Research Article Open Access
Pro-Oxidant and Inflammatory Mediators Produced In Transgenic Mice (App/ Ps1)
| Aguirre-Rueda D, Aldasoro M, Santonja S, Guerra S, Iradi A and Valles SL* | |
| Department of Physiology, Faculty of Medicine, University of Valencia, Blasco Ibañez, 15. 46010 Valencia, Spain | |
| *Corresponding Author : | Soraya L Valles, Ph.D. Department of Physiology, School of Medicine University of Valencia, Blasco Ibañez 15. 46010 Valencia, Spain Tel: 34-96-3983813 Fax: 34-96-3864642 E-mail: lilian.valles@uv.escom |
| Received November 16, 2013; Accepted December 16, 2013; Published December 20, 2013 | |
| Citation: Aguirre-Rueda D, Aldasoro M, Santonja S, Guerra S, Iradi A, et al. (2013) Pro-Oxidant and Inflammatory Mediators Produced In Transgenic Mice (App/Ps1) . Biochem Physiol 2:121. doi:10.4172/2168-9652.1000121 | |
| Copyright: © 2013 Aguirre-Rueda D, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Biochemistry & Physiology: Open Access
Abstract
Amyloid precursor protein plus presenilin-1 (APP/PS1) transgenic mice are a frequently model for amyloid deposition studies in Alzheimer disease (AD). We determined gene expression in these transgenic mice by Westernblot technique. Protein expression was similar in both transgenic and wild type mice and also transgenic expression was similar in old and young mice. In mice with 12 months, transgenic animals developed cognitive dysfunction and reduced protein expression of several genes essentials in oxidative stress, such as MnSOD without changes in expression of Cu/ZnSOD protein. Also, we are unable to detect changes between wild type and transgenic mice for PPAR-γ antiinflammatory protein expression. Loss of neurons and synapses are contributing to the AD illness and here, we noted increased expression of AIF, contributing to the loss of neurons, astrocytes and probably synapses function inside the brain. Furthermore, we detect an increase in Sir-2 protein expression only significant in limbic brain area of APP/ PS1 mice compared with wild type mice, area first involved in AD patients. On the other hand, a reduction on p-53 protein expression is noted in all brain APP/PS1 areas compared to wild type mice. Our results demonstrate increase in pro-oxidant proteins and no changes in anti-inflammatory, with increase expression in HIF demonstrating increase in apoptosis in transgenic mice compared to wild type mice. All changes indicated loss of neurons and astrocytes with an unbalance between pro- and anti-oxidant proteins with induction of protecting proteins such as Sir-2 and reduction of p-53, which is normally increased in cancer cells.
| Keywords |
| Alzheimer’s Diseases; APP/PS1; mnSOD; HIF; P-53; Cu- ZnSOD |
| Abbreviations |
| AD: Alzheimer’s disease; Mn-SOD: Manganeso- Super-Oxido-Dismutase; Cu/Zn-SOD: Cu/Zn-Super-Oxido- Dismutase; HIF: Hypoxia Inducible Factor; P-53: P-53 protein; PPAR-γ: Peroxisoma Proliferator-Activated Receptor |
| Introduction |
| Alzheimer’s disease is a common multifactorial neurodegenerative disorder that occurs with aging. The neuropathological hallmarks of Alzheimer’s disease (AD) are amyloid plaques, intra-neuronal tangles, and activation of glial cells [1-3]. Glial swelling and astrogliosis are a characteristic response of astrocytes to inflammation, oxidative stress and trauma, leading to secretion of several potentially toxic products, including inflammatory [4] and oxidative stress mediators [5]. |
| Beta amyloid (Aβ) deposition can result in brain damage and neurodegeneration, but whether astrocytes activation participates in the Aβ-induced brain damage, and furthermore, its intervention in inflammation and in oxidative stress in AD is poorly known. Also in vivo studies have been demonstrated the involvement of oxidative stress and inflammation in the induction of Alzheimer phenotypes. We demonstrate here oxidative stress and inflammation changes in APP/PS1 transgenic mice [transgenic mice, APP (amyloid precursor protein) and PS1 (Preseniline 1) protein] compared with wild type mice. We determine by western-blot MnSOD and Cu/ZnSOD, such as pro-oxidant proteins, PPAR-γ such as anti-inflammatory protein, p-53 and Sir-2 proteins, in transgenic compared with wild type mice. We previously found that oestradiol or genistein attenuate inflammation and oxidative processes, preventing expression of inflammatory mediators and production of peroxide levels, demonstrating antioxidant and anti-inflammatory effects of oestrogens in neurons and astrocytes in primary culture [6]. Furthermore, these compounds promote PPAR-γ activation as a regulator of inflammatory responses and consequently protecting cells from Aβ deleterious effects [7]. Our results here, demonstrate a decreased expression of Mn-SOD, HIF and p-53 with increase in Sir-2 and without changes in Cu/Zn-SOD and PPAR-γ expression in transgenic compared with wild type mice. |
| Materials and Methods |
| Materials |
| Dulbecco’s modified Eagle’s medium (DMEM) and fetal bovine serum (FBS) were obtained from Gibco (Gibco Invitrogen Corparation, Barcelona, Spain). Western Blot Chemiluminescent Detection System (ECL) was from Amersham (Amersham Biosciences, Barcelona, Spain). Antibodies: monoclonal anti-peroxisome proliferator-activated receptor antibody (anti-PPARγ) (1:250) and monoclonal anti-α- tubulin antibody (1:1000) (Santa Cruz Biotechnology, Madrid, Spain), polyclonal anti-Mn-SOD protein antibody (anti-Mn-SOD) (1:500), polyclonal anti-Cu/Zn-SOD antibody (anti-Cu/Zn-SOD) (1:500), polyclonal anti-HIF protein antibody (anti-HIF) (1:250), monoclonal anti-p-53 antibody (anti-p-53) (Sigma Aldrich, Madrid, Spain). All other reagents are analytical or culture grade purity. |
| Western blot analysis |
| Protein extracts from brain cells were mixed with equal volumes of SDS buffer (0.125 M Tris-HCl, pH 6.8, 2% SDS, 0.5% (v/v) 2-mercaptoethanol, 1% bromophenol blue and 19% glycerol) and then boiled for 5 min. Protein concentration was determined using a modified Lowry method [8]. Proteins were separated by SDS-PAGE gels and transferred to nitrocellulose membranes using standard techniques. Membranes were blocked with 5% dried milk in TBS containing 0.05% Tween-20 and then incubated with the corresponding antibodies following manufacturer’s recommendations. The blots were washed three times with a washing buffer (phosphate-buffered saline, 0.2% Tween 20) for 15 min each and then incubated for 1 h with a secondary horseradish peroxidase-linked anti-rabbit or antimouse IgG antibody (Cell Signaling Technologies, Barcelona, Spain). As above, the blots were washed three times and developed using the enhanced chemiluminescence (ECL) procedure as specified by the manufacturer (Pharmacia biotechnology, San Francisco, California, USA). Autoradiographic signals were assessed using a Bio-Rad scanning densitometer. |
| Data analysis and statistics |
| Measurements are expressed as means ± SD. Data were determined by a Student’s t-test or by one-way ANOVA. Analysis of variance followed by a post hoc Tukey–Kramer test for multiple-comparisons between groups were did it. Statistical significance was designated as P<0.05. Also Graph Pad Prism 5 Program was used if necessary. |
| Results |
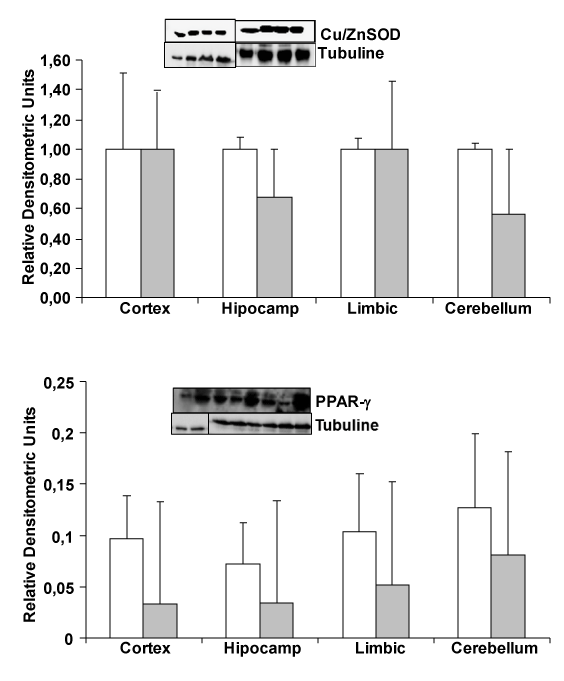
| Change in PPAR-γ and Cu/ZnSOD protein expression |
| To know whether oxidative stress and inflammation proteins, such as Cu/ZnSOD and PPAR-γ can change between transgenic and wild type mice, such as we published in cells in culture induced by amyloid β addition, we determine protein expression by Westernblot. In Figure 1, we demonstrate no changes in PPAR-γ (an anti inflammatory protein) and Cu/ZnSOD protein (expressed in cell cytoplasm) expression between wild type and transgenic mice. We use hippocampus, cerebellum, cortex and limbic brain areas. |
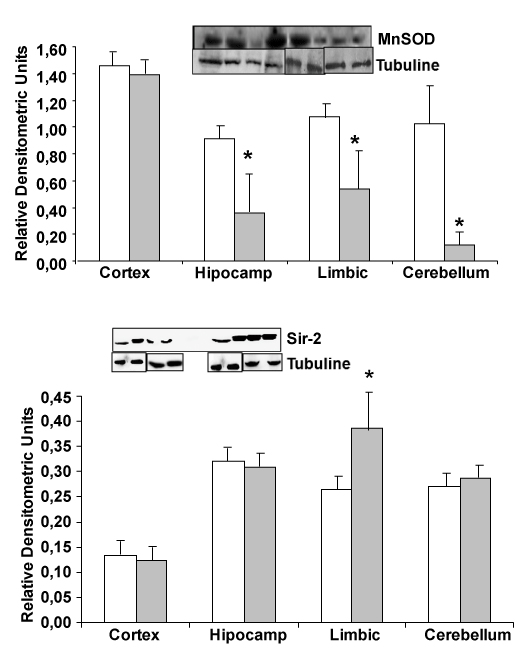
| Change in MnSOD and Sir-2 protein expression |
| Looking for MnSOD protein, expressed in mitochondria, we note a protein decrease in hippocampus, limbic and cerebellum from transgenic mice compared with wild type mice (Figure 2). On the other hand, increase in Sir-2 limbic protein expression was detected comparing APP/PS1 with wild type mice, indicating a decrease in antioxidant proteins and an increase in Sir-2 protein (Figure 2). |
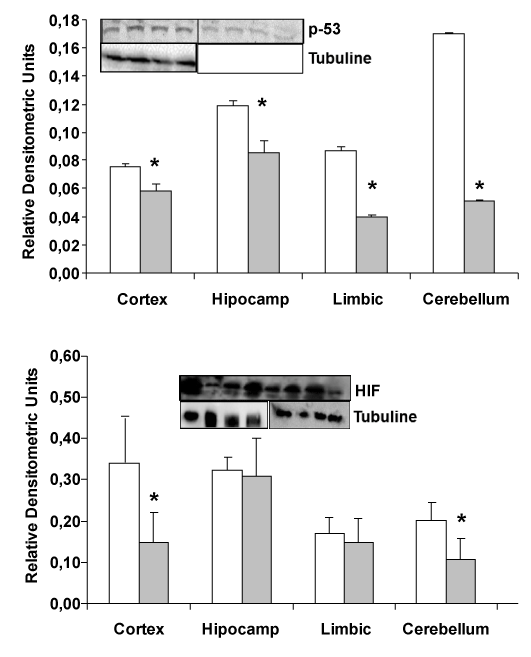
| Change in HIF and P-53 protein expression in different parts of the brain |
| In transgenic mice (APP/PS1), a decrease in HIF and p-53 expression is detected compared wild type mice (Figure 3). Protein expression by western-blot in different areas of the brain: hippocampus, cerebellum, cortex and limbic shown that HIF and p-53 protein are decremented in APP/PS1 transgenic mice compared with wild type. |
| Discussion |
| Our previous results demonstrated that Amyloid β (Aβ) peptide causes oxidative stress in neurons in primary culture [6] and others demonstrated secretion of reactive oxygen species (ROS)/reactive nitrogen species (RNS) by inflammatory cells. Those results are the major mechanism for attacking opsonised targets and furthermore, activated glial cells have the potential to produce large amounts of ROS/RNS using different mechanisms [9]. Induction of iNOS gene and activation of astrocytes can produce large amounts of nitric oxide (NO) which can react with superoxide to form peroxynitrite [10]. Increased expression of iNOS is also detected in astrocytes surrounding plaques in AD [11,12]. Furthermore, Wyss-Corey and his collaborators [3] have shown that astrocytes could play a major role in Alzheimer’s disease. Authors showed the possible role of astrocytes as defense cells against Aβ toxicity. It is proposed that astrocytes may eliminate and destroy Aβ peptide [3] and here we have detected a decrease in expression of prooxidant molecules (Figure 2, MnSOD) and reduction in HIF (Figure 3), which are expressed in hypoxic situations, in transgenic APP/ PS1 compared with wild type mice. The decrease in MnSOD in the mitochondria can increase the level of peroxide inside cells producing an increase in oxidative stress damage. Respect to Cu/ZnSOD, our results demonstrated non changes in this cytoplasmic protein, probably because mitochondria have important roles in oxidative stress damage. Also we cannot detect changes in other proteins showed in Figure 1. |
| Our group has demonstrated that estradiol prevents neuronal death by lowering oxidative stress [6] and also that Amyloid β can induce oxidative stress and pro-inflammatory proteins. All together demonstrated the influence of inflammation and oxidative stress in development AD and the possible future strategies to des-accelerate the illness in patients. Many authors have been recommending the use of polyphenols, not only in neurodegeneration patients, in cancer and others destructive cell illness [13]. |
| Recent studies clearly showed the involvement of peroxisome proliferator-activated receptor (PPARγ) gene in the pathogenesis of Alzheimer’s disease [14,15]. It is interesting to note that activation of PPARγ by non-steroid anti-inflammatory drugs (NSAIDs) suppress pro-inflammatory Aβ actions in AD animal models and in patients with long-term intake of certain NSAIDs (e.g., ibuprofen, indomethacin, naproxen). Furthermore, PPAR-γ activation produce reduced risk and manifest delayed development of Alzheimer’s disease [16-18]. In this study we noted not changes in PPAR-γ protein expression in transgenic compared to wild type mice (Figure 2), indicating that this protein can increase after inflammation occurs in cells in culture [13] but not in our transgenic mice. That discrepancy probably is because other cells and protective mechanisms are actuating inside our brain. In inflammation NFκB expression is increased in cells in culture after Aβ peptide induction but in in vivo studies, authors not always detect high increase in this transcription factor [19,20], demonstrating a control of the brain to reduce quickly NFκB. |
| Sir-2 protein is increased in elderly people. That protein has been related with a good health in older healthy people. An increase of Sir- 2 protein only in limbic brain area, which is the most devastate area in Alzheimer’s disease, is demonstrated in Figure 2. On the other hand, reduction of p53 in transgenic mice compared with wild type could indicate a protective effect in these mice, because decrease in the possibility to suffer cancer in patients with Alzheimer’s disease has been published. |
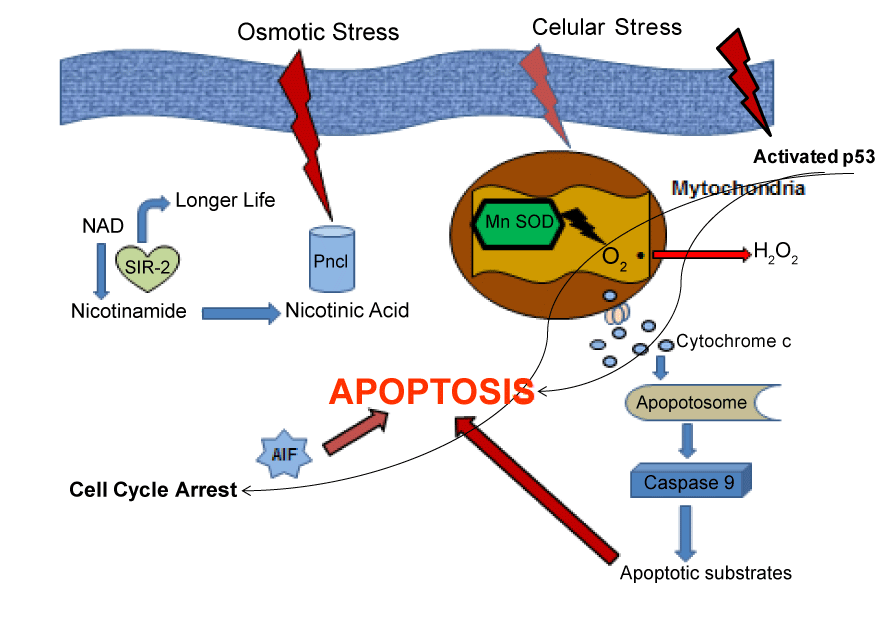
| In summary, our results demonstrate that in transgenic mice APP/ PS1 there are an increase in oxidative stress compared with wild mice, demonstrated by induction of proteins involved in that mechanisms of cell damage. We detect here a decrement in Sir-2 protein expression, such as occurs in AD patients. Also a diminished expression in p-53 protein demonstrated here, is according with previous results published for other authors, indicating the possibility to suffer less cancer in AD patients (Figure 4). All these findings reinforce the hypothesis that oxidative stress significantly contribute to the pathogenesis of AD and also another mechanisms can act producing the phenotype of that illness. |
| Acknowledgements |
| This work was supported by: GVcs2007-AP-001 (SV), BFU 2005/230 (FP), BFU 2007 6583/BFI (JV). |
References
|
Figures at a glance
 |
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 |
Relevant Topics
- Analytical Biochemistry
- Applied Biochemistry
- Carbohydrate Biochemistry
- Cellular Biochemistry
- Clinical_Biochemistry
- Comparative Biochemistry
- Environmental Biochemistry
- Forensic Biochemistry
- Lipid Biochemistry
- Medical_Biochemistry
- Metabolomics
- Nutritional Biochemistry
- Pesticide Biochemistry
- Process Biochemistry
- Protein_Biochemistry
- Single-Cell Biochemistry
- Soil_Biochemistry
Recommended Journals
- Biosensor Journals
- Cellular Biology Journal
- Journal of Biochemistry and Microbial Toxicology
- Journal of Biochemistry and Cell Biology
- Journal of Biological and Medical Sciences
- Journal of Cell Biology & Immunology
- Journal of Cellular and Molecular Pharmacology
- Journal of Chemical Biology & Therapeutics
- Journal of Phytochemicistry And Biochemistry
Article Tools
Article Usage
- Total views: 13547
- [From(publication date):
December-2013 - Apr 05, 2025] - Breakdown by view type
- HTML page views : 9057
- PDF downloads : 4490
