Progressive Fulminant Demyelinating Neuropathy Associated with Paranodal Antibodies
Received: 30-Mar-2018 / Accepted Date: 24-Apr-2018 / Published Date: 01-May-2018
Abstract
Chronic inflammatory demyelinating polyneuropathy (CIDP) is a clinical condition with complex immune based pathology. There are different types of immune mediated markers associated with CIDP that describe different subtypes of the disease but data on paranodal antibodies associated with striational and potassium channel antibodies is lacking.
Keywords: Chronic inflammatory demyelinating polyneuropathy; Paranodal antibodies; Immunoglobulin; Hematoxylin
Case Report
71 year old male presented with 3 weeks of gradual worsening of right arm weakness and right facial droop. Pertinent positives on examination were weakness, MRC strength graded: 3+/5 of right deltoid, biceps and triceps, subtle right facial droop and diffuse hyporeflexia. MRI brain was negative. Cerebrospinal fluid (CSF) was obtained which revealed elevated protein, thus diagnosis of Guillian Barre Syndrome (GBS) was made due to albumino-cytologic dissociation. Intravenous immunoglobulin (IVIG) was tried but over the next few days’ deterioration in weakness, involving bilateral upper and lower extremities was noted. Inpatient nerve conduction studies were obtained and findings were confirmatory for an acute demyelinating polyneuropathy with sural sparing. No further intervention, as the thought was disease will probably hit nadir within the next few weeks.
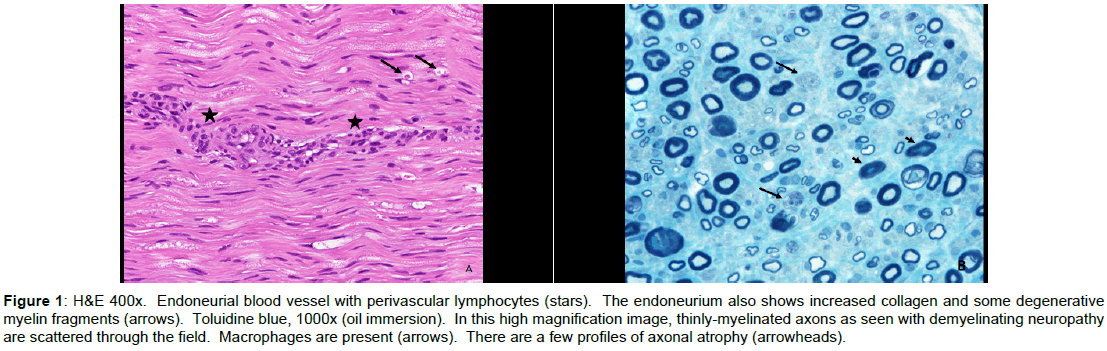
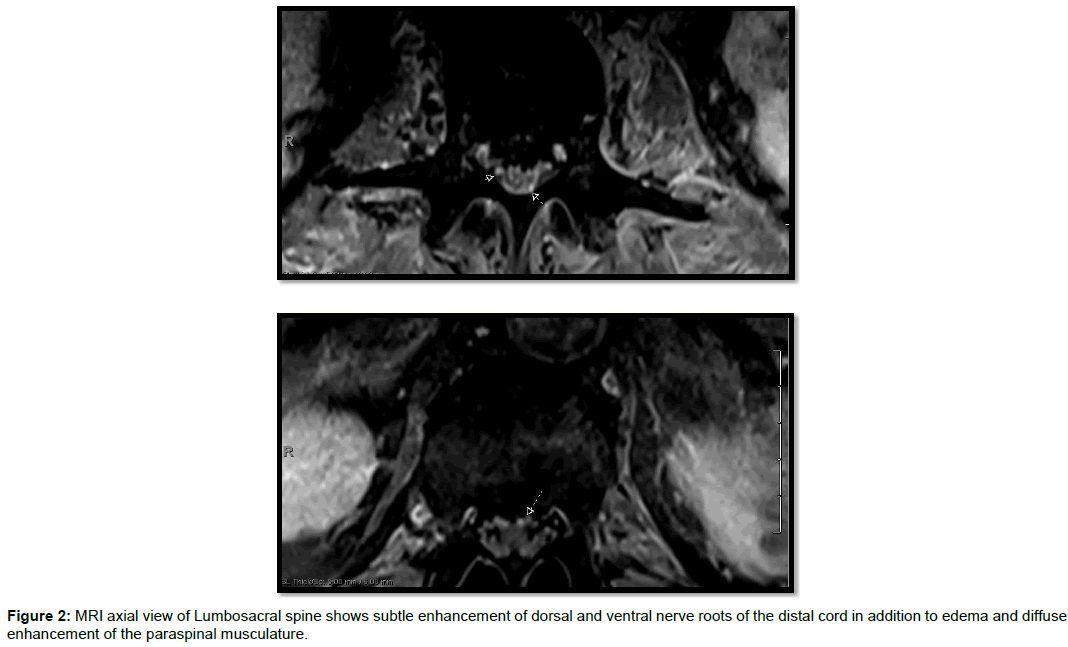
After a total of 2 cycles of IVIG 4 weeks apart and 9 week duration of progressively worsening symptoms, patient developed difficulty swallowing, increased weakness and numbness in all extremities (lower>upper). Dysautonomia involving sinus tachycardia and uncontrolled hypertension was evident. Pertinent examination findings showed strength of 0/5 in bilateral lower extremities. In bilateral upper extremities strength was graded as follows: Deltoid, biceps, triceps, wrist flexors 4/5 and wrist extensors and interossei were 3/5. Neck flexion was 3/5 and neck extension was 5-/5. Diffuse hyporeflexia was noted. Sensory examination showed decreased pinprick distal to the belly button. Due to lack of plateauing of symptoms and poor response to IVIG he underwent repeat electro diagnostic testing which revealed findings consistent with progression of an acquired demyelinating, motor and sensory polyneuropathy with secondary axonal loss. Sural nerve biopsy was done and showed evidence for mixed axonal and demyelinating neuropathy with moderate to marked axonal loss and perivascular lymphocytes along endoneurial blood vessels. Hematoxylin and eosin staining revealed endoneurial blood vessel with perivascular lymphocytes, increased collagen and degenerative myelin fragments. Toluidine blue staining revealed thinly myelinated axons with atrophy (Figure 1). Pertinent repeat abnormal CSF findings showed elevated protein >600. Repeat imaging of brain was unremarkable but MRI lumbosacral spine (Figure 2) showed subtle enhancement of dorsal and ventral nerve roots of the distal cord in addition to edema and diffuse enhancement of the paraspinal musculature.
Figure 1: H&E 400x. Endoneurial blood vessel with perivascular lymphocytes (stars). The endoneurium also shows increased collagen and some degenerative myelin fragments (arrows). Toluidine blue, 1000x (oil immersion). In this high magnification image, thinly-myelinated axons as seen with demyelinating neuropathy are scattered through the field. Macrophages are present (arrows). There are a few profiles of axonal atrophy (arrowheads).
Above clinical presentation and studies were consistent with the diagnosis of chronic inflammatory demyelinating polyneuropathy (CIDP) or one of its variants. Patient then received 5 days of Plasma exchange and was started on high dose steroids. No improvement was noted.
To rule out other etiologies, patient underwent full body bone scan to look for organomegaly and malignancies which were ruled out. Normal serum immunofixation studies. Management with IVIG, Plasma exchange and high dose steroids was already attempted but increasing dyspnea mandated intubation, later tracheostomy and PEG placement.
Further testing including paraneoplastic panel on serum revealed high titer of Striational antibodies and Potassium channel antibody. Paranodal antibodies including Anti-contactin was elevated at 62000(normal<2000). Diagnosis of chronic inflammatory demyelinating polyneuropathy (CIDP) refractory to conventional treatment was made based on above studies. Patient was eventually started on Rituximab with noteworthy signs of improvement.
Discussion
CIDP is a heterogeneous clinical entity with numerous forms described [1]. Widely used treatments for CIDP are intravenous immunoglobulin, plasmapheresis and steroids, and each of these therapies have different response in individuals. Because of emerging variants of CIDP which in some cases do not respond to conventional therapies, more aggressive therapy with immunomodulators have to be instituted [2].
Paranodal antibodies in recent years have frequently been linked to CIDP patients. There have been reports of patients with CIDP where antibodies are directed against the nodes of Ranvier or the paranodal axo-glial apparatus. Notably, neurofascin-186, gliomedin and contactin 1 (CNTN1) are the targets of autoantibodies [3]. Also, association of these autoantibodies with CIDP patients showed aggressive symptom and poor response to intravenous immunoglobulin but good response to Rituximab as seen in our case [4].
Our case differs from previously noted ones as in addition to paranodal antibody our patient has positive striational antibodies and potassium channel antibodies. Striational antibodies have been reported in myasthenia gravis and thymoma thus they may support an underlying autoimmune diagnosis [5]. To this date, studies investigating the association between striational antibodies and neurological disease including CIDP are lacking. Antibodies against voltage-gated potassium channels have strongly been associated with paraneoplastic syndromes including peripheral nerve hyperexcitability syndrome, POEMS (polyneuropathy, organomegaly, endocrinopathy, monoclonal gammopathy and skin changes) syndrome, neuromyotonia and limbic encephalitis [6]. Patient underwent whole body scanning which showed no evidence of organomegaly. No evidence of endocrinopathy, monoclonal gammopathy, or skin changes were seen as well.
In CIDP it has been postulated that autoantibodies towards axoglial adhesion molecules may disrupt potassium voltage-gated channels clustering leading to a failure of saltatory conduction [7].
Paranodal antibodies have also been reported in patients with Guillian Barre syndrome [8]. Recurrent GBS which is relapsing and remitting in nature, can be confused with CIDP frequently. Recurrent GBS usually presents in patients under age 30 with milder symptoms and associated with miller fisher variant syndrome. Our patient was 71 year old with severe symptoms and had negative GQ1B antibody. Also our patient had clinical deterioration 3 or more times without any signs of improvement between the episodes, which would be the case with recurrent GBS where patients would improve between episodes with accumulated small neurological deficits [9-11].
Thus, CIDP with severe respiratory embarrassment, autonomic dysfunction, cranial nerve involvement not associated with POEMS syndrome, presence of anti-nodal, striational and potassium-channel antibodies raise the possibilities of new faces of this disease with protean manifestations and associations. Nodes of Ranvier are a key target of autoantibodies in chronic inflammatory neuropathies. Most importantly, these patients have aggressive presentation and poor response to IVIG. These results should motivate physicians to implement testing of the anti-CNTN1 IgG4 antibodies [12]. Further studies need to be conducted on the role of striational and potassium channel antibodies. Our case highlights that specific biomarkers are crucial for correctly identifying CIDP subtypes for accurate patient diagnosis and initiating treatment of choice earlier.
References
- Koller H, Kieseier BC, Jander S, Hartung HP (2005) Chronic inflammatory demyelinating polyneuropathy. N Engl J Med 352: 1343-1356.
- Bright RJ, Wilkinson J, Coventry BJ (2014) Therapeutic options for chronic inflammatory demyelinating polyradiculoneuropathy: a systematic review. BMC Neurol 14: 26.
- Devaux JJ (2012) Antibodies to gliomedin cause peripheral demyelinating neuropathy and the dismantling of the nodes of Ranvier. Am J Pathol 18: 1402-1413.
- Querol L, Rojas-Garcia R, Diaz-Manera J, Barcena J, Pardo J, et al. (2015) Rituximab in treatment-resistant CIDP with antibodies against paranodal proteins. Neurol Neuroimmunol Neuroinflamm 2: e149.
- Anwar F (2015) Significance of Striational antibodies in the context of neuropathy. Mark Flemmer: J Neurol Neurosci 6.
- Paterson RW, Zandi MS, Armstrong R, Vincent A, Schott J (2013) Clinical relevance of positive voltage-gated potassium channel (VGKC)-complex antibodies: experience from a tertiary referral centre. J Neurol Neurosurg Psychiatry 85: 625-630.
- Svahn J, Antoine JC, Camdessanche JP (2014) Pathophysiology and biomarkers in chronic inflammatory demyelinating polyradiculoneuropathies. Rev Neurol (Paris) 170: 808-817.
- Devaux JJ, Odaka M, Yuki N (2012) Nodal proteins are target antigens in Guillain-Barre' syndrome. J Peripher Nerv Syst 17: 62-71.
- Hantson P, Kevers L, Fabien N, Van Den Bergh P (2010) Acute-onset chronic inflammatory demyelinating polyneuropathy with cranial nerve involvement, dysautonomia, respiratory failure, and autoantibodies. Muscle Nerve 41: 423-426.
- Kuitwaard K, Koningsveld R, Ruts L, Jacobs BC, Doorn PA (2009) Recurrent Guillain-Barre syndrome. J Neurol Neurosurg Psychiatry 80: 56-59.
- Almadani A, Alboudi A, Alrukn S, Sarathchandran P, Inshasi J (2015) Recurrent Guillain Barre syndrome- A report of three cases with clinical electrophysiological and CSF profile. Neurology 84: P2.070.
- Miura Y, Devaux JJ, Fukami Y, Manso C, Belghazi M, et al. (2015) Contactin 1 IgG4 associates to chronic inflammatory demyelinating polyneuropathy with sensory ataxia. Brain 138: 1484-1491.
Citation: Tiwana HK, Bandyopadhyay S, Kaur D, Specht CS, Ahmed A (2018) Progressive Fulminant Demyelinating Neuropathy Associated with Paranodal Antibodies. Neurol Clin Therapeut J 2: 104.
Copyright: © 2018 Tiwana HK, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Open Access Journals
Article Usage
- Total views: 4377
- [From(publication date): 0-2018 - Apr 07, 2025]
- Breakdown by view type
- HTML page views: 3496
- PDF downloads: 881