Proning in Unilateral Pulmonary Edema
Received: 04-Apr-2023 / Manuscript No. roa-23-95510 / Editor assigned: 06-Apr-2023 / PreQC No. roa-23-95510 (PQ) / Reviewed: 20-Apr-2023 / QC No. roa-23-95510 / Revised: 22-Apr-2023 / Manuscript No. roa-23-95510 (R) / Published Date: 29-Apr-2023 DOI: 10.4172/2167-7964.1000439
Abstract
We describe a case of unilateral pulmonary edema in a patient of acute M.I. with acute M.R. and cardiogenic shock which was managed with an intra-aortic balloon pump, vasodilators, diuretics and proning. Usually, pulmonary edema presenting due to acute mitral regurgitation requires mitral valve repair for resolution of pulmonary edema but in our patient we were able to manage it conservatively without the need for any mechanical circulatory support device like VA- ECMO, LVAD or surgery.
Keywords
Heart failure; Pulmonary edema; Radiology
Introduction
Pulmonary edema is a broad term which is defined as the accumulation of fluid in the extravascular compartment of the lung. Cardiogenic pulmonary edema is usually caused by an increased hydrostatic pressure either due to left ventricular systolic or diastolic dysfunction. It is most commonly caused by acute decompensated heart failure. Chest radiograph remains the most practical tool for assessing and quantifying pulmonary edema [1]. Unilateral pulmonary edema is a rare and easily mistaken imaging finding which can mimic unilateral lung infiltrative diseases like pneumonia. A study has shown that unilateral pulmonary edema is seen in only 2% of all pulmonary edema cases [2]. The most common cause is mitral regurgitation with an asymmetric jet. It is associated with a delayed diagnosis of heart failure and is associated with high mortality [3].
Herein we describe a case of unilateral pulmonary edema in a patient of acute M.I. with acute M.R. and cardiogenic shock which was managed conservatively with an intra-aortic balloon pump, vasodilators, diuretics and Proning.
Case report
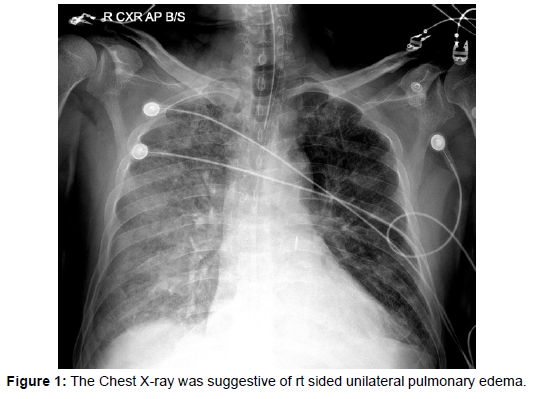
An 80 yr old male patient who was a known case of hypertension presented with abdominal pain and chest pain of 4 hours duration. He was admitted to a nearby hospital where he was evaluated and discharged with treatment for gastritis. On the same day, he developed severe breathlessness along with orthopnea, following which he visited the emergency department of our hospital. Laboratory tests indicated a white blood cell count of 21970/μL with 91.8% neutrophils, haemoglobin of 9.5 g/dL and serum creatinine of 1.53 mg/dl. He was in respiratory failure (pH 7.2, PaO2 94 mm Hg, pCO2 54 mm Hg, FiO2 0.6) and was initiated on NIV support. ECG showed ST depression from V1 to V5. Echocardiography indicated hypokinesia in the basal septum, inferior wall, and posterior wall along with acute severe mitral regurgitation and mild LV systolic dysfunction. The Chest X-ray was suggestive of rt. sided unilateral pulmonary edema (Figure 1). A diagnosis of unilateral pulmonary edema due to acute mitral regurgitation was made as LA and LV were normal in size. The patient’s dyspnoea worsened, and he failed to respond to NIV therapy (pH 7.05, PaO2 58.3 mm Hg, pCO2 80.5 mm Hg, FiO2 0.7) and he required invasive mechanical ventilation. In view of hemodynamic instability and worsening heart failure he underwent coronary angiography which was suggestive of triple vessel disease.
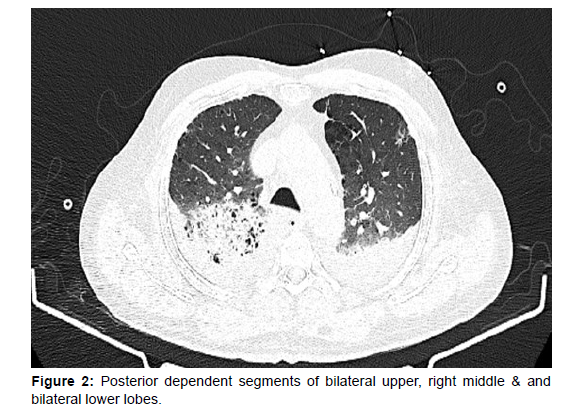
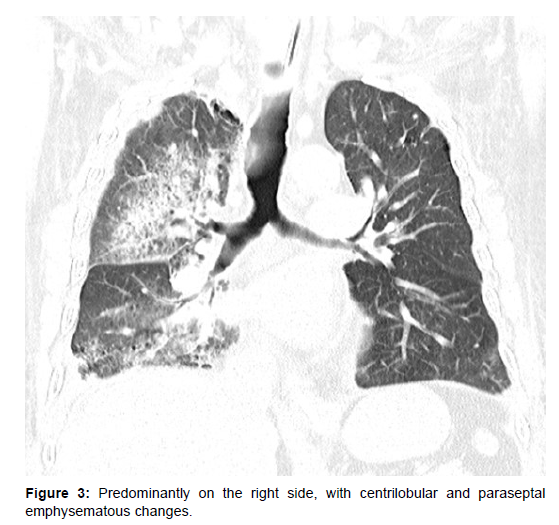
In view of his critical condition emergency PCI was planned for culprit vessel with IABP support. His cardiogenic shock was managed with inotropes, diuretics and high dose vasopressors and IABP. Despite optimal mechanical ventilation strategy and IABP support, his oxygenation was unsatisfactory. In view of worsening renal function and poor response to diuretics CRRT was also initiated. To assess further he was evaluated with a CT chest which was suggestive of areas of consolidation in the posterior dependent segments of bilateral upper, right middle & and bilateral lower lobes, predominantly on the right side, with centrilobular and paraseptal emphysematous changes (Figure 2 and 3).
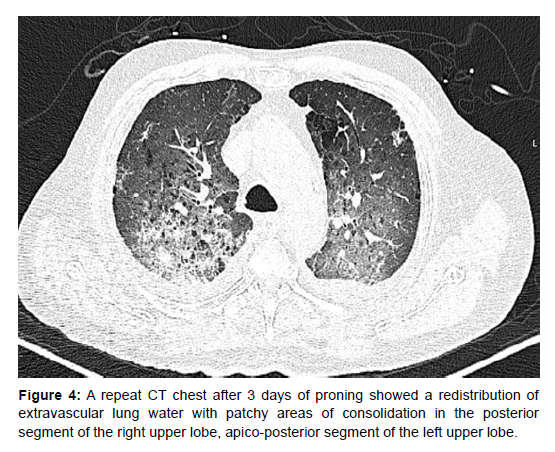
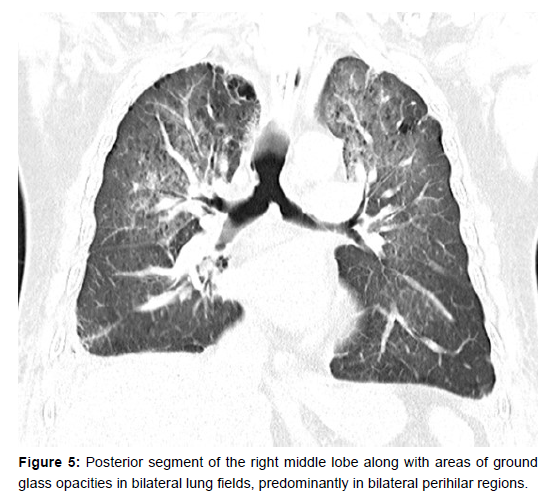
Due to his continued poor oxygenation status, a trial of proning was given. His oxygenation improved drastically after proning for 2 days with PaO2/FiO2 ratios almost doubling from 80 to 150. A repeat CT chest after 3 days of proning showed a redistribution of extravascular lung water with patchy areas of consolidation in the posterior segment of the right upper lobe, apico-posterior segment of the left upper lobe and posterior segment of the right middle lobe along with areas of ground glass opacities in bilateral lung fields, predominantly in bilateral perihilar regions (Figure 4 and 5). The patient gradually improved and could be extubated onto NIV on day 9. NIV support was also gradually weaned and ceased. Repeat echocardiography showed a reduction in the severity of mitral regurgitation to mild mitral regurgitation. He was discharged on day 14.
Discussion
Pulmonary edema is rarely unilateral but may cause confusion and present diagnostic challenges. It is most commonly mistaken as acute pneumonia. It is often helpful to obtain a detailed history of symptoms. Peribronchovascular consolidations and elevated BNP with the absence of elevated procalcitonin often help in differentiation. Physical examination to detect systolic murmur of acute mitral regurgitation is important but may be misleading and even absent due to equilibration of LA and LV pressures. The most important test to differentiate it at bedside is echocardiography. The differentiating features between pneumonia and unilateral pulmonary edema have been described in (Table 1).
| Unilateral Cardiogenic Pulmonary Edema | Pneumonia | |
|---|---|---|
| Symptom | Sudden onset/mostly afebrile | Gradual onset/febrile |
| Physical examination | pansystolic murmur | No murmur |
| Unilateral “bat wing” consolidation with peripheral field spared | Diffuse, lobar, or multifocal consolidation | |
| Sputum staining | Negative | Often positive |
| Biomarkers | PCT - negative BNP - elevated |
PCT - positive BNP - normal |
| Echocardiography | Acute and severe mitral regurgitation | Normal |
Table 1: Differences between unilateral cardiogenic pulmonary edema and pneumonia.
In our case, though the leucocyte count was elevated, lung ultrasound was suggestive of C profile, and echocardiography confirmed the presence of severe mitral regurgitation.
The development of pulmonary edema is a result of complex interactions between capillary hydrostatic pressure, capillary permeability, and capillary oncotic pressures. When the balance of these forces is altered and the capacity of pulmonary interstitial lymphatics is exceeded, fluid extravasates into alveolar spaces.
Depending on the vascular or parenchymal abnormalities, edema can occur on the same side or opposite side. Various pathologies and their mechanistic origin has been described in (Table 2 and 3) respectively.
S. No |
Condition | Mechanism |
|---|---|---|
| 1 | Papillary dysfunction | Retrograde flow of blood directed across left atrium towards rt upper lobe pulmonary vein orifices |
| 2 | Dependent lung of an unconscious patient | Gravity aided |
| 3 | A rapid removal of fluid/ air by thoracocentesis [3] | Swift re-expansion causes a change in hydrostatic pressure in pulmonary capillaries |
| 4 | Aspiration of gastric content | Damage to surfactant |
| 5 | Bronchial obstruction | Hypoxic damage to alveolar cells producing surfactant |
Table 2: Pulmonary edema due to ipsilateral pathology.
S. No |
Condition | Mechanism |
|---|---|---|
| 1. |
|
Reduced blood flow in pulmonary capillaries |
| 2. | Emphysema due to any aetiology | pulmonary capillaries may be reduced or damaged |
Table 3: Pulmonary edema due to contralateral pathology.
Another important cause of unilateral pulmonary edema is neurogenic pulmonary edema. Several clinicopathologic theories have been proposed to explain this clinical syndrome: 1) Neuro-cardiac 2) Neuro-hemodynamic 3) “blast theory” and 4) pulmonary venule adrenergic hypersensitivity [4]. It has also been reported after multiple sclerosis exacerbation [5].
Most cases of unilateral cardiogenic pulmonary edema affect the right lung. A possible explanation is the poorer lymphatic drainage of the right lung by the small-calibre right bronchomediastinal trunk in comparison with that of the left lung by the large-calibre thoracic duct. Another explanation relates to the enlargement of left heart that develops in most patients with heart failure and that may physically impede blood flow in the left pulmonary artery, thereby reducing capillary volume [2,6]. The main mechanism of MR in UPE is acute chordal rupture, but functional MR may also be involved [7].
The regurgitant flow may be directed toward the right pulmonary veins, frequently the superior right pulmonary vein, in patients with severe mitral regurgitation from the posterior leaflet [8].
It is important to have a high level of suspicion for acute MR in any patient with acute pulmonary edema in the setting of acute myocardial infarction, especially if LV systolic function is well preserved [6].
The anterolateral papillary muscle of the mitral valve is supplied by the diagonal branch of the LAD coronary artery. During the early phases of a myocardial infarction involving the LAD coronary artery transient mitral regurgitation (MR) is common and can cause haemodynamic compromise.
For patients with acute MR, immediate surgical intervention is recommended, but the risks of acute MR surgery are very high. A multi-centre study involving 279 patients who underwent emergency surgery for acute severe MR reported a mortality rate of 22.5% [9]. Therefore, the primary goal of the medical treatment of acute MR is to stabilize the patient’s condition and prepare the patient for surgery. Sodium nitroprusside can reduce systemic vascular resistance, thereby alleviating MR; however, the use of vasodilators in patients with systemic hypotension is usually limited. Moreover, shortterm circulatory assistive devices, such as the intra-aortic balloon counterpulsation, Impella percutaneous left ventricular assist device [10] and extracorporeal membrane oxygenation (ECMO), can be used as a temporary measure before emergency valve surgery to temporarily stabilize the patient’s condition.
In our case, even after 4 days of medical treatment, our patient’s condition had not improved. His PaO2/Fio2 fell to 71 mm and a trial of proning was given and the patient’s oxygenation and CO2 exchange showed drastic improvement. There is evidence that prone ventilation improves oxygenation in hydrostatic pulmonary edema [11]. Prone positioning allows the heart to lay on the sternum and the mechanical effect on the left lower lobe is relieved. It leads to improved alveolar ventilation, and better V/Q matching. Prone ventilation also leads to improved cardiac index by causing the reduction in pulmonary vascular resistance leading to improved performance of the right ventricle which could have a beneficial effect on the diastolic function of the left ventricle. Furthermore, the improvement of oxygenation as well as inotropic agents could affect the systolic function of the left ventricle. The reduction in pulmonary vascular resistance in HPE probably is related to the decreased hypoxic pulmonary vasoconstriction, owing to the recruitment of atelectatic areas.
Furthermore, the improvement in alveolar ventilation is more in hydrostatic pulmonary edema compared with ARDS patients, as the enlarged hearts of patients with HPE could compress the left lower lobe more than the normal-sized hearts of patients with ARDS. Furthermore, the excess lung water is more easily removed in patients with HPE rather than ARDS.
In addition to this, there is also a reduction in lung stress in the prone position. The reduction in gravitational stress in the prone lung is a function of oedema volume, location and height of the isogravitational section. This implies that in patients with a larger anteroposterior lung diameter, gravitational stress might be greater than in those with smaller lungs and that these patients might benefit more from the prone posture. Likewise, a patient with a larger volume of oedema fluid might experience a greater reduction in gravitational stress from a change to prone positioning than someone with less oedema, as long as some functional lung units remain uncompromised. Studies have also indicated a change in posture to prone might redistribute that pulmonary oedema, resulting in a more homogeneous distribution of regional inflation and density [12,13] Such changes could reduce and redistribute gravitational stress further and cause the redistribution of extravascular lung water, potentially increasing the impact of prone positioning.
2021 ESC/EACTS guidelines recommend immediate surgical treatment for acute severe mitral regurgitation [14]. Valve repair may be selected as the surgical method. Compared with valve replacement, mitral valve repair has lower perioperative mortality, preserves left ventricular function, and results in better longterm survival. In addition, long-term oral anticoagulation drug therapy may be avoided after artificial valve replacement. The possibility of valve repair was suggested but was refused by the family in view of the high perioperative risk.
Conclusion
UPE is easily misdiagnosed, and timely diagnosis is essential. CT imaging findings, BNP, and echocardiography may be used for differential diagnosis. Mitral valve regurgitation is the most common cause. Surgery is the recommended treatment; however, the intraoperative and postoperative risks of emergency surgery are extremely high. IABP, proning, LVAD, and ECMO may be used to support transitional treatment.
References
- Ware LB, Matthay MA (2005) Acute Pulmonary Edema. N Engl J Med 353: 2788-2796.
- Attias D, Mansencal N, Auvert B, Vieillard-Baron A, Delos A, et al. (2010) Prevalence, characteristics, and outcomes of patients presenting with cardiogenic unilateral pulmonary edema. Circulation 122: 1109-1115.
- Childress ME, Moy G, Mottram M (1971) Unilateral pulmonary edema resulting from treatment of spontaneous pneumothorax. Am Rev Respir Dis 104: 119-121.
- Davison DL, Terek M, Chawla LS (2012) Neurogenic pulmonary edema. Crit Care 16: 212.
- Makaryus JN, Kapphahn S, Makaryus AN (2009) Unilateral neurogenic pulmonary oedema and severe left ventricular dysfunction secondary to acute multiple sclerosis exacerbation. Heart Lung Circ 18: 155-158.
- Shin JH, Kim SH, Park J, Lim YH, Park HC, et al. (2012) Unilateral pulmonary edema: a rare initial presentation of cardiogenic shock due to acute myocardial infarction. J Korean Med Sci 27: 211-214.
- Legriel S, Tremey B, Mentec H (2006) Unilateral pulmonary edema related to massive mitral insufficiency.
- Raman S, Pipavath S (2009) Asymmetric Edema of the Upper Lung Due to Mitral Valvular Dysfunction. N Engl J Med 361: e6.
- Lorusso R, Gelsomino S, de Cicco G, Beghi C, Russo C, et al. (2008) Mitral valve surgery in emergency for severe acute regurgitation: analysis of postoperative results from a multicentre study. Eur J Cardiothorac Surg 33: 573-582.
- Jalil B, El-Kersh K, Frizzell J, Ahmed S (2017) Impella percutaneous left ventricular assist device for severe acute ischaemic mitral regurgitation as a bridge to surgery. BMJ Case Rep 2017: bcr2017219749.
- Nakos G, Tsangaris I, Kostanti E, Nathanail C, Lachana A, et al. (2000) Effect of the Prone Position on Patients with Hydrostatic Pulmonary Edema Compared with Patients with Acute Respiratory Distress Syndrome and Pulmonary Fibrosis 161: 360-368.
- Kallet RH (2015) A comprehensive review of prone position in ARDS. Respir Care 60: 1660-1687.
- Gattinoni L, Taccone P, Carlesso E, Marini JJ (2013) Prone position in acute respiratory distress syndrome. Rationale, indications, and limits. 188: 1286-1293.
- Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, et al. (2022) 2021 ESC/EACTS Guidelines for the management of valvular heart disease: Developed by the Task Force for the management of valvular heart disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Rev Esp Cardiol 43: 561-632.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: Krishna MR, Kola VR (2023) Proning in Unilateral Pulmonary Edema.OMICS J Radiol 12: 439. DOI: 10.4172/2167-7964.1000439
Copyright: © 2023 Krishna MR, et al. This is an open-access article distributedunder the terms of the Creative Commons Attribution License, which permitsunrestricted use, distribution, and reproduction in any medium, provided theoriginal author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 2580
- [From(publication date): 0-2023 - Dec 20, 2025]
- Breakdown by view type
- HTML page views: 2236
- PDF downloads: 344