Review Article Open Access
Progress in Treatment and Prevention of Trichinellosis
| Yan-Rong Yu1, Yong-Fen Qi1,2,3* | |
| 1Department of Pathogen Biology, School of Basic Medical Sciences, Peking University Health Science Center, Beijing 100191, China | |
| 2Laboratory of Cardiovascular Bioactive Molecule, School of Basic Medical Sciences, Peking University Health Science Center, Beijing, 100191, China | |
| 3Key Laboratory of Molecular Cardiovascular Science, Ministry of Education, Beijing 100191, China | |
| Corresponding Author : | YF Qi Department of Pathogen Biology School of Basic Medical Sciences Peking University Health Science Center 38 Xueyuan Rd Haidian District, Beijing 100191, P. R. China Tel: +8610 82805627 E-mail: yongfenqi@163.com |
| Received: November 16, 2015 Accepted: November 30, 2015 Published: November 30, 2015 | |
| Citation: Rong-Yu Y, Feng-qi Y (2015) Progress in Treatment and Prevention of Trichinellosis. J Infect Dis Ther 3:251. doi:10.4172/2332-0877.1000251 | |
| Copyright: © 2015 Rong-Yu Y, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
| Related article at Pubmed, Scholar Google | |
Visit for more related articles at Journal of Infectious Diseases & Therapy
Abstract
Trichinellosis is a foodborne parasitic zoonosis caused by eating raw or uncooked meat of animals infected with Trichinella species. The disease is widely distributed all over the world. It is a public health hazard by affecting humans and represents an economic problem in porcine animal production and food safety. Drugs used to treat Trichinellosis include anthelmintics and glucocorticosteroids. Benzimidazole derivatives or adding excipients, medical plant extracts and some biological agents have shown good insecticidal effects. Preventing infection is crucial for combating human and mammal trichinellosis. Designing effective vaccines and developing promising probiotics may be future preventive strategies against infection with Trichinella spiralis infection. This paper reviews new progress in the treatment and prevention of trichinellosis.
| Keywords |
| Trichinellosis; Trichinella spiralis ; Treatment; Prevention |
| Introduction |
| Trichinellosis is a foodborne parasitic zoonosis widely distributed all over the world in most climates, except for deserts, with a burden of approximately 10,000 people per year and a 0.2% mortality rate [1,2]. It transmitted to humans by the consumption of raw or undercooked meat contaminated with nematodes of Trichinella species [3]. Trichinellosis is a public health hazard by affecting human patients and represents an economic problem in porcine animal production and food safety [4]. In the international ranking of foodborne parasites, Trichinella spiralis (T. spiralis ) was among the top 10. T. spiralis is the most pathogenic and prevalent species causing trichinellosis in humans [5]. |
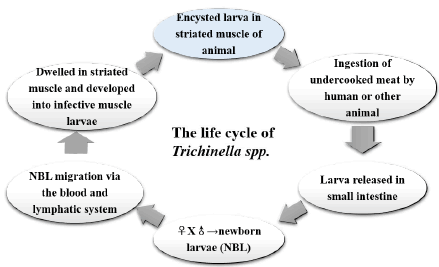
| Infection of humans occurs with the ingestion of Trichinella larvae that are encysted in muscle tissue of meat from domestic or wild animals. After ingestion of contaminated meat, infective muscle larvae (ML) are released into the stomach of the host. The ML invade the intestinal columnar epithelial cells and undergo four moults to reach sexual maturity. The adult worms mate within the intestinal epithelium, and produce newborn larvae (NBL). In 2 to 3 weeks, the fertilized females release ~1500 NBL that penetrate the intestine and migrate via the blood and lymphatic system to skeletal muscle. The NBL then develop into infective ML, and the infected muscle cells develop into nurse cells that contain a collagen capsule [3]. The adult worms live in the intestinal mucosa and persist for 10 to 20 days in mice and rats or 4 to 6 weeks in humans [6]. The ML encapsuled in muscle fibers remain for months to years. The life cycle of Trichinella spp. summarized in Figure 1. |
| Trichinella infection in the human host can be divided into two phases: an intestinal (or enteral) phase and a muscular (or parenteral or systemic) phase. Low-intensity infection can remain asymptomatic, but parasite burdens greater than a few hundred larvae can initially cause gastroenteritis associated with diarrhea and abdominal pain approximately 2 days postinfection (p.i.) (intestinal acute phase of disease). Subsequently, migrating larvae and their metabolites provoke an immediate reaction, with an inflammatory and allergic response. Pyrexia, eyelid or facial edema, myalgia, and eosinophilia are the most prominent manifestations, occasionally complicated by myocarditis, thromboembolic disease, and encephalitis. Months or even years after the acute stage, chronic trichinellosis may yield persistent formication, numbness, and excessive sweating as well as impaired muscle strength and conjunctivitis, which may persist up to 10 years p.i. in people not treated early during the acute phase of infection [4,6]. |
| Therefore, the administration of efficacious anthelmintic drugs at the stage of intestinal invasion or in the acute phase is crucial for effective therapy. In addition, because of the predominantly zoonotic importance of infection, the main efforts in many countries have focused on the control or elimination of Trichinella from the food chain [4]. Domestic pork and related products remain the most important source of Trichinella infection in humans, especially when pigs are raised under free-range or backyard production conditions. Therefore, the prevention and treatment of humans and animals are all important. In following text, we review new progress in the treatment and prevention of trichinellosis. |
| Therapies |
| Anthelmintics |
| Anthelmintics, primarily albendazole and mebendazole, are the principal drugs for the treatment of trichinellosis. The mode of action is inhibition of microtubule polymerization by selectively binding to the β-tubulin monomer of the parasite, with little effect on binding the tubulin of the mammalian host [7]. The recommended dose of albendazole is 400 mg twice daily for 8 to 14 days; for mebendazole, it is 200 to 400 mg three times a day for 3 days, followed by 400 to 500 mg three times a day for 10 days. Both treatment schemes are suitable for adults and children; however, they are contraindicated during pregnancy and are not recommended in children less than 2 years old. Albendazole has a slight advantage in that for most patients, recommended plasma levels are achieved and thus do not require monitoring, whereas mebendazole plasma levels can vary considerably among patients and might require individual monitoring and dosing [4]. |
| The effectiveness of albendazole or mebendazole strongly depends on the time of administration; application at the early stage of infection is effective. Unfortunately, for most infected people, the diagnosis is considered only several weeks after the infection, when the larvae have already established themselves in the muscles. As a general rule, the later a treatment is prescribed, the greater the probability that the infected person will already harbor viable larvae in their muscles, which can then survive for years despite treatment, with possible persistent myalgia [8]. |
| For pregnant women and children, pyrantel can be used. The mode of action is inhibition of cholinesterase, which make the helminth neuromuscular depolarization, spasm and paralysis. Then, the helminth loses the ability of adhesion intestinal wall and was expelled from the body. Pyrantel (Combantrin) is given in a single dose of 10 to 20 mg/kg body weight, repeated for 2 to 3 days. However, it is active against only worms in the gut and has no effect against NBL and ML [4]. The main features of these anthelmintics summarized in Table 1. |
| Since the middle of the 1970s, the new class of macrocyclic lactones has revolutionized the animal health market. Ivermectin, abamectin, eprinomectin, doramectin, milbemycin, moxidectin, and selamectin belong to this class. In human medicine, only ivermectin has been used against some nematodes. The gamma-aminobutyric acid receptor of Trichinella as a target for ivermectin’s mode of action displayed sufficient binding activity in vitro to suggest further investigation. El- Azzouni demonstrated some effect of ivermectin against experimental T. spiralis infection in Swiss albino mice at an early stage of infection [9]. Soliman et al. found an efficacy of 97.75% and 86.23% for dormectin in eliminating mature worms and migrating larvae, respectively. Ivermectin had an efficacy of 94.99% and 83.85%, respectively. Both drugs failed to inhibit the encysted larvae in the diaphragm [10]. Therefore, new drugs for ML are urgently needed. |
| Benzimidazole derivatives or excipients |
| Albendazole and mebendazole are widely used in the chemotherapy of human and animal trichinellosis, with a high therapeutic index and low toxicity. Nevertheless, their effectiveness is limited by poor water solubility (1 μg/mL at 25â�¦C) and the consequent low bioavailability, producing in some cases an unpredictable therapeutic response [11]. Chemotherapy failure has been reported in trichinellosis patients treated with mebendazole [8]. A very active area of research has focused on the development of new pharmaceutical formulations to increase the solubility, dissolution rate, and bioavailability of agents used to treat trichinellosis. Solid dispersion, polymeric microencapsulation and complexation with cyclodextrins (CDs) are good choice [1]. |
| The use of CDs as suitable pharmaceutical excipients in liquid and solid formulations is attractive to enhance the dissolution rate and bioavailability of poorly soluble drugs. Hydroxypropyl-β-CD (HP-β- CD) is more water-soluble and toxicologically benign than the natural β-CD, and its most important use is the intravenous route. The benzimidazole derivative 5-(2,3-dichlorophenoxy)-1-methyl-2- (trifluoromethyl)-1H-benzimidazole was synthesized and tested in vitro against T. spiralis ML. It showed higher activity in vitro than albendazole (80% vs 67%) but had low efficacy in vivo (46% reduction of ML load). Its activity was significantly increased (84%) in complex with HP-β-CD. T. spiralis ML recovered from mice treated with this complex showed motility loss and cuticle damage [12]. The complex of 6-chloro-5-(1-naphthyloxy)-2-(trifluoromethyl)-1H-benzimidazole (RCB20) and HP-β-CD showed better activity than RCB20 alone against the adult and ML phase of T. spiralis [13]. Randomly methylated-β-CD inclusion complexes also greatly increased the dissolution efficiency of albendazole and improved the in vivo therapeutic activity for trichinellosis [1]. The albendazole-β-CD citrate inclusion complex achieved 90% reduction in muscle burden as compared to 79% with albendazole and increased the bioavailability and effectiveness of albendazole against encapsulated Trichinella larvae [11]. |
| García et al. evaluated the in vivo antiparasitic activity of three novel solid microencapsulated formulations, designed to improve albendazole dissolution rate, in a murine model of trichinellosis. The microparticulate formulations significantly decreased ML burden measured in the parenteral phase. Moreover, two of the three microencapsulated formulations strongly and consistently reduced worm burden [14]. From these results, developing new derivatives or suitable pharmaceutical excipients could increase the effectiveness of anthelmintics. |
| Glucocorticosteroids |
| Worsening symptoms in patients during treatment with antihelminthic therapy for trichinellosis is common. Prednisone is given with antihelminthic drugs to prevent worsening symptoms and to shorten the symptomatic period [15]. Administered at 30 to 60 mg/day for 10 to 15 days for severe symptoms is the standard chemotherapy. Prednisone was shown to be safe and to alleviate symptoms due to active tissue larvae [4]. |
| Medical plant extracts or other biological agent |
| Because of the increasing spread of anthelminthic resistance and/or decreasing activity against encapsulated larval stages of parasites, there has been a growing interest in developing newer anthelminthics from medicinal plants, particularly those used in traditional medicine in many parts of the world. Yadav et al. showed that leaf extracts of Lasia spinosa possess significant anthelminthic efficacy against the adult stages and migrating larvae of T. spiralis , but the encysted ML are comparatively less sensitive to L. spinosa leaf extract treatment [16]. |
| Artemisia species have traditionally been used as anthelmintics, and different extracts and components (such as artemisinin from Artemesia annua ) have been reported as plant and animal nematocidal agents. The essential oils (Eos) from two cultivated A. absinthium populations showed strong ex vivo activity against the infective ML of T. spiralis , with a reduction in infectivity between 72% and 100% at 0.5 to 1 mg/ml and without cytotoxicity to mammalian cells. Moreover, the in vivo activity of the Eos against T. spiralis showed a 66% reduction of intestinal adults [17]. |
| Yu et al. found that 1% taurine supplementation in drinking water for mice infected with T. spiralis could alleviate the burden of intestinal adult worms on days 7 and 10 p.i. and reduce the formation of infective ML in striated muscle [18]. Wang et al. found that siRNAmediated silencing of nudix hydrolases expression in T. spiralis significantly reduced the larval infectivity, development, and survival in the host [19]. These findings all imply the potential for treatment and prevention of trichinellosis. |
| Prevention |
| All these medications are active against adult worms in the gut and are ineffective against the larvae embedded in tissues, so there is serious concern about the currently used therapeutic agents. Therefore, preventing the infection in the first place can be considered a promising tool against trichinellosis [5]. Significant efforts have been made to design effective vaccines against T. spiralis infection. The main obstacle seems to be related to the complicated antigenic components of the parasite. |
| Vaccines |
| Induction of therapeutic and protective responses in T. spiralis infection should activate both innate and acquired immunological mechanisms to block the establishment of the parasite in the host. The life cycle of T. spiralis differs from that of other nematodes because all of the developmental stages occur in the same host. However, the nematode's antigens vary during the different developmental stages within the host [20]. In trichinellosis, host-parasite interplay is complicated by the T. spiralis life cycle, which includes a diversity of stage-specific antigens, immune evasion strategies and modulatory effects on host responses. Hence, achieving effective protective responses is challenging [21]. Eliciting high levels of protection or sterile immunity with only a single antigen is difficult. Therefore, vaccination approaches against T. spiralis primarily with murine experimental models have involved a wide range of strategies that include whole extracts and excretory-secretory products, recombinant proteins, epitope-peptides and DNA vaccines, along with various adjuvants and different routes of antigen administration. Table 2 summarized currently researched typical vaccines used in experimental T. spiralis infection in animal model. The detailed strategies are reviewed in reference 21. |
| Probiotics |
| The potential use of probiotics to control enteric infections has generated tremendous interest in the last decade. Probiotics can prevent enteric infections by three major strain-specific mechanisms relying on modulation of the intestinal environment, immune modulation and secretion of active molecules [22]. Lactobacilli are the most commonly used probiotics. The inherent biological features allow them to predominate and overcome the potential pathogens infecting the human digestive tract. Lactobacillus casei is the most popularly used probiotic for protection against T. spiralis infection. Several strains of L. casei that have proven efficacy against trichinellosis include L. casei ATCC 7469, L. casei ATCC469 and L. casei Shirota strains [23-25]. L. casei administered to mice before T. spiralis infection protected against T. spiralis infection, both in adult worms in the intestine and ML, ranging from 78.6% to 100%, depending on the challenging dose. In addition, immunoglobulin G (IgG) and IgA antibody levels and interleukin 4 levels increased significantly in L. casei -treated mice as compared with controls [24]. Temsahy et al. reported the protective efficacy of new safe probiotic strains, L. plantarum P164 and L. acidophilus P110, isolated from faeces of breastfed infants, against experimental trichinellosis. L. plantarum P164 was superior in parasitologic and histopathologic improvement against T. spiralis infection [5]. This promising probiotic strain may be a safe natural protective agent against T. spiralis infection. |
| Conclusion |
| Since its discovery in 1835 by Owen and Piaget, trichinellosis has not been eradicated. There are many factors involved: the T. spiralis complex life cycle, its lack of host specificity, different structural forms and diverse ecological niches throughout the life cycle. Additionally, the acute stage of infection often has no pathognomonic signs and in most infected people, the diagnosis is concluded long after the infection is established, when larvae have invaded the skeletal muscle cells. With the growing evidence of the poor effect of drugs against larvae and the difficulties in producing vaccines, new alternatives are needed to control trichinellosis. Clearly, more basic research on the pathogenic mechanism and molecular nature and function of Trichinella antigens is needed to provide insights into new strategies. In addition, emphasis should be given to applications of probiotics, medical plant extracts and other biological agents. |
| Acknowledgement |
| This work was supported by the Leading Academic Discipline Project of Beijing Education Bureau (BMU20110254) and the National Natural Science Foundation of China (no. 30901247). |
| References |
References
- García A, Leonardi D, Vasconi MD, Hinrichsen LI, Lamas MC (2014) Characterization of albendazole-randomly methylated-β-cyclodextrin inclusion complex and in vivo evaluation of its antihelmitic activity in a murine model of Trichinellosis. PLoS One 9: e113296.
- Ashour DS, Elbakary RH (2011) Pathogenesis of restricted movements in trichinellosis: an experimental study. ExpParasitol 128: 414-418.
- Tang B, Liu M, Wang L, Yu S, et al. (2015) Characterisation of a high-frequency gene encoding a strongly antigenic cystatin-like protein from Trichinellaspiralis at its early invasion stage. Parasit Vectors 8: 78.
- Gottstein B, Pozio E, Nöckler K (2009) Epidemiology, diagnosis, treatment, and control of trichinellosis. ClinMicrobiol Rev 22: 127-145, Table of Contents.
- Temsahy MM, Ibrahim IR, Mossallam SF, Mahrous H, Bary AA, et al. (2015) Evaluation of newly isolated probiotics in the protection against experimental intestinal trichinellosis. Vet Parasitol .
- Sun GG, Wang ZQ, Liu CY, Jiang P, Liu RD, et al. (2015) Early serodiagnosis of trichinellosis by ELISA using excretory-secretory antigens of Trichinellaspiralis adult worms. Parasit Vectors 8: 484.
- Aguayo-Ortiz R, Méndez-Lucio O, Medina-Franco JL, Castillo R, Yépez-Mulia L, et al. (2013) Towards the identification of the binding site of benzimidazoles to β-tubulin of Trichinellaspiralis: insights from computational and experimental data. J Mol Graph Model 41: 12-19.
- Pozio E, Sacchini D, Sacchi L, Tamburrini A, Alberici F (2001) Failure of mebendazole in the treatment of humans with Trichinellaspiralis infection at the stage of encapsulating larvae. Clin Infect Dis 32: 638-642.
- el-Azzouni MZ (1997) Effect of ivermectin on experimental trichinosis. J Egypt SocParasitol 27: 331-340.
- Soliman GA, Taher ES, Mahmoud MA (2011) Therapeutic efficacy of Dormectin, Ivermectin and Levamisole against different stages of Trichinellaspiralis in rats. TurkiyeParazitolDerg 35: 86-91.
- Codina AV, García A, Leonardi D, Vasconi MD, Di Masso RJ, et al. (2015) Efficacy of albendazole:β-cyclodextrin citrate in the parenteral stage of Trichinellaspiralis infection. Int J BiolMacromol 77: 203-206.
- Matadamas-Martínez F, Nogueda-Torres B, Hernández-Campos A, Hernández-Luis F, Castillo R, et al. (2013) Analysis of the effect of a 2-(trifluoromethyl)-1H-benzimidazole derivative on Trichinellaspiralis muscle larvae. Vet Parasitol 194: 193-197.
- Rojas-Aguirre Y, Yépez-Mulia L, Castillo I, López-Vallejo F, Soria-Arteche O, et al. (2011) Studies on 6-chloro-5-(1-naphthyloxy)-2-(trifluoromethyl)-1H-benzimidazole/2-hydroxypropyl-ß-cyclodextrin association: Characterization, molecular modeling studies, and in vivo anthelminthic activity. Bioorg Med Chem 19: 789-797.
- García A, Barrera MG, Piccirilli G, Vasconi MD, Di Masso RJ, et al. (2013) Novel albendazole formulations given during the intestinal phase of Trichinellaspiralis infection reduce effectively parasitic muscle burden in mice. ParasitolInt 62: 568-570.
- Shimoni Z, Klein Z, Weiner P, Assous MV, Froom P (2007) The use of prednisone in the treatment of trichinellosis. Isr Med Assoc J 9: 537-539.
- Yadav AK, Temjenmongla (2012) Efficacy of Lasiaspinosa leaf extract in treating mice infected with Trichinellaspiralis. Parasitol Res 110: 493-498.
- García-Rodríguez JJ, Andrés MF, Ibañez-Escribano A, Julio LF, Burillo J, et al. (2015) Selective nematocidal effects of essential oils from two cultivated Artemisia absinthium populations. Z Naturforsch C 70: 275-280.
- Yu YR, Liu XC, Zhang JS, Ji CY, Qi YF (2013) Taurine drinking attenuates the burden of intestinal adult worms and muscle larvae in mice with Trichinellaspiralis infection. Parasitol Res 112: 3457-3463.
- Wang ZQ, Zhang SB, Jiang P, Liu RD, Long SR, et al. (2015) The siRNA-mediated silencing of Trichinellaspiralisnudix hydrolase results in reduction of larval infectivity. Parasitol Res 114: 3551-3557.
- Gu Y, Wei J, Yang J, Huang J, Yang X, et al. (2013) Protective immunity against Trichinellaspiralis infection induced by a multi-epitope vaccine in a murine model. PLoS One 8: e77238.
- Ortega-Pierres G, Vaquero-Vera A, Fonseca-Liñán R, Bermúdez-Cruz RM, Argüello-García R (2015) Induction of protection in murine experimental models against Trichinellaspiralis: an up-to-date review. J Helminthol 89: 526-539.
- Travers MA, Florent I, Kohl L, Grellier P (2011) Probiotics for the control of parasites: an overview. J Parasitol Res 2011: 610769.
- Randazzo V, Costamagna SR (2005) Effect of oral administration of probiotic agents on Trichinellaspiralis infected mice. Rev Patol Trop 34: 129-135.
- Martínez-Gómez F, Fuentes-Castro BE, Bautista-Garfias CR (2011) The intraperitoneal inoculation of Lactobacillus casei in mice induces total protection against Trichinellaspiralis infection at low challenge doses. Parasitol Res 109: 1609-1617.
- Martínez-Gómez F, Santiago-Rosales R, Ramón Bautista-Garfias C (2009) Effect of Lactobacillus caseiShirota strain intraperitoneal administration in CD1 mice on the establishment of Trichinellaspiralis adult worms and on IgA anti-T. spiralis production. Vet. Parasitol 162: 171-175.
Tables and Figures at a glance
| Table 1 | Table 2 |
Figures at a glance
 |
| Figure 1 |
Relevant Topics
- Advanced Therapies
- Chicken Pox
- Ciprofloxacin
- Colon Infection
- Conjunctivitis
- Herpes Virus
- HIV and AIDS Research
- Human Papilloma Virus
- Infection
- Infection in Blood
- Infections Prevention
- Infectious Diseases in Children
- Influenza
- Liver Diseases
- Respiratory Tract Infections
- T Cell Lymphomatic Virus
- Treatment for Infectious Diseases
- Viral Encephalitis
- Yeast Infection
Recommended Journals
Article Tools
Article Usage
- Total views: 15648
- [From(publication date):
December-2015 - Nov 23, 2024] - Breakdown by view type
- HTML page views : 14768
- PDF downloads : 880
