Research Article Open Access
Prospective, Longitudinal Study to Evaluate the Clinical Utility of a Predictive Algorithm to Detect Opioid Use Disorder in Chronic Pain Patients
Katrina Lewis1, Chee Lee2, John Blanchard2, Svetlana Kantorovich2, Brian Meshkin2and Ashley Brenton2*1Benefis Health System, Great Falls, MT, USA
2Proove Biosciences, Irvine, CA, USA
- *Corresponding Author:
- Ashley Brenton
Proove Biosciences, Irvine
CA, USA
Tel: 443-699-9951
Fax: (888) 971-4221
E-mail: abrenton@proove.com
Received date: May 16, 2017; Accepted date: June 02, 2017; Published date: June 09, 2017
Citation: Lewis K, Lee C, Blanchard J, Kantorovich S, Meshkin B, et al. (2017) Prospective, Longitudinal Study to Evaluate the Clinical Utility of a Predictive Algorithm to Detect Opioid Use Disorder in Chronic Pain Patients. J Addict Res Ther 8:329. doi:10.4172/2155-6105.1000329
Copyright: © 2017 Lewis K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Addiction Research & Therapy
Abstract
A prospective, longitudinal study was conducted to determine the clinical utility of an algorithm-based precision medicine profile designed to assess risk associated with opioid use disorder in 5,315 patients in a clinical setting. Specifically, we sought to assess how physicians were using the profile and how its use affected patient outcomes. Ninety percent of all clinicians surveyed reported some benefit to their patient care, with the most utilization for changing the prescribed opioid and the most significant benefits from discontinuing opioids. Patients who received profile-guided care reported on average a 42% reduction in pain, and almost 40% of patients had >50% reduction in pain.
Keywords
Chronic pain; Precision medicine; Personalized medicine; Opioids; Pain management; Opioid use disorder
Introduction
The World Health Organization estimates that up to 22% of patients in primary care clinics suffer from chronic pain and in the United States, chronic pain affects 11% of adults [1,2]. Since the 1990s, spurred by several studies that reported that opioids pose little addiction harm and pressured to not undertreat pain in patients, physicians have gradually adopted opioids as the mainstay of chronic pain management [3]. The Center for Disease Control and Prevention (CDC) reports that opioid prescriptions increased by 300% in recent years [4] while data from the RADARS (Researched Abuse, Diversion and Addiction-Related Surveillance) System programs show that opioid prescriptions increased from 47 million per quarter in 2006 to 60 million per quarter in 2011 and stayed so until 2013 [5]. From 2002 to 2011, an estimated 25 million Americans used opioids for nonmedical purposes [6].
Opioid-related abuse and deaths have also escalated with prescription numbers. From 2004 to 2011, opioid abuse related emergency medical cases almost tripled, with 420,040 emergency department visits in 2011 [7]. In 2013, about 1.9 million people abused or were dependent on prescription opioid pain medication [8]. Opioidrelated overdose deaths tripled between 2000 and 2014 in the United States, with more than 165,000 deaths in the period [9] and about 28,000 deaths in 2014 [10]. Between 2004 and 2011, rates of drug diversion, opioid abuse, and opioid use among college students all at least doubled. Opioid abuse costs the economy between $53-$72 billion annually [11].
These statistics reflect the conundrum in which physicians find themselves, particularly those in the United States. Physicians need to alleviate pain in patients while avoiding opioid abuse. Surveys of primary care physicians reveal that most felt stress from the risk of opioid abuse and addiction in their patients; younger physicians were particularly distressed and lacked confidence in making opioid-related decisions [12]. Notably, about half of the physicians felt they lacked adequate training in prescribing opioids. In another survey, authors found that while most physicians support using clinical tests and regulations to curb opioid abuse, only one-third of them believed that such interventions would work [13]. More education and training in opioid-related interventions were especially welcomed [14].
Opioid use disorder (OUD) is the diagnostic term for chronic opioid abuse and dependence, which includes using opioids for longer than intended, an increased tolerance to opioids, having an uncontrollable craving for opioids and using opioids despite detrimental effects to one’s physical, emotional, and social well-being. To prevent OUD, physicians are advised to check for and monitor opioid risk in patients. A variety of tools are available. Patient selfreported questionnaires like the Opioid Risk Tool (ORT) and Screener and Opioid Assessment for Patients with Pain-Revised (SOAPP®-R) use family, social and medical history to evaluate the risk of aberrant opioid behavior and addiction [15,16]. Although easy to use, these subjective questionnaires pose variable reliability [17]. Regardless of how accurate patients answer the questions, physicians still have only a 50% chance of predicting the development of OUD [18]. For more objective information, physicians can run random urine drug tests (UDT) to monitor medication metabolites in urine or query the database of the Prescription Drug Monitoring Program (PDMP) for drug prescription records [19]. Although there is no conclusive evidence that such checks and interventions reduce opioid-related deaths, a drop in opioid prescriptions, abuse and deaths have been observed [5].
In view of the high burden of OUD on healthcare and the economy, tools that help physicians assess opioid risks are greatly needed. The profile is a patent-protected algorithm that evaluates a patient’s risk for OUD based on a panel of SNP genotypes and phenotypic factors selected from the ORT [20,21]. Several studies of pain patients demonstrate that the profile identifies those at high risk of OUD with greater accuracy, sensitivity and specificity than either the ORT or SOAPP®-R [20,21]. In this study, we further evaluate the clinical utility and action ability of the profile through examination of how physicians use the profile results to guide treatment and evaluating patient outcomes [12].
Methods
Study population
A prospective, longitudinal study was conducted to assess the utility of precision medicine testing in 5,315 patients across 76 clinics in the USA. This study was reviewed, approved, and overseen by Solutions IRB (Protocols 1JUL14-62CR, 1JAN15-14CR, 1JAN15-20CR), an institutional review board licensed by the United States Department of Health and Human Services, Office for Human Research Protections. All participants signed informed consent forms prior to data collection. The research sites were stratified into four different specialty groups: Family Medicine/Primary Care/Internal Medicine, Neurology/ Psychiatry, Orthopedic Surgery and Pain Medicine/PMR/ Anesthesiology. Per protocol, exclusion criteria were significant diminished mental capacity, recent febrile illness that precludes or delays participation by more than one month, pregnancy or lactation, incomplete gene report, invalid profile score, participation in a clinical study that may interfere with participation in this study and anything that would place the individual at increased risk or preclude full compliance.
Data collection
Genomic DNA was isolated from buccal swabs obtained from each patient using a proprietary DNA isolation technique and DNA isolation kit (Macherey Nagel GmbH and Co., KG; Germany), according to the manufacturer’s instructions. Genotyping was performed using pre-designed TaqMan® assays (Applied Biosystems; Foster City, CA). Allele-specific fluorescence signals were distinguished by measuring endpoint 6-FAM or VIC fluorescence intensities at 508 nm and 560 nm, respectively, and genotypes were generated using Genotyper® Software V 1.3 (Applied Biosystems; Foster City, CA). The DNA Elution Buffer was used as a negative control, and K562 Cell Line DNA (Promega Corporation; Madison, WI), was included in each batch of samples tested as positive control.
Age and behavioral information was also collected, including whether subjects had a personal or family history of alcoholism, illegal drug abuse, prescription drug abuse, mental health disorders and/or depression.
Physicians who requested a profile assessment for their patients were given questionnaires for their patient’s baseline and follow-up study visits to document their actions, decisions and perceptions regarding the utility of the precision medicine tests. Baseline visits were conducted when physicians received their patients’ profile results. A follow-up visit occurred approximately one month later. During both the baseline and follow-up visits, physicians completed the questionnaires, which consisted of a 12-item checklist of actions or decisions that the physician might have made using profile guidance (Table S1). Physicians could also describe any other decisions not listed. The questionnaire queried the physicians for any dosage or medication selection changes they made for the patients and their patients’ response to medication, and evaluated the degree to which the profile benefitted both clinical decision making and patient are on a 5-point scale: 1=no benefit; 5=significant benefit.
To assess patient outcomes, patients were asked approximately one month after receiving guided decisions from their physicians to assess their pain levels before and after receiving care using the pain numerical rating scale (NRS). The NRS ranges from 0-10, where 0 is “no pain” and 10 is “agonizing” pain. NRS scores of 7-10 correspond to severe pain, 4-6 to moderate pain and 1-3 to mild pain [22].
The profile algorithm
A profile score and its associated OUD risk stratification were calculated for each subject. The profile algorithm is a patent-protected, validated measure of opioid use disorder risk [20,21]. In short, it combines phenotypic and genotypic information to calculate a risk score that correlates to low-, moderate- or high-risk stratifications of opioid use disorder [20,21]. A profile score of 1-11 is associated with low risk, 12-23 with moderate risk and ≥ 24 with high risk. The genetic markers used in the algorithm include 11 different single nucleotide polymorphisms (SNPs) that have been implicated in opioid abuse, misuse, dependence or addiction (Table 1). This approach, which focuses on validated genetic variants, as opposed to comprehensive next-generation sequencing, is the preferred approach of many in the field [23]. The phenotypic factors tested include an age of 16-45 years [24,25] personal history of alcohol abuse, personal history of illegal drug abuse, personal history of prescription drug abuse [26-29] and personal history of other mental health diseases including attention deficit disorder, obsessive compulsive disorder [30], bipolar disorder [31] and schizophrenia [32]. The algorithm is 42% genetic information and 58% phenotypic information [20,21].
| Protein Name | Gene | SNP | Associated Neuro-Psychiatric Disorders |
|---|---|---|---|
| Catechol-O-Methyltransferase | COMT | rs4680 | Alcohol and Drug Abuse [39,40] |
| Anxiety [41] | |||
| Depression [42] | |||
| Dopamine Beta-Hydroxylase | DBH | rs1611115 | Cocaine Addiction [43,44] |
| ADHD | |||
| Schizophrenia [45] | |||
| Dopamine D1 Receptor | DRD1 | rs4532 | Depression [46] |
| Heroin Addiction [47] | |||
| Ankyrin Repeat and Kinase Domain Containing 1/Dopamine Receptor D2 | ANKK1/DRD2 | rs1800497 | Alcohol and Cocaine Dependence [48] |
| Dopamine D4 Receptor | DRD4 | rs3758653 | Anxiety [49,50] |
| Dopamine Transporter SLC6A3 | COMT | rs27072 | Methamphetamine Addiction [51] |
| Gamma Aminobutyric Acid Receptor A, gamma2 subunit | GABRG2 | rs211014 | Alcohol Abuse [52] |
| Opioid Receptor, Kappa 1 | OPRK1 | rs1051660 | Mood Disorders [53] |
| Alcohol Dependence [54] | |||
| Methylenetetrahydrofolate Reductase | MTHFR | rs1801133 | Bipolar Disorder Depression [55] |
| Opioid Receptor, Mu 1 | OPRM1 | rs1799971 | Heroin Addiction [56] |
| Serotonin Receptor 2A | HTR2A | rs7997012 | Drug Abuse [39] |
| Depression [57] | |||
| Phenotypic Traits | Risk Factors | ||
| Age | 16-45 years old | ||
| Personal history | Mental health disorders [27,39,58,59] | ||
| Depression [28,29,60] | |||
| Alcoholism [24,61] | |||
| Illicit drug use [25,62] | |||
| Prescription drug abuse [63] |
Table 1: Profile algorithm test panel markers.
Statistical methods
For each patient, an aggregate rating of the benefit of the profile was calculated, as there was no difference in the mean or distribution of scores across visits. Chi-squared test was used to assess any differences in sex and if physicians used the profile to guide decisions. The Student’s t-test was used to assess any differences in age and if physicians used the profile to guide decisions. The Wilcoxon ranksums test was used to assess the difference in physicians’ average ratings by those who used the profile to guide decisions. Ordinal logistic regression was used to test for associations between profilepredicted risk of opioid abuse and ratings, and between ratings and specific decisions, adjusting for possible confounders: age, sex, race, and clinic specialty. The Wilcoxon signed rank sums test for paired data was used to test for significant differences in before and after pain NRS scores. All tests were two-sided and p ≤ 0.05 was considered significant. Statistical analysis was performed with R Statistical Software version 3.2.3.
Results
Study population
A total of 5,315 patients were assessed in the study (Table 2). There was no sex bias and patient ages were normally distributed around a mean age of 57 years old.
| Specialty | Total Patients | Low (%) | Moderate (%) | High (%) |
|---|---|---|---|---|
| Pain Medicine/Physical Medicine and Rehabilitation/Anesthesiology | 2822 | 47.8 | 47.7 | 4.5 |
| Family Medicine/Primary Care/Internal Medicine | 2066 | 48.5 | 46.7 | 4.8 |
| Orthopedic Surgery | 396 | 66.7 | 32.1 | 1.3 |
| Neurology/Psychiatry | 31 | 19.4 | 61.3 | 19.4 |
Table 2: Opioid risk categories of patients from 76 clinics assessed by the profile, clinics were grouped according to specialties. Orthopedic surgery had the greatest proportion of patients in the low risk category, while neurology/psychiatry had the highest proportion in the high risk category.
Opioid risk category distribution of patients by specialty
Among the four categories of clinics, patients in Orthopedic Surgery had primarily low-risk profile test results (66.7%), while over 50% of the patients from the other three categories of clinics, including pain medicine, primary care and neurology, had moderate-to high-risk results.
Profile use by physicians
Physicians rated the benefit of the profile for clinical decisionmaking and patient care during the baseline and follow up study visits. An average benefit rating (referred to as rating from here on) was calculated across both visits in order to have one rating per patient. There were no significant differences between the ratings of each follow-up visit. Physicians rated the benefit of the profile an average of 3.5 on a scale of 1-5 (1: no benefit, 5: significant benefit; Table 3), with 90% of physicians reporting that the test provided some benefit and 27% reporting significant benefit.
| Specialty | Total Patients | Rating Distribution (%) | Mean Rating | ||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |||
| Pain Medicine/Physical Medicine and Rehabilitation/Anesthesiology | 2822 | 11.3 | 8.2 | 16.7 | 36.5 | 27.3 | 3.6 |
| Family/Primary Care/Internal Medicine | 2066 | 9.4 | 20 | 27.8 | 19.6 | 23.2 | 3.3 |
| Orthopedic Surgery | 396 | 2.5 | 2.5 | 21.5 | 34.1 | 39.4 | 4.1 |
| Neurology/Psychiatry | 31 | 0 | 0 | 0 | 3.2 | 96.8 | 5 |
Table 3: Benefit ratings of the profile tool by physicians, physicians from 76 clinics used the profile and rated its benefit for clinical decision making and patient care on a scale of 1-5 (1=no benefit; 5=significant benefit), clinics were grouped according to specialties, the mean benefit rating differed by specialty, with significant differences in reported benefit between the 4 groups of practices, at least 90% of physicians thought the profile provided some benefit, with 27% indicating they felt the test provided significant benefit.
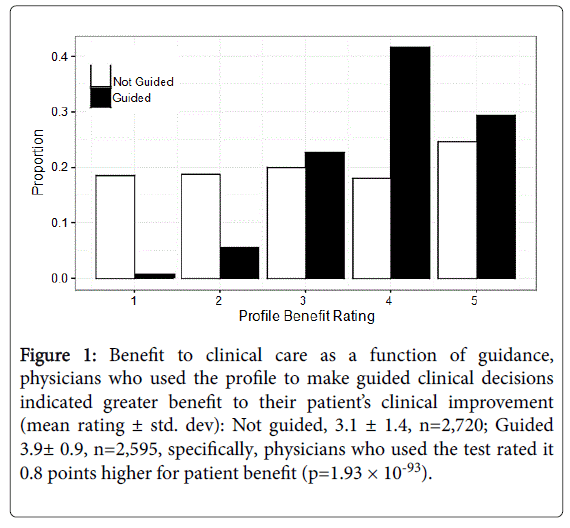
The mean benefit ratings of the profile were dependent on 3 variables: clinic specialty, profile risk stratification results, and whether profile results were used to guide clinical decisions (Table S1). Orthopedic Surgery and Neurology/Psychiatry physicians rated the profile more favorably than those in Pain Medicine and Family Medicine; physicians treating patients with a high profile score rated the benefit of profile more favourably (p=1.37 × 10-5); and physicians rated the profile as more beneficial by 0.8 points when used for making specific clinical actions or decisions (p=1.93 × 10-93; Figure 1). In particular, the benefit of the profile to patient care was greater if physicians discontinued or initiated an opioid prescription, made a change to an opioid prescription or dosage, advised another provider to make changes in the patient’s prescriptions and/or used the results to verify and document their medical regimen with more confidence (Table 4). After adjusting for any confounding due to age, sex, race and clinic specialties, physicians who implemented profile guidance still rated the profile to be on average 3.2 times more beneficial for patient care than physicians who did not follow profile guidance (Table 4).
Figure 1:Benefit to clinical care as a function of guidance, physicians who used the profile to make guided clinical decisions indicated greater benefit to their patientâÂ?Â?s clinical improvement (mean rating ± std. dev): Not guided, 3.1 ± 1.4, n=2,720; Guided 3.9± 0.9, n=2,595, specifically, physicians who used the test rated it 0.8 points higher for patient benefit (p=1.93 ÃÂ? 10-93).
| Action/Decision | n=5,315 patients | Adjusted model: age+sex+race+specialty | ||||
|---|---|---|---|---|---|---|
| Count "Yes" | Percent "Yes" | Avg. Rating Yes/No | ||||
| OR | P-value | |||||
| No changes were implemented (i.e., not guided) | 3,242 | 61 | 3.1/3.9 | 0.31 | 7.34x10-107 | *** |
| n=2,595 patients (guided only) | ||||||
| Confidence in medical regimen | 1,852 | 71.4 | 4.0/3.7 | 2 | 1.81x10-15 | *** |
| Discontinued opioids | 78 | 3 | 4.4/3.9 | 3.6 | 1.17x10-6 | *** |
| Changed opioid or dosage | 455 | 17.5 | 4.2/3.9 | 1.66 | 1.39x 10-6 | *** |
| Advised another provider | 66 | 2.5 | 4.0/3.9 | 2.15 | 3.16x10-3 | *** |
| Initiated opioid | 40 | 1.5 | 4.3/3.9 | 2.13 | 0.019 | * |
| Changed urine toxicology test frequency | 28 | 1.1 | 4.3/3.9 | 2.14 | 0.059 | trending |
| Spent more time with patient | 1,769 | 68.1 | 4.0/3.9 | 1.01 | 0.92 | |
Table 4: Benefit of profile-specific guidance in clinical management, any answer of “yes” on survey questions indicated that the physician used the profile to guide decisions, overall, physicians who made any decision rated the benefit of the profile higher than physicians who did report any guidance, odds ratios (OR) are proportional odds that may be interpreted as the average odds comparing consecutive ratings (i.e., the overall average of the odds of having a rating of 5 versus 4, 4 versus 3 and etc.), an OR<1 indicates that the decision correlated with decreased ratings, the OR of 0.31 for physicians who made no changes indicates that physicians who used the profile to guide decisions rated the profile to be on average 3.2 times higher (1/0.31) than physicians who made no changes, significance levels are indicated as * =p = 0.05, *** =p = 0.001 and trending” =p = 0.10.
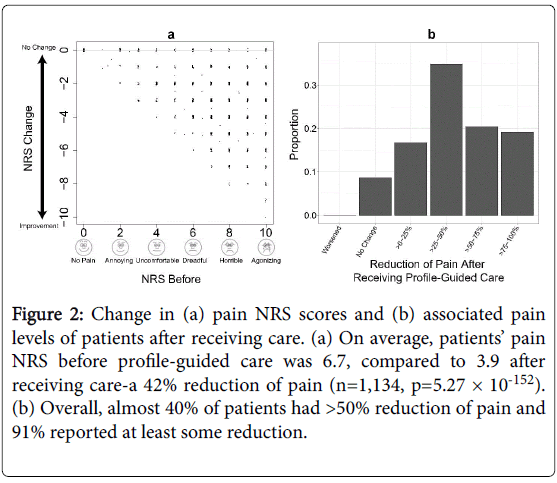
Figure 2:Change in (a) pain NRS scores and (b) associated pain levels of patients after receiving care. (a) On average, patientsâÂ?Â? pain NRS before profile-guided care was 6.7, compared to 3.9 after receiving care-a 42% reduction of pain (n=1,134, p=5.27 ÃÂ? 10-152). (b) Overall, almost 40% of patients had >50% reduction of pain and 91% reported at least some reduction.
Patient outcomes after receiving profile-guided decisions
Overall, patients improved significantly after receiving guided care from their physicians. Patients’ pain NRS before profile-guided care was 6.7 (on a scale of 0-10, where 0 was “no pain” and 10 was “agonizing” pain), compared to 3.9 after receiving care-a 42% reduction of pain (n=1,134, p=5.27 × 10-152). Almost 40% of patients had >50% reduction and 91% reported at least some reduction of pain. Additionally, 13% of patients reported 100% or complete reduction of pain. Whereas no patients reported higher pain and only 8.6% reported no change in pain (Figure 2).
Discussion
The prevalence of OUD in primary care ranges from 3%-26%, [23,31,33] and physicians prescribing opioids are under stringent scrutiny from federal and state regulations. Although strict policies are meant to curb opioid abuse, they inadvertently place huge stress on physicians who thread the fine line between treating chronic pain and preventing opioid abuse. Guidelines for opioid prescribing for chronic pain management recommend physicians evaluate the patients for opioid risk factors. The profile is a patent-protected tool that predicts patient risk of OUD based on a combination of genetic and phenotypic information. Compared to other tests based exclusively on self-report, the profile can better identify and stratify opioid use disorder in patients Previous studies [20,21] have demonstrated that the profile identifies those at risk of OUD with high sensitivity (>95%) and specificity (~90%). Furthermore, previous studies have found that the profile performs with Receiver Operating Characteristic (ROC) Area Under the Curve (AUC) measurements ranging from 0.75-0.97, which demonstrates that the profile correctly identifies those at risk of OUD between 75% and 97% of the time [20,21]. This is in contrast with published studies describing the specificity of the SOAPP®-R 52%, [34] and the sensitivities of the SOAPP ranging from 72% to 80% [34,35] and the ORT 45% [35]. The published AUC of the SOAPP-R ranges from 0.67-0.76 [36] which is lower than the AUC of the profile, in all cases except one.
Analyzing rating patterns sheds light on how physicians use and appreciate the profile. In this study, 90% of physicians agreed that the profile benefited their practice, with 27% reporting a significant benefit to patient care. Physicians rated the benefit of the profile an average of 3.5 on a 1-5 point scale (“5” indicates that physicians received significant benefits from using profile). Physicians rated the benefit of the profile more favorably for high-risk patients. While it may be intuitive that the result would be most useful for taking action in highrisk cases, we found that the specialty of the clinic makes a difference in the utility of the profile. The trend towards higher benefit in highrisk cases was driven by physicians specializing in pain medicine and those in primary care. For orthopedic surgeons, though not significant, the trend towards higher benefit of the test for treatment decision support leaned towards the low-risk test results. This may be because orthopedic surgeons, unlike pain management physicians, are using the profile as a screening test for surgical cases. Pain management clinics, on the other hand, may be more focused on making differential opioid utilization decisions based on high-risk cases.
Furthermore, physicians reported the profile as more beneficial to patient care-0.8 points higher on a 5-point scale-when they used the tool to guide a treatment decision. The clinical actions that attributed most towards physician reported benefit (raising benefit score by 0.5 points or more) were discontinuing opioids, changing frequency of urine toxicology tests, and changing the opioid selection or dosage. These results demonstrate the benefit of using the profile over other methods used to predict aberrant behavior to opioids.
In both the baseline and follow-up visits, between 23%-34% of physicians felt that the profile facilitated confidence in their medical regimen. Physicians who responded so tended to rate profile more favorably. Although these responses are not direct clinical effects, they indicate that the nebulous nature of prescribing opioids based on selfreport can benefit from a more objective, documented assessment. Moreover, a physician’s confidence in their prescribing or diagnostic practices can strongly affect doctor-patient interactions. If and how opioid therapy works for a patient depends on a myriad of factors, and one factor in the success of opioid therapy-particularly in terms of avoiding aberrant opioid behaviors-hinges on effective and honest communication between physicians and patients. CDC guidelines recommend physicians discuss opioid risks and benefits in transparent and realistic terms with patients [19]. Other practitioners encourage physicians to win patients’ cooperation through empathy and establishing trust [37,38]. Higher confidence and spending more time with patients would help physicians make better opioid prescribing decisions through the establishment of stronger doctor-patient relationships.
Pain is a huge burden on healthcare. In 2010, pain direct and indirect costs exceeded $560-635 billion than those of injury, cardiovascular disease and respiratory. Incorporation of the profile in physician decisions to guide treatment of pain can have immense impact on healthcare costs. Along with establishing improved provider-patient relationships, profile-guided treatment resulted in improved patient outcomes through decreased pain. Patients whose physician used the profile to guide treatment experienced an average pain decrease of 2.8 points on the NRS, equivalent to an average decrease from moderately-high to low pain levels.
Conclusion
A patent-protected opioid risk assessment profile combining known genetic risk factors with proven phenotypic risk factors is beneficial and relevant for physicians in clinics. Physicians rated the profile favourably, with 90% stating the profile was beneficial to clinical decision-making and patient care and 27% of them indicating that the profile resulted in significant improvements in their patients’ status. The actions ranked most highly by physicians included: making decisions regarding opioid prescriptions and increasing confidence in opioid prescribing - resulting in improved patient-physician relationships. Most importantly, patients whose physicians used the profile to guide treatment experienced a reduction in overall pain. The results of this study demonstrate the clinical utility of the profile in a naturalistic, multi-specialty setting.
Acknowledgement
The authors would like to acknowledge Yao Hua Law, Ph.D. for drafting the manuscript. The authors also gratefully acknowledge all patients and physicians who agreed to take part in the study, without whose participation and cooperation this work would not have been possible.
Disclosure and Conflicts of Interest
This study was sponsored by Proove Biosciences Inc.; KL is a member of the Proove Biosciences Medical Advisory Board. JB is a former employee of Proove Biosciences. CL, SK, BM and AB are employees of Proove Biosciences.
References
- Gureje OVKM, Simon GE, Gater R (1998) Persistent pain and well-being: A world health organization study in primary care. JAMA 280: 147-151.
- Nahin RL (2015) Estimates of pain prevalence and severity in adults: United States, 2012. J Pain 16: 769-780.
- Wilkerson RG, Kim HK, Windsor TA, Mareiniss DP (2016) The opioid epidemic in the United States. Emerg Med Clin North Am 34: e1-1e23.
- Center for disease control and prevention (CDC) (2013) Vital signs: Overdoses of prescription opioid pain relievers and other drugs among women-United States, 1999-2010. MMWR Morb Mortal Wkly Rep 62: 537-542.
- Dart RC, Severtson SG, Bucher-Bartelson B (2015) Trends in opioid analgesic abuse and mortality in the United States. N Engl J Med 372: 1573-1574.
- Administration (SAMHS) (2012) Results from the 2011 national survey on drug use and health: Summary of national findings US. Department of health and human services.
- Administration (SAMHS) (2013) The DAWN report: Highlights of the 2011 drug abuse warning network (DAWN) Findings on drug-related emergency department visits.
- Administration (SAMHS) (2014) Results from the 2013 national survey on drug use and health: Summary of national findings.
- Wonder C (2016) Multiple causes of death data. Centers for disease control and prevention.
- Rudd RA, Aleshire N, Zibbell JE, Gladden RM (2016) Increases in drug and opioid overdose deaths, United States, 2000-2014. MMWR Morb Mortal Wkly Rep 64: 1378-1382.
- Hansen RN, Oster G, Edelsberg J, Woody GE, Sullivan SD (2011) Economic costs of nonmedical use of prescription opioids. Clin J Pain 27: 194-202.
- Jamison RN, Sheehan KA, Scanlan E, Matthews M, Ross EL (2014) Beliefs and attitudes about opioid prescribing and chronic pain management: Survey of primary care providers. J Opioid Manag 10: 375-382.
- Hwang CS, Turner LW, Kruszewski SP, Kolodny A, Alexander GC (2016) Primary care physicians' knowledge and attitudes regarding prescription opioid abuse and diversion. Clin J Pain 32: 279-284.
- Kennedy-Hendricks ABS, McGinty EE, Bachhuber MA, Niederdeppe J, Gollust SE, et al. (2016) Primary care physiciansâÂ?Â? perspectives on the prescription opioid epidemic. Drug Alcohol Depend 165: 61-70.
- Webster LR (2005) Predicting aberrant behaviors in opioid-treated patients: Preliminary validation of the opioid risk tool. Pain Med 6: 432-442.
- Butler SF, Fernandez K, Benoit C, Budman SH, Jamison RN (2008) Validation of the revised screener and opioid assessment for patients with pain (SOAPP-R). J Pain 9: 360-372.
- Arthur JA, Edwards T, Waletich-Flemming J, Reddy S, Bruera E, et al. (2016) Aberrant opioid use and urine drug testing in outpatient palliative care. J Palliat Med 19: 778-782.
- Bronstein K, Passik S, Munitz L, Leider H (2011) Can clinicians accurately predict which patients are misusing their medications? Pain 12: 3.
- Dowell D, Haegerich TM, Chou R (2016) CDC guideline for prescribing opioids for chronic pain, United States. JAMA 315: 1624-1645.
- Brenton A, Richeimer S, Sharma M, Lee C, Kantorovich S, et al. (2017) Observational study to calculate addictive risk to opioids: A validation study of a predictive algorithm that detects opioid use disorder. Pharmacogenomics Pers Med.
- Farah R (2017) Evaluation of a predictive algorithm that detects aberrant use of opioids in an addiction treatment centre. J Addict Res Ther 8.
- McCaffery M, Beebe A (1993) Pain: Clinical manual for nursing practice. J Pain Symptom Manage 5: 338-339.
- Fleming MF, Balousek SL, Klessig CL, Mundt MP, Brown DD (2007) Substance use disorders in a primary care sample receiving daily opioid therapy. J Pain 8: 573-582.
- Cleland CM, Rosenblum A, Fong C, Maxwell C (2011) Age differences in heroin and prescription opioid abuse among enrolees into opioid treatment programs. Subst Abuse Treat Prev Policy 6: 11.
- Sproule B, Li S, Catz-Biro L (2009) Changing patterns in opioid addiction: Characterizing users of oxycodone and other opioids. Can Fam Physician 55: 68-69.
- Brown RL (1995) Conjoint screening questionnaires for alcohol and other drug abuse: Criterion validity in a primary care practice. Wis Med J 94: 135-140.
- Compton WM, Volkow ND (2006) Abuse of prescription drugs and the risk of addiction. Drug Alcohol Depend 83: S4-S7.
- Manchikanti L, Giordano J, Boswell MV, Fellows B, Manchukonda R, et al. (2007) Psychological factors as predictors of opioid abuse and illicit drug use in chronic pain patients. J Opioid Manag 3: 89-100.
- Brooner RK, King VL, Kidorf M, Schmidt CW, Bigelow GE (1997) Psychiatric and substance use comorbidity among treatment-seeking opioid abusers. Arch Gen Psychiatry 54: 71-80.
- Tobin DG, Andrews R, Becker WC (2016) Prescribing opioids in primary care: Safely starting, monitoring and stopping. Cleve Clin J Med 83: 207-215.
- Boscarino JA, Hoffman SN, Han JJ, Erlich PM, Gerhard GS, et al. (2010) Risk factors for drug dependence among out-patients on opioid therapy in a large US health-care system. Addiction 105: 1776-1782.
- Kern AM, Akerman SC, Nordstrom BR (2014) Opiate dependence in schizophrenia: Case presentation and literature review. J Dual Diagn 10: 52-57.
- Banta-Green CJ, Merrill JO, Doyle SR, Boudreau DM, Calsyn DA (2009) Opioid use behaviors, mental health and pain-Development of a typology of chronic pain patients. Drug Alcohol Depend 104: 34-42.
- Butler SF, Budman SH, Fernandez KC, Fanciullo GJ, Jamison RN (2009) Cross-validation of a screener to predict opioid misuse in chronic pain patients (SOAPP-R). J Addict Med 3: 66-73.
- Moore TM, Jones T, Browder JH, Daffron S, Passik SD (2009) A comparison of common screening methods for predicting aberrant drug-related behavior among patients receiving opioids for chronic pain management. Pain Med 10, 1426-1433.
- Finkelman MD, Kulich RJ, Butler SF, Jackson WC, Friedman FD, et al. (2016) An investigation of completion times on the screener and opioid assessment for patients with pain-revised (SOAPP-R). J Pain Res 9: 1163-1171.
- Gallagher RM (2006) Empathy: A timeless skill for the pain medicine toolbox. Pain Med 7: 213-214.
- Chen JT, Fagan MJ, Diaz JA, Reinert SE (2007) Is treating chronic pain torture? Internal medicine residentsâÂ?Â? experience with patients with chronic non-malignant pain. Teach. Learn. Med 19: 101-105.
- Tassin JP (2008) Uncoupling between noradrenergic and serotonergic neurons as a molecular basis of stable changes in behavior induced by repeated drugs of abuse. Biochem Pharmaco 75: 85-97.
- Kranzler HR, Modesto-Lowe V, Van Kirk J (2000) Naltrexone vs. nefazodone for treatment of alcohol dependence. A placebo-controlled trial. Neuropsychopharmacology 22: 493-503.
- Kõks S, Vasar E (2002) Deramciclane (Egis). Curr Opin Investig Drugs 3: 289-294.
- Wade AG (2011) Citalopram plus low-dose pipamperone versus citalopram plus placebo in patients with major depressive disorder: An 8 week, double-blind, randomized study on magnitude and timing of clinical response. Psychol Med 41: 2089-2097.
- Brousse G (2010) Could the inter-individual variability in cocaine-induced psychotic effects influence the development of cocaine addiction? Towards a new pharmacogenetic approach to addictions. Med Hypotheses 75: 600-604.
- Fernandez-Castillo N (2010) Association study between the DAT1, DBH and DRD2 genes and cocaine dependence in a Spanish sample. Psychiatr Genet 20: 317-320.
- Cubells JF, Sun X, Li W, Bonsall RW, McGrath JA, et al. (2011) Linkage analysis of plasma dopamine ÃÂ?-hydroxylase activity in families of patients with schizophrenia. Hum Genet 130: 635-643.
- Nyman ES (2011) Interaction of early environment, gender and genes of monoamine neurotransmission in the aetiology of depression in a large population-based Finnish birth cohort. BMJ Open 1: e000087.
- Le Foll B, Gallo A, Le Strat Y, Lu L, Gorwood P (2009) Genetics of dopamine receptors and drug addiction: A comprehensive review. Behav Pharmacol 20: 1-17.
- Noble EP (2003) D2 dopamine receptor gene in psychiatric and neurologic disorders and its phenotypes. Am J Med Genet B Neuropsychiatr Genet 116b: 103-125.
- Cao BJ, Rodgers RJ (1997) Dopamine D4 receptor and anxiety: Behavioural profiles of clozapine, L-745,870 and L-741,742 in the mouse plus-maze. Eur J Pharmacol 335: 117-125.
- Navarro JF, Luna G, Garcia F, Pedraza C (2003) Effects of L-741,741, a selective dopamine receptor antagonist, on anxiety tested in the elevated plus-maze in mice. Methods Find Exp Clin Pharmacol 25: 45-47.
- Gross NB, Duncker PC, Marshall JF (2011) Striatal dopamine D1 and D2 receptors: Widespread influences on methamphetamine-induced dopamine and serotonin neurotoxicity. Synapse 65: 1144-1155.
- Han DH, Bolo N, Daniels MA, Lyoo IK, Min KJ, et al. (2008) Craving for alcohol and food during treatment for alcohol dependence: modulation by T allele of 1519T>C GABAAalpha6. Alcohol Clin Exp Res 32: 1593-1599.
- Carlezon WA Jr, Béguin C, Knoll AT, Cohen BM (2009) Kappa-opioid ligands in the study and treatment of mood disorders. Pharmacol Ther 123: 334-343.
- Gelernter J (2007) Opioid receptor gene (OPRM1, OPRK1 and OPRD1) variants and response to naltrexone treatment for alcohol dependence: Results from the VA Cooperative Study. Alcohol Clin Exp Res 31: 555-563.
- Peerbooms OL (2011) Meta-analysis of MTHFR gene variants in schizophrenia, bipolar disorder and unipolar depressive disorder: Evidence for a common genetic vulnerability? Brain Behav Immun 25: 1530-1543.
- Nelson EC, Lynskey MT, Heath AC, Wray N, Agrawal A, et al. (2014) Association of OPRD1 polymorphisms with heroin dependence in a large case-control series. Addict Biol 19: 111-121.
- Celada P, Puig M, Amargós-Bosch M, Adell A, Artigas F (2004) The therapeutic role of 5-HT1A and 5-HT2A receptors in depression. J Psychiatry Neurosci 29: 252-265.
- Daigre C (2013) Attention deficit hyperactivity disorder in cocaine-dependent adults: A psychiatric comorbidity analysis. Am J Addict 22: 466-473.
- Fontenelle LF, Oostermeijer S, Harrison BJ, Pantelis C, Yucel M (2011) Obsessive-compulsive disorder, impulse control disorders and drug addiction: Common features and potential treatments. Drugs 71: 827-840.
- Burke JD, Burke KC, Rae DS (1994) Increased rates of drug abuse and dependence after onset of mood or anxiety disorders in adolescence. Hosp Community Psychiatry 45: 451-455.
- Lauschke VM, Ingelman-Sundberg M (2016) Requirements for comprehensive pharmacogenetic genotyping platforms. Pharmacogenomics 17: 917-924.
- Edlund MJ, Steffick D, Hudson T, Harris KM, Sullivan MD (2007) Risk factors for clinically recognized opioid abuse and dependence among veterans using opioids for chronic non-cancer pain. Pain 129: 355-362.
- Institute of Medicine Committee on Advancing Pain Research, C. a. E. (2011) The national academies collection: Reports funded by national institutes of health, in relieving pain in America: A blueprint for transforming prevention, care, education and research. National academies press, USA.
Relevant Topics
- Addiction Recovery
- Alcohol Addiction Treatment
- Alcohol Rehabilitation
- Amphetamine Addiction
- Amphetamine-Related Disorders
- Cocaine Addiction
- Cocaine-Related Disorders
- Computer Addiction Research
- Drug Addiction Treatment
- Drug Rehabilitation
- Facts About Alcoholism
- Food Addiction Research
- Heroin Addiction Treatment
- Holistic Addiction Treatment
- Hospital-Addiction Syndrome
- Morphine Addiction
- Munchausen Syndrome
- Neonatal Abstinence Syndrome
- Nutritional Suitability
- Opioid-Related Disorders
- Relapse prevention
- Substance-Related Disorders
Recommended Journals
Article Tools
Article Usage
- Total views: 3433
- [From(publication date):
August-2017 - Jul 06, 2025] - Breakdown by view type
- HTML page views : 2605
- PDF downloads : 828