Properties and Characteristics of an Optimum/Ideal Non-active Surgical Implant
Received: 03-Feb-2018 / Accepted Date: 12-Feb-2018 / Published Date: 27-Feb-2018
Abstract
Essence of properties and characteristics rendering a non-active surgical implant optimum or ideal has hitherto been lacking and requires further consideration. Definitions governing medical devices given in leading geo-political regulatory domains are insufficient to specify fundamental attributes of non-active surgical implants. Equally, voluntary, non-majoritarian goal-oriented standards on non-active surgical implants are devoid of fundamental attributes of such product. Exploration of fundamentals of non-active surgical implants, their properties and characteristics permits formulation of implant rules based on the concept of implant potential, also proposed herein. Implant rules deduced from implant potential promote uniform, scientific assessment and comparison, offering numerous obvious benefits. In this article, we explore fundamentals of non-active surgical implant, their properties and characteristics and propose implant rules based on implant potential, from which concepts of optimum physiological function and implant potential, respectively, are proponed. They might even improve standards on or relevant to non-active surgical implant published by standard organisations.
Keywords: Implants; Surgical; Non-active; Optimum; Ideal; Rules; Regulation
Introduction
Extant legislation excludes specific logic and rules by which properties and characteristics of an optimum / ideal non-active surgical implant (NASI) may be deduced and subsequently regulated.
This article explores fundamentals of NASI, their properties and characteristics and proposes implant rules based on implant potential, from which concepts of optimum physiological function and implant potential, respectively, are proponed. They might even improve standards published by standard organisations [1,2].
Ideal/optimal anatomy and physiology
Surgery is performed for many reasons, whether elective or emergency. In the case of non-active surgical implant (NASI) medical devices, this may include to replace or restore diseased organs and tissues, remove or create obstruction, reposition structures to their normal position or redirect conduits, improve physical appearance or compensate disability or abnormality.
Therefore, what is the ideal / optimal anatomy and physiology for a NASI? From this, is it possible to deduce properties and characteristics of optimum / ideal NASI?
Functionally, the body may be considered organised into structures and systems, the breadth of which yields insight on conduciveness to surgery involving medical devices.
Fully-organised or highly-organised organs and tissues seem most conducive if not optimally-conducive to surgical intervention; such increasing organisation making it easier to conceive morphologically and structurally analogous physical substitutes and albeit nonidentically, functionally close constructs. In certain cases, indeed, for example, restorative dental implants, it can be argued certain properties of materials are superior to native ones.
Among the best examples of ideal or optimum anatomy and physiology conducive to NASI surgery is the lens. The lens is a biconvex, transparent, flexible structure enclosed in a thin, elastic capsule and held in place just posterior to the iris by the ciliary zonule. Like the cornea, it is avascular (blood vessels interfere with transparency). It is a discrete body, functionally self-contained, operates mechanically by simple, singular physics and importantly, is easily accessed surgically [3]. Additionally, complicated or prolonged medical procedures, medication and mechanisms are unnecessary to access, explore, excide and replace it.
Further, materials possessing suitable properties are available to manufacture anatomical and physiological substitutes, moreover, visually indistinguishable or invisible in daily life. It is no surprise that intra-ocular lens implants constitute among the most popular NASI in the world.
None of the leading international legislation governing medical devices specifies fundamental attributes (taken to mean properties and characteristics) for non-active surgical implants. European Council directives on medical devices do specify Essential requirementssubsequently mimicked by other nations and at global harmonisation task force levels- however, such requirements are general, do not constitute logic rules and are not conducive to determine optimum / ideal characterisation (because they are goal-oriented). Equally, the position concerning voluntary, non-majoritarian goal-oriented standards in NASI is devoid of fundamental attributes of such product. Consequently, it is opined the vast majority of NASI placed on the market (PoM) manifest immaculately by spontaneous assumption of implant potential during their conception.
We propose the concept of implant potential as: the extent of qualities of an implant determining its suitability, safety, performance and usefulness throughout its service and total implant life without undesirable physiological loss, malfunction or detriment to an organ or organ system of the body.
Consequently, it is necessary to understand physiological function from which optimum implant potential may be deduced. Physiological function may be defined as: activity natural to or purpose of a person or thing. Consequently, optimum physiological function is highest conduciveness of such function to a favourable physiological outcome. It is therefore conjectured defining NASI by implant potential might improve such products, evaluation of suitability and (criteria for) regulatory compliance.
Consequently, it is possible to deduce implant rules from implant potential, thereby promoting uniform, scientific assessment and comparison. If successful, such approaches connote obvious benefits.
This article explores fundamentals of NASI, their properties and characteristics and proposes implant rules based on implant potential, from which concepts of optimum physiological function and implant potential, respectively, are proponed. They might even improve standards published by standard organisations.
Implant rules
Initially, it might seem obvious what constitutes a NASI and its role in medicine, yet, closer examination of the subject indicates critical scientific investigation of these matters is lacking [4-6] and requires further consideration. Among factors which might be considered, it is first necessary to establish understanding of physiology as it relates to NASI treatments.
Physiology is the study of the function of living organisms a branch of biology concerned with normal function of living organisms and their parts and a way in which a living organism or bodily functions.
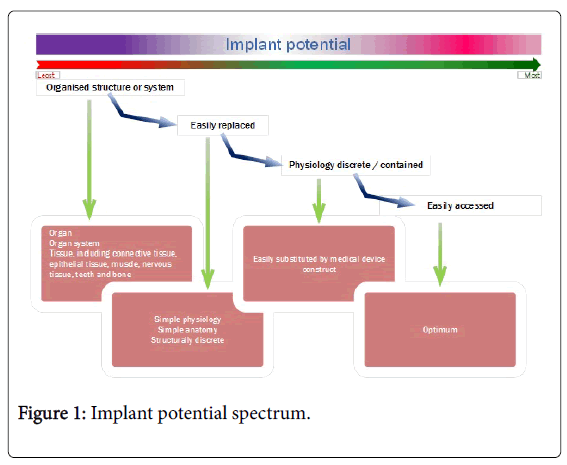
Thus, a NASI designed to remedy disordered physiological (function) can do so optimally if it adheres to certain rules (Figure 1).
Present regulatory position of NASI may be illustrated by studying leading jurisdictions and certain goal-oriented voluntary standards. In the European Union, the following are pertinent:
Definition implantable device regulation (EU) 2017/745 [1]
Article (2) ‘Implantable device’: ‘Implantable device’ means any device, including those that are partially or wholly absorbed, which is intended to be totally introduced into the human body, or — to replace an epithelial surface or the surface of the eye, by clinical intervention and which is intended to remain in place after the procedure. Any device intended to be partially introduced into the human body by clinical intervention and intended to remain in place after the procedure for at least 30 days shall also be deemed to be an implantable device. The term implant is used synonymously with implantable device in the regulation.
Whereas Annex IX 1.2. Council Directive 93/42/EEC defines:
Definition surgically invasive device council directive 93/42/EEC [2]
An invasive device which penetrates inside the body through the surface of the body, with the aid or in the context of a surgical operation.
For the purposes of this Directive devices other than those referred to in the previous subparagraph and which produce penetration other than through an established body orifice, shall be treated as surgically invasive devices.
Definition implantable device council directive 93/42/EEC
Annex IX 1.2. ‘Implantable device’: Any device which is intended to be totally introduced into the human body or to replace an epithelial surface or the surface of the eye. By surgical intervention which is intended to remain in place after the procedure. Any device intended to be partially introduced into the human body through surgical intervention and intended to remain in place after the procedure for at least 30 days is also considered an implantable device.
The term implant is used synonymously with implantable device in the directive.
In the USA, by comparison, the term implant is defined as follows:
Definition implant title 21 – code of federal regulations part 860 [7]
Subchapter I Part 860 Subpart A - General § 860.3 (d) ‘implant’: A device that is placed into a surgically or naturally formed cavity of the human body. A device is regarded as an implant for the purpose of this part only if it is intended to remain implanted continuously for a period of 30 days or more, unless the Commissioner determines otherwise in order to protect human health.
EN ISO 14630:2012 [8], however, defines the following:
1. Non-active surgical implant
2. Surgical implant
According to EN ISO 14630, an implant is (defined as):
Definition non-active surgical implant EN ISO 14630
3.6 EN ISO 14630 ‘non-active surgical implant’: Surgical implant, the operation of which does not depend on a source of electrical energy or any source of power other than that directly generated by the human body or gravity.
Definition surgical implant EN ISO 14630
3.8 EN ISO 14630 ‘surgical implant’: Device that is intended to be totally introduced into the human body, or to replace an epithelial surface or the surface of the eye, by means of surgical intervention and that is intended to remain in place after the procedure, or any medical device that is intended to be partially introduced into the human body by means of surgical intervention and that is intended to remain in place after the procedure for at least 30 days.
Meaning a NASI (by deduction is defined as) must be:
Definition non-active surgical implant EN ISO 14630
3.6 and 3.8 (merged) EN ISO 14630 ‘non-active surgical implant’: Surgical implant, the operation of which does not depend on a source of electrical energy or any source of power other than that directly generated by the human body or gravity, that is intended to be totally introduced into the human body, or to replace an epithelial surface or the surface of the eye, by means of surgical intervention and that is intended to remain in place after the procedure, or any medical device that is intended to be partially introduced into the human body by means of surgical intervention and that is intended to remain in place after the procedure for at least 30 days.
It is argued these definitions, while they might be suited for the respective ISO/TR (ISO/TR 14283) and standard (ISO 14630) [9], also directives on medical devices do not conduce precise properties and characteristics necessary for design rules on such products. Further, it is opined temporality used to discriminate implants allow classification into regulatory classes is irrelevant in determining implant potential. The following rules allow improved definition (Table 1):
| Rule | Principle |
|---|---|
| 0 | Anatomical structure or physiology (function of the body) shall be (sufficiently) discrete that dysfunction to account for symptoms thereof shall be such that a NASI can correct such dysfunction, malfunction or abnormality; restore; replace or substitute; deliver substances; modify or augment, indistinguishably from normal |
| 1 | A NASI shall correct one or more discrete dysfunction, malfunction or abnormality or combination thereof |
| 2 | A NASI shall restore one or more discrete anatomical structure or physiology (function of the body) |
| 3 | A NASI shall replace or substitute for one or more discrete anatomical structure or physiology (function of the body) |
| 4 | A NASI shall deliver an intended substance to a specified anatomical structure or system in a manner that neither disrupts nor corrupts native physiology (function of the body) |
| 5 | A NASI shall modify one or more discrete anatomical structure or physiology (function of the body) in a manner that does not deleteriously affect structure or physiology intended, surrounding or systemic structure or physiology |
| 6 | A NASI shall augment one or more discrete anatomical structure or physiology (function of the body) isotropically, including where such NASI is used to increase size of a structure or cavity, tissue, organ or organ system, correct disfigurement, improve shape, size, feel, or replace loss mass, eg, bone in jawbone or insufficient natural bone |
| 7 | A temporary NASI shall be explanted / removed from its implant site in a manner that does not cause the original condition for which it was implanted originally to return, cause morbidity, either local or systemic or elicit dysfunction, etc |
Table 1: Implant rules (applied to non-active surgical implants).
From these implant rules, properties and characteristics of optimum / ideal NASI can be postulated (Table 2).
| Property / characteristic | Optimum / ideal state |
|---|---|
| Detectability | Undetectable implanted |
| Imperceptible presence in organ or organ system following explant of NASI that can be explanted | |
| Physiological identity | Physiologically indistinguishable from native organ or organ system |
| Biological identity | Biochemically indistinguishable from native organ or organ system biochemical function |
| Immunologically indistinguishable from native organ or organ system immunological function | |
| Histo-compatibly indistinguishable from native tissue, organ or organ system | |
| Anatomical mimic, as required | |
| Isotropically indistinguishable from native tissue, organ or organ system | |
| Functional identity | Fully-functional mimic |
| Accommodation | Able to adapt or adjust in situ to changes especially of a bodily part (as an organ); automatically adjust to physiological changes over which such adjustment is possible, including as a recipient grows unless NASI intended to integrate in tissue or recede, in which case, it shall be neutral and non-attritional |
| Neutrality | Disambiguous or indifferent, not evoke responses affecting comfort, well-being or quality of life of recipient, including physiologic motion and articulation |
| Attrition | Wear over time, if any, consistent and concurrent with native implant environment |
| Depreciation | Functional loss over time, if any, consistent and concurrent with native implant environment |
Table 2: Properties and characteristics of optimum / ideal NASI.
It is not the purpose of this article to argue only autologous NASI can substitute, however implied.
Implant Rule 0 constitutes the quintessential criterion from which sub-ordinate rules are deduced:
Rule 0
Anatomical structure or physiology (function of the body) shall be (sufficiently) discrete that dysfunction to account for symptoms thereof shall be such that a NASI can correct such dysfunction, malfunction or abnormality; restore; replace or substitute; deliver substances; modify or augment, indistinguishably from normal.
It is conjectured these implant rules sufficiently describe a suitable implant site. Naturally, it would be up to designers to determine which implant rules to apply and show how a given NASI fulfils.
Summary and Future Research
Essence of properties and characteristics rendering a non-active surgical implant (NASI) optimum or ideal has hitherto been lacking and requires further consideration [10-13].
Exploration of fundamentals of NASI, their properties and characteristics permits formulation of implant rules based on the concept of implant potential, also proposed herein. Implant rules deduced from implant potential promote uniform, scientific assessment and comparison, offering numerous obvious benefits.
Areas for future research include NASI explant rules, implant suitability index and active surgical implant rules. Such research is underway. Additionally, framework of superial goal-oriented standard on NASI based on implant rule and implant potential is in preparation.
References
- European Parliament (2017) Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017 on medical devices, amending Directive 2001/83/EC, Regulation (EC) No 178/2002 and Regulation (EC) No 1223/2009 and repealing Council Directives 90/385/EEC and 93/42/EEC [http://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX:32017R0745] Official Journal of the European Union. Accessed on: February 12, 2018
- The Council Of The European Communities (1993) Council Directive 93/42/EEC of 14 June 1993 concerning medical devices [http://data.europa.eu/eli/dir/1993/42/oj] Official Journal of the European Communities. Accessed on: February 12, 2018
- Bramhill JH, Tighe BJ (2011) Towards a functional accommodating IOL: Bulk properties of the crystalline lens and synthetic analogues. Cont Lens Anterior Eye 34: s1-s43.
- Choroszynski M, Choroszynsk MR, Skrzypek SJ (2017) Biomaterials for hip implants - Important considerations relating to the choice of materials. Bio Alg Med Sys 13: 133-145.
- Todros S, Pavan PG, Natali AN (2017) Synthetic surgical meshes used in abdominal wall surgery: Part I-materials and structural conformation. J Biomed Mater Res 105: 689-699.
- Zhu LM, Schuster P, Klinge U (2015) Mesh implants: An overview of crucial mesh parameters. World J Gastrointest Surg 7: 226-236.
- Code of Federal Regulations (2012) Food and Drugs [https://www.emergogroup.com/sites/default/files/file/usa-fda-labeling-21-cfr-part-801.pdf?action] U.S. Food & Drug. Accessed on: February 12, 2018
- International Organization for Standardization (2012) Non-active surgical implants -- General requirements [https://www.iso.org/standard/61749.html] International Organization for Standardization. Accessed on: February 12, 2018
- Haroon Atchia (2017) An analysis of the standard on non-active surgical implants and practicalities in its application. Quality First International. Accessed on: February 12, 2018
- Jones AA, Cochran DL (2006) Consequences of implant design. Dent Clin North Am 50: 339-360.
- Peel S, Bhatia S, Eggbeer D, Morris DS and Hayhurst C (2016) Evolution of design considerations in complex craniofacial reconstruction using patient-specific implants. Sage J 231: 509-524
- Khan W, Muntimadugu E, Jaffe M and Domb AJ (2014) Implantable medical devices. Focal Controlled Drug Delivery, Advances in Delivery Science and Technology XVII. Controlled Release Society, pp:33-59.
Citation: Atchia H (2018) Properties and Characteristics of an Optimum / Ideal Non-active Surgical Implant. J Med Imp Surg 3: 118.
Copyright: © 2018 Atchia H. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Conferences
42nd Global Conference on Nursing Care & Patient Safety
Toronto, CanadaRecommended Journals
Open Access Journals
Article Usage
- Total views: 4219
- [From(publication date): 0-2018 - Apr 09, 2025]
- Breakdown by view type
- HTML page views: 3369
- PDF downloads: 850