactase unit (ALU) is defined as the quantity of enzyme that will liberate o-nitrophenol at a rate of 1 μmol/min under the conditions of the assay.
Determination of enzyme stability in acid
For the pH stability experiments, the soluble fractions of the Gastroval® Capsules (at the dilution of 1.0 mg/ml for determination of protease enzymatic activity, 0.1 mg/ml for α-amylase and 0.1 mg/ml for lactase) were placed at pH 2.0, pH 4.0 and pH 7.0 and incubated for 0, 10, 20, 30, 40, 60 and 120 min at room temperature (20°C). Reduced pH was achieved by: pH 2.0–20 mM H3PO4, pH 4.0–10 mM acetic acid, pH 7.0–0.1 M Na2HPO4 and Na2HPO4 mixed in equal amounts. After incubation, the solutions were neutralized and the remaining activity was determined as described above for each enzyme group, protease, amylase and lactase.
Determination of enzyme stability at elevated temperature
For the temperature stability experiments, the soluble fraction from Gastroval® Capsules (at the dilution of 1.0 mg/ml for the determination of protease enzymatic activity, 0.1 mg/ml for α-amylase and 0.1 mg/ml for lactase) were placed at -20, +4, +20, +50 and +70°C and incubated for 0, 10, 20, 30, 40, 60 and 120 min. The tablet soluble fraction was obtained by dissolving in water and thus the pH of the solution was not controlled during the incubation at various temperatures. After the incubation, the remaining enzymatic activity was determined for each protein at a constant pH as described above.
For the statistical reliability of the results, all enzymatic activity and stability experiments were repeated at least twice, while the key data points repeated three to five times. Graphs present average values and the standard error did not exceed 15%.
Results and Discussion
To determine whether plant-derived human digestion-alleviating enzymes could survive and bypass the stomach and help food digestion, the stomach environment was mimicked by the enzyme incubation at acidic pH 2.0 and body temperature. In reality, human stomach may exhibit slightly more acidic and alkaline environment and contain numerous other components such as human enzymes, salts and other materials that may affect plant-derived enzyme stability. However, the incubation conditions are good approximation of the human stomach. Thus the plant-derived enzyme solution was exposed to acidic and neutral pH conditions, then neutralized and the remaining enzyme activity measured for proteolytic, amylolytic, and lactase total activity according to the procedure described in the materials and methods section and schematically shown in Figure 1. In addition, the enzyme resistance to unfolding and inactivation by temperature was determined by following the remaining activity after exposure to elevated and reduced temperature for various time periods.
Proteases
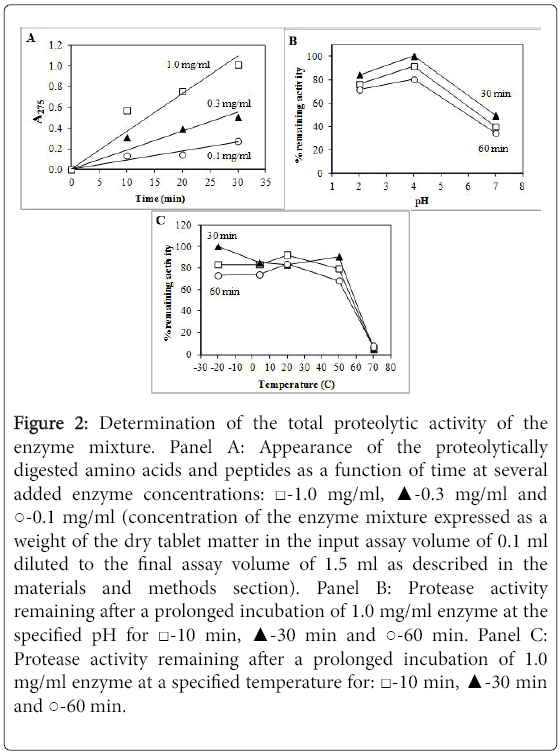
The proteolytic activity of the enzyme mixture causes the appearance of digested fragments, amino acids and peptides, derived from haemoglobin. Protease activity as a function of time at three different enzyme concentrations is shown in Figure 2. The appearance of digestion fragments is approximately linear with some saturation at higher enzyme concentrations (Figure 2A). It was important to determine the linear range of the enzyme concentration in order to calculate the total activity of the enzyme in the preparation.
Figure 2: Determination of the total proteolytic activity of the enzyme mixture. Panel A: Appearance of the proteolytically digested amino acids and peptides as a function of time at several added enzyme concentrations: ‚?°-1.0 mg/ml, ‚?≤-0.3 mg/ml and ‚??-0.1 mg/ml (concentration of the enzyme mixture expressed as a weight of the dry tablet matter in the input assay volume of 0.1 ml diluted to the final assay volume of 1.5 ml as described in the materials and methods section). Panel B: Protease activity remaining after a prolonged incubation of 1.0 mg/ml enzyme at the specified pH for ‚?°-10 min, ‚?≤-30 min and ‚??-60 min. Panel C: Protease activity remaining after a prolonged incubation of 1.0 mg/ml enzyme at a specified temperature for: ‚?°-10 min, ‚?≤-30 min and ‚??-60 min.
The protease activity was reduced by about 10-20% after 60 min incubation at pH 4.0. There was 20-30% loss of activity after 60 min incubation at pH 2.0. The same result was observed after 120 min incubation (data not shown). Based on the fact that the swallowed food remains in the stomach for about 2-4 h at pH 2.0-4.0, it appears that the proteases in the tablet would survive the acidic environment of the stomach and a significant portion of their proteolytic activity would remain.
Surprisingly, incubation at neutral pH 7.0 showed a loss of approximately 50-60% of activity. Thus it is more likely that proteases would lose some of their activity in the duodenum where the pH is 7.0-8.0 than in the acidic environment of the stomach. It is also possible that only certain proteases lose their activity at neutral pH because there is an observation that most of the loss occurs within the first 10-20 min and there is no more loss of activity observed in the following 120 min.
The proteases were also resistant to the exposure at -20, +4, +20 and +50°C for up to 120 min. There was some loss of activity observed after incubation at these temperatures. Interestingly, there was a minor loss of activity after incubation at +4°C as compared to +20°C indicating that some proteases may be slightly unstable in the cold. Approximately 10-30% of activity was lost after incubation at -20 or +4°C and also at +50°C. However, nearly all proteolytic activity was irreversibly lost after incubation of the enzyme mixture at +70°C.
Amylases
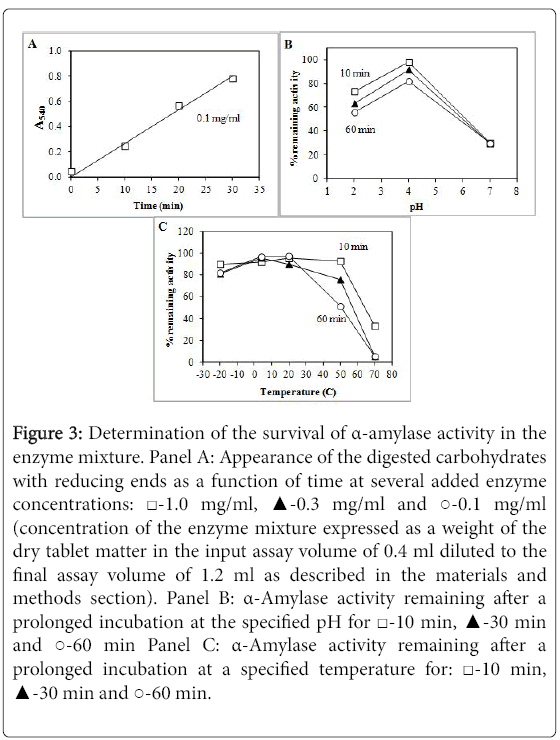
Figure 3 shows the activity of α-amylase. This group of enzymes behaved rather similar to proteases. However, there was a substantial loss of some 30% of activity after incubation at pH 2.0 while incubation at pH 4.0 preserved essentially all activity. Similar to proteases, there was significant loss of some 70% of activity after incubation at neutral pH 7.0.
Figure 3: Determination of the survival of α-amylase activity in the enzyme mixture. Panel A: Appearance of the digested carbohydrates with reducing ends as a function of time at several added enzyme concentrations: ‚?°-1.0 mg/ml, ‚?≤-0.3 mg/ml and ‚??-0.1 mg/ml (concentration of the enzyme mixture expressed as a weight of the dry tablet matter in the input assay volume of 0.4 ml diluted to the final assay volume of 1.2 ml as described in the materials and methods section). Panel B: α-Amylase activity remaining after a prolonged incubation at the specified pH for ‚?°-10 min, ‚?≤-30 min and ‚??-60 min Panel C: α-Amylase activity remaining after a prolonged incubation at a specified temperature for: ‚?°-10 min, ‚?≤-30 min and ‚??-60 min.
There was also some loss of enzymatic activity after incubation at +50°C. However, some activity could still be observed after incubation for 10 min at +70°C. Therefore, the α-amylases are also highly stable and are likely to pass through the stomach without losing a substantial portion of their activity.
Lactases
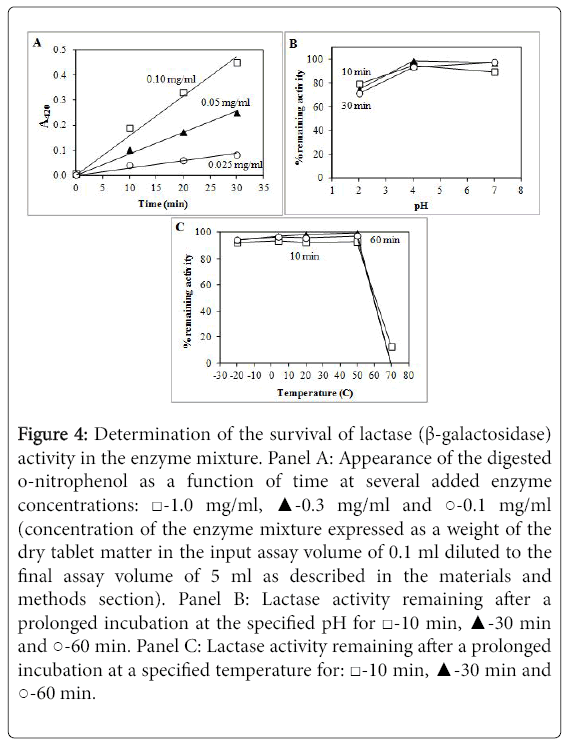
Figure 4 shows the activity determination of lactases. The appearance of o-nitrophenol as a function of time at various enzyme concentrations was essentially linear. There was only a minor loss of activity after up to 120 min incubation at pH 2.0 while there was nearly 100% enzyme survival after incubation at pH 4.0 and pH 7.0. Differently from proteases and α-amylases, the lactases did not lose their activity after incubation at neutral pH. Lactases also exhibited no loss of activity after incubation at temperatures from -20°C to +50°C. However, nearly all lactase activity was irreversibly lost after incubation at +70°C.
Figure 4: Determination of the survival of lactase (β-galactosidase) activity in the enzyme mixture. Panel A: Appearance of the digested o-nitrophenol as a function of time at several added enzyme concentrations: ‚?°-1.0 mg/ml, ‚?≤-0.3 mg/ml and ‚??-0.1 mg/ml (concentration of the enzyme mixture expressed as a weight of the dry tablet matter in the input assay volume of 0.1 ml diluted to the final assay volume of 5 ml as described in the materials and methods section). Panel B: Lactase activity remaining after a prolonged incubation at the specified pH for ‚?°-10 min, ‚?≤-30 min and ‚??-60 min. Panel C: Lactase activity remaining after a prolonged incubation at a specified temperature for: ‚?°-10 min, ‚?≤-30 min and ‚??-60 min.
The reason for such significant stability of the studied enzymes in Gastroval® Capsules might originate from Aspergillus fungus, source of enzymes. Other studies also demonstrated that enzymes from Aspergillus species showed a high acid stability [22,23]. The stability was significantly higher when using enzymes from vegetarian food sources than from animals [22,23].
The results of temperature stability show that the tested soluble enzymes from Gastroval® Capsules survive exposure to both elevated and cold temperature and therefore it could be approximated that the Capsules in solid form could also be stored at both refrigerator and room temperature. Higher environmental temperature (40–50°C) reduces the remaining activity of the enzymes in solution by up to 30%. Capsules are generally expected to be significantly more stable in the solid form than the dissolved enzymes.
Conclusion
To determine the stability of digestion-alleviating enzymes in stomach-like conditions, the total remaining enzymatic activity of proteases, α-amylases and lactases (β-galactosidase) was measured after incubation at neutral and acidic pH, at human body temperature and also elevated and cold temperatures. All three groups of enzymes (α-amylases, proteases, and lactases (β-galactosidase) were sufficiently stable at reduced pH and therefore are likely to survive the acidic environment of the human stomach. Furthermore, nearly all enzymatic activity was retained after incubation at temperatures in the range from -20 to +50°C.
References
- Walther B, Sieber R (2011) Bioactive proteins and peptides in foods. Int J Vitam Nutr Res 81: 181-192.
- Li L, Somerset S (2014) Digestive system dysfunction in cystic fibrosis: Challenges for nutrition therapy. Dig Liver Dis 46: 865-874.
- Somaraju UR, Solis-Moya A (2015) Pancreatic enzyme replacement therapy for people with cystic fibrosis (Review). Paediatr Respir Rev 16: 108-109.
- Avigan MI, Mozersky RP, Seeff LB (2016) Scientific and regulatory perspectives in herbal and dietary supplement associated hepatotoxicity in the United States. Int J Mol Sci 17: 331.
- Raschi E, Ponti DF (2015) Drug and herb induced liver injury: Progress, current challenges and emerging signals of post-marketing risk. World J Hepatol 7: 1761-1771.
- Stickel F, Kessebohm K, Weimann R, Seitz HK (2011) Review of liver injury associated with dietary supplements. Liver Int 31: 595-605.
- Cabrita LD, Bottomley SP (2004) How do proteins avoid becoming too stable? Biophysical studies into metastable proteins. Eur Biophys J 33: 83-88.
- Brömme D, Nallaseth FS, Turk B (2004) Production and activation of recombinant papain-like cysteine proteases. Methods 32: 199-206.
- Li Q, Yi L, Marek P, Iverson BL (2013) Commercial proteases: Present and future. FEBS Lett 587: 1155-1163.
- Storer AC (1991) Engineering of proteases and protease inhibition. Curr Opin Biotechnol 2: 606-613.
- Vojcic L, Pitzler C, Körfer G, Jakob F, Ronny Martinez, et al. (2015) Advances in protease engineering for laundry detergents. N Biotechnol 32: 629-634.
- Fitter J (2005) Structural and dynamical features contributing to thermostability in alpha-amylases. Cell Mol Life Sci 62: 1925-1937.
- Fried M, Abramson S, Meyer JH (1987) Passage of salivary amylase through the stomach in humans. Dig Dis Sci 32: 1097-1103.
- Janecek S (1997) α-Amylase family: Molecular biology and evolution. Prog Biophys Mol Biol 67: 67-97.
- Lonsane BK, Ramesh MV (1990) Production of bacterial thermostable alpha-amylase by solid-state fermentation: A potential tool for achieving economy in enzyme production and starch hydrolysis. Adv Appl Microbiol 35: 1-56.
- Prakash O, Jaiswal N (2010) Alpha-amylase: An ideal representative of thermostable enzymes. Appl Biochem Biotechnol 160: 2401-2414.
- Shaw A, Bott R, Day AG (1999) Protein engineering of alpha-amylase for low pH performance. Curr Opin Biotechnol 10: 349-352.
- Singh K, Shandilya M, Kundu S, Kayastha AM (2015) Heat, acid and chemically induced unfolding pathways, conformational stability and structure-function relationship in wheat‚?Įα-amylase. PLoS One 10: e0129203.
- Svensson B (1994) Protein engineering in the alpha-amylase family: Catalytic mechanism, substrate specificity and stability. Plant Mol Biol 25: 141-157.
- Husain Q (2010) Beta galactosidases and their potential applications: A review. Crit Rev Biotechnol 30: 41-62.
- Sathya TA, Khan M (2014) Diversity of glycosyl hydrolase enzymes from metagenome and their application in food industry. J Food Sci 79: 2149-2156.
- Salamanova LS, Zhdanova AA (1975) Effect of pH on the enzymic activity of the fungi Trichothecium roseum and Aspergillus niger hydrolyzing non-starch polysaccharides. Prikl Biokhim Mikrobiol 11: 214-218.
- De-Castro RJS, Sato HH (2014) Protease from Aspergillus oryzae: Biochemical characterization and application as a potential biocatalyst for production of protein hydrolysates with antioxidant activities. Journal of Food Processing 2014: 372352.
Relevant Topics
- Analytical Biochemistry
- Applied Biochemistry
- Carbohydrate Biochemistry
- Cellular Biochemistry
- Clinical_Biochemistry
- Comparative Biochemistry
- Environmental Biochemistry
- Forensic Biochemistry
- Lipid Biochemistry
- Medical_Biochemistry
- Metabolomics
- Nutritional Biochemistry
- Pesticide Biochemistry
- Process Biochemistry
- Protein_Biochemistry
- Single-Cell Biochemistry
- Soil_Biochemistry
Recommended Journals
- Biosensor Journals
- Cellular Biology Journal
- Journal of Biochemistry and Microbial Toxicology
- Journal of Biochemistry and Cell Biology
- Journal of Biological and Medical Sciences
- Journal of Cell Biology & Immunology
- Journal of Cellular and Molecular Pharmacology
- Journal of Chemical Biology & Therapeutics
- Journal of Phytochemicistry And Biochemistry
Article Tools
Article Usage
- Total views: 6832
- [From(publication date):
December-2016 - Apr 26, 2025] - Breakdown by view type
- HTML page views : 5890
- PDF downloads : 942