Progress in the Study of the Role of ncRNA on Osteogenic Differentiation of Human Periodontal Stem Cells
Received: 01-May-2024 / Manuscript No. cmb-24-136814 / Editor assigned: 04-May-2024 / PreQC No. cmb-24-136814 / Reviewed: 18-May-2024 / QC No. cmb-24-136814 / Revised: 25-May-2024 / Manuscript No. cmb-24-136814 / Published Date: 30-May-2024
Abstract
Repair of bone defects, orthodontic tooth movement, and alveolar bone remodelling involve bone, and the osteogenic differentiation potential of human periodontal stem cells closely affects alveolar bone repair and bone regeneration. The ncRNA (non-coding RNA) play an important role in the regulation of osteogenic differentiation genes of human periodontal stem cells, which is expected to be a new strategy for solving clinical bone-related problems in dentistry. In this paper, we review the progress related to the role of ncRNA on osteogenic differentiation of human periodontal stem cells.
keywords
ncRNA; Human periodontal stem cells; Osteogenesis; Orthodontics
Introduction
Alveolar bone defect has always been a common problem in oral cavity. Bone defect caused by maxillofacial trauma and alveolar bone resorption poses certain difficulties to maxillofacial surgery and periodontal treatment. At the same time, bone remodelling also affects the efficiency of orthodontic tooth movement. Stem cells are a research hotspot in tissue engineering in recent years. Stem cells are usually derived from bone marrow. Because of their self-renewal properties, plasticity of differentiation potential, immunosuppressive and anti-inflammatory functions, stem cells have gradually become the key to cell therapy and are applied in regenerative medicine. However, the difficulty of obtaining stem cells limits their research applications. Therefore, it is essential to seek efficient and large number of stem cell sources. human periodontal ligament stem cells (hPDLSCs), as a kind of undifferentiated mesenchymal cells in periodontal ligament, have the potential to differentiate into osteoblasts, adipocytes, chondrocytes and other types of cells in vitro [1].
Therefore, it is widely used in experimental research. PDLSCs are widely found in periodontal tissues and have the potential of osteogenic differentiation and can retain the ability to generate cemento-like tissue when transplanted in vitro. Feng et al. reported that the transplantation of autologous human PDLSCs into the body can obtain a stable regeneration effect, and PDLSCs can be used as an alternative therapy to repair defects with stable effects and few ethical complications. More importantly, PDLSCs are easy to obtain [2]. PDLSCS can be collected by minimally invasive separation of periodontal tissues during clinical flap surgery, root planning and standard scaling, and can also be obtained as clinical discarded materials. The process of collecting PDLSCs from clinic is minimally invasive or even non-invasive, and the wound is too small or even no wound so that the donor can heal without scar. The unique advantages of ethics may be an important reason for choosing PDLSCs for clinical application in the future [3].
Many researchers have focused on using the osteogenic differentiation potential of hPDLSCs to repair alveolar bone defects, providing a promising strategy for the treatment of clinical bone defects. At present, a variety of measures to promote the osteogenic differentiation of hPDLSCs have been studied, such as the synthesis of doxycycline doped polymer nanoparticles, copper (II) ion loaded mineralized diatoms (Cu-DBs) [4] and other materials to promote the osteogenic differentiation of hPDLSCs. There are also studies using existing materials such as graphene quantum dots, gold nanocomposites (AuNCs), and natural substances such as crocin and irisin to promote osteogenesis. It is worth noting that in recent years, the measures to promote the osteogenic differentiation of hPDLSCs under microscopic conditions have been continuously explored, among which the research based on genetic engineering has been greatly developed. Non-coding (nc) RNA is a class of RNA without protein coding function. ncRNA accounts for about 98% of the whole genome and regulates a variety of physiological processes by controlling the transcriptional, post-transcriptional and epigenetic aspects of gene expression, such as the occurrence and development of periodontitis, cleft lip and palate and oral cancer. Therefore, the research based on ncRNA to promote the osteogenic differentiation of hPDLSCs and repair alveolar bone defects has gradually come into view [5-10].
ncRNAs can be subdivided into micrornas (miRNAs/miRs), long ncRNAs (lncRNAs), and circular Rnas (circRNAs). MicroRNA (miRNA), a highly conserved endogenous non-protein-coding RNA of about 19-25 nucleotides (nt) in length, can degrade or inhibit gene expression at the post-transcriptional level by binding to the 3' untranslated region (3'-UTR) of the target mRNA [11]. lncRNAs are a class of ncrnas with a length of >200 nucleotides, which can act as an epigenetic mechanism to regulate miRNA function, acting as miRNA "sponges", thereby inhibiting miRNA targets and causing additional levels of post-transcriptional regulation. It can also bind substrates through its own nucleotide sequence or folded secondary structure and regulate gene expression through transcription. Circular RNA (circRNA) is a newly discovered RNA involved in the regulation of gene expression from transcription to translation. It has high stability and acts as a "sponge" when combined with complementary sequences on miRNA. It can also bind and change specific regulatory proteins or translate into small regulatory peptides to regulate cell development, growth, proliferation and differentiation [12-15].
ncRNA are actively involved in the regulation of osteogenic genes in human periodontal ligament derived cells, and can also be developed as clinical therapeutic drugs. For example, LncRNA Nron is considered to be a potential therapeutic drug for osteoporosis, and it was recently reported to significantly inhibit bone resorption in mouse periodontitis model [16]. The study of lncRNA, miRNA, circRNA and mRNA during the osteogenic induction of PDLSCs will provide a prospective perspective for PDLSCS-based molecular therapy of alveolar bone regeneration. This review aims to explore the ncRNA that may play a role in the osteogenic differentiation of human periodontal ligament stem cells under a variety of different conditions. It is expected to be applied in oral and maxillofacial surgery, periodontology, and orthodontics, which is of great significance for the precise regulation of periodontal tissue regeneration in the future [17].
ncRNA that promotes osteogenic differentiation of hPDLSCs in bone defect repair
miRNA promoting osteogenic differentiation of PDLSCs
Studies have shown that miRNA plays an important role in the osteogenic differentiation of PDLSCs, and abnormal miRNA expression has an important impact on its osteogenic differentiation. In the PDLSCs mineralization induction experiment, Hao et al. found that 116 mirnas were differentially changed, and 31 of them were predicted to have osteogenic-related target genes. In recent years, more and more studies have shown that miRNA has a significant role in promoting the osteogenic differentiation of PDLSCs. For example, miR-589-3p can down-regulate the expression of ATF1, thereby promoting the proliferation and osteogenic differentiation of PDLSCs. Liu Fen et al. found that the expression of miR-30a in PDLSCs induced by enamel matrix protein derivative (EMD) could affect the expression of cathepsin K (CTSK), an important regulator of cementagenic differentiation of PDLSCs. It was confirmed that EMD increased the expression of CTSK by increasing the expression of miR-30a to promote the osteogenic differentiation of PDLSCs [18-21].
miRNA related pathways in regulating osteogenic differentiation of PDLSCs
Wnt/β-catenin signaling pathway: The Wnt pathway is involved in the development of periodontal tissue and regulates bone homeostasis, and is also crucial in the pathogenesis of periodontitis and periodontal regeneration. The expression of miR-30a induced by EMD is related to the activation of wnt signaling pathway, and the activation of Wnt/β-catenin signaling pathway increases the expression of CTSK. The activated Mir-30a-Wnt /β-catenin signaling axis can promote the osteogenic differentiation of PDLSCs. The increase or decrease of miR-17 expression level has also been confirmed to affect the osteogenic effect of classical Wnt signal transduction. Cao F et al. found that knockdown of miR-214 promoted the osteogenic differentiation of PDLSCs. A series of studies have shown that miR-214 directly interacts with the 3' -untranslated region of the β-catenin gene CTNNB1 and inhibits Wnt/β-catenin signaling by inhibiting β-catenin. Thus, the regulation of miR-214/Wnt/β-catenin axis on osteogenic differentiation of PDLSCs was concluded [22-25].
TGF-β/BMP signaling pathway: histone deacetylase (HDAC) is a kind of protease that plays an important role in the structural modification of chromosomes and the regulation of gene expression. Targeting HDAC by miRNA has been proved to regulate the osteogenic differentiation of stem cells. In the TGF-β signaling pathway, HDAC complex acts as a target and cooperates with miRNA to regulate gene expression [26]. Studies have found that miR-20a can promote the osteogenic differentiation of PDLSCs in inflammatory microenvironment by inhibiting the expression of Mir-17-92 family at the transcriptional level through its upstream histone deacetylase 9, and miR-22 targets HDAC6 to promote the osteogenic differentiation of PDLSCS. miR-543 negatively targets the 3-UTR of the transducer of ERBB2 2 (TOB2) and promotes osteogenic differentiation of PDLSCs by targeting HDAC [30].
As an upstream regulator, Smad5 promotes the expression of osteogenic markers such as Runx2 and plays an important role in the process of osteogenic differentiation of various stem cells. Some mirnas target SMAD1/5 induced by TGF-β/BMP signaling to affect osteogenic differentiation. Down-regulation of miR-133a, -133b and -135a found in human dental pulp stem cells (DPSC) leads to the expression of target genes RUNX2 and Smad5, leading to osteogenic differentiation [31]. miR-222-3p and miR-135 can also regulate the osteogenic process of mesenchymal stem cells by targeting Smad5 [26]. The down-regulation of miR-21, miR-17-5p and miR-106b-5p in PDLSCs has been confirmed to directly target Smad5 to mediate osteogenic differentiation. Other studies have shown that miR-24-3p targeting SMAD5 can promote the osteogenic potential of PDLSC. As the most widely studied ncRNA, miRNA targets related osteogenic genes or related pathways in the process of osteogenic differentiation of PDLSCs and plays a very important regulatory role, which is expected to provide new ideas for bone defect repair and periodontal regeneration [32].
lncRNA promoting osteogenic differentiation of PDLSCs
miRNA usually directly binds to target gene mRNA at the post-transcriptional level and inhibits its expression, while lncRNA can regulate the expression of target gene by directly affecting the target gene or acting as a competing endogenous RNA (ceRNA) to affect miRNA to achieve an important regulatory role in the osteogenic differentiation of PDLSCs. In recent years, the role of lncRNA as a competitor of mirNA-target gene loop has received more and more attention, and emerging studies are emerging. Zhang et al. showed that 63 lncrnas were highly expressed in PDLSCs [33] in the lncRNA expression profile of PDLSCs in osteogenic differentiation, among which 149 lncrnas were up-regulated and 169 lncrnas were down-regulated. Some lncrnas, such as POIR, ANCR and XIST, can regulate the osteogenic differentiation of PDLSCs, but the underlying mechanisms still need to be further explored. lncRNA-POIR has been shown to compete with the osteogenic related target gene Fox O1 to bind miR-182 and promote the osteogenic differentiation of PDLSCs through lncRNA-POIR/miR-182 /Fox O1 axis. Jianbin Guo et al. verified the molecular function of LINC00707/miR-490-3p/FOXO1 regulatory network in PDLSC osteogenic differentiation, and provided new ideas for the prevention of bone defects and periodontal bone tissue regeneration and repair [34].
Cytoskeleton regulator RNA (lncRNA CYTOR) positively regulates the expression of SOX3 by competitive binding to miR-11-6512p, thereby promoting the osteogenic differentiation of PDLSC. CYTOR/miR-6512-3p/SOX11 axis provides a promising target for regulating osteogenic differentiation of PDLSC to treat periodontal and bone diseases. Moreover, long non-coding RNA FER1L4 positively regulates osteogenic differentiation of PDLSC through miR-874-3p and VEGFA, and long non-coding RNA X-inactive specific transcript (lncRNA XIST) participated in the competition of miR-214-3p for the osteogenic differentiation of PDLSCs and other studies have proved that lncRNA plays a regulatory role through spongifying miRN [35].
In addition, the direct transcriptional regulation of lncRNA can also affect the osteogenic differentiation of PDLSCs. lncRNA taurine upregulated gene 1 (TUG1) promotes PDLSC osteogenesis by targeting Lin28A. Overexpression of growth arrest-specific transcript 5 (GAS5) can enhance the expression of growth differentiation factor 5 (GDF5) and activate the p38/JNK signaling pathway to promote the phosphorylation of JNK and p38 in hPDLSCs, thereby mediating the osteogenic differentiation of PDLSCs [36].
lncRNA related pathways in regulating osteogenic differentiation of PDLSCs
Wnt/β-catenin signaling pathway: Several signaling pathways play important roles in the process of lncrnas acting as "sponges". For example, lncRNAH19 can promote the osteogenic differentiation of PDLSCs through Wnt/ β-catenin signaling pathway. Down-regulation of lncRNA DANCR promotes osteogenesis of PDLSC through Wnt signaling pathway [37].
It has been suggested that lncRNA-POIR and miR-182 inhibit each other to form a network to regulate FoxO1. FoxO1 increases bone formation in PDLSC by competing with TCF-4 for β-catenin and inhibiting the classical Wnt pathway. As one of the important pathways regulating bone differentiation, wnt/β-catenin signaling pathway has also received more and more attention in the process of related gene function [38].
TGF-β/BMP signaling pathway: lncRNA-PCAT1, which is related to the occurrence and development of osteosarcoma, can act as ceRNA to bind miR-106-5p and activate TGF-β/Smad pathway to promote osteogenic differentiation of PDLSCs. LncNEAT1 may target miR-214-5p/SMAD4 to regulate the osteogenic differentiation of PDLSC. Recently, it was found that the expression of lncRNA metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) and SMAD5 was up-regulated, while miR-93-5p was down-regulated after PDLSC osteogenic induction. Smad5, a member of the TGF-β superfamily, regulates cell proliferation, differentiation and apoptosis. Related experiments have verified that lncRNA MALAT1 can activate BMP2/SMAD signaling pathway through lncRNA MALAT1/miR-93-5p/SMAD5 axis to promote the differentiation of human PDLSC. Similarly, Wu revealed that lncRNA TUG1 accelerates osteogenic differentiation by targeting miRNA-222-3p to regulate Smad2/7 [39].
Whether lncRNA as ceRNA binds miRNA to regulate mRNA indirectly or directly affect the transcription of target genes, the strategy of lncRNA regulating key osteogenic genes and improving the efficiency of osteogenic differentiation of PDLSCs has become a research hotspot in recent years. In the future, more research on the regulatory relationship between lncRNA and miRNA and determination of miRNA target sequences can be carried out to develop small molecule drugs, providing practical solutions for clinical maxillofacial surgery and periodontal diagnosis and treatment [40].
circRNA promoting osteogenic differentiation of PDLSCs
The ends of circRNA are covalently bound together to form a circular single strand, which has gradually become a research hotspot in recent years because of its high stability without the influence of exonuclease. Some analysis showed that 766 circrnas were up-regulated and 690 circrnas were down-regulated after 7 days of osteogenic induction of PDLSCs. The multi-expression of circRNA and the diversity of changes in different stages of osteogenic differentiation make it worthy of in-depth study. Both in vitro and in vivo studies have confirmed that circRNA is involved in regulating the osteogenic differentiation of PDLSC. Earlier studies have shown that antisense cerebellar degeneration-related protein 1 transcript, CDR1as) inhibited the effect of miR-7 by targeting growth and differentiation factor-5 (GDF5) and activated p38 MAPK signaling pathway. Enhancing the phosphorylation of Smad1/5/8 to promote the osteogenic differentiation of PDLSCs, Gu X et al. found that CDR1as maintained the stemness of PDLSCs through miR-7 / KLF4 and participated in the regulation of molecular pathways. Since then, CDR1as has been described as the first circRNA with miRNA sponge activity, which has been confirmed to be applied to the treatment of osteoporosis by the skull defect experiment in nude mice. Therefore, more studies on circRNA with miRNA sponge activity have been gradually carried out [41].
Regulation of circRNA related pathways during osteogenic differentiation of PDLSCs
In PDLSC osteogenic differentiation, 1382 circrnas were combined with 148 mirnas and competed with 744 mrnas for miRNA binding sites. Therefore, circrnas mainly function as ceRNA in vivo. Ye Y et al. found that circFAT1 may participate in the regulation of osteogenic differentiation of PDLSCs by targeting miR-4781-3p and activating SMAD5. It has also been shown that circBIRC6 activates the PI3K/AKT/mTOR signaling pathway through sponge miR-543 to regulate the osteogenic differentiation of PDLSC. It is worth noting that circRNA may be cell-specific in periodontal tissues. The regulatory role of circRNA in PDLSCs is still in its infancy (Table1.). For example, miR-4781-3p, miR-34a, miR-146a, etc., and regulate many cellular pathways, including wnt/β-catenin, MAPK, BMP/Smad, TGF-β, NF-κB, PI3 / AKT and other pathways inducing osteogenesis in vitro and in vivo. Therefore, in the future, with the further study of circRNA derived from periodontal tissue, to explore the target and the exact mechanism of circRNA, and to evaluate its potential as a therapeutic target for alveolar bone defect repair, it is expected to develop circRNA as a suitable inducer to promote the osteogenic differentiation of stem cells in alveolar bone repair, and solve the difficult problems in oral clinical diagnosis and treatment (Tab. 1.) [42].
| Species | Target gene/protein | Mechanism | References |
|---|---|---|---|
| miR-589-3p | ATF1 | Direct targeting | [21] |
| miR-30a | CTSK | Mir-30a-wnt/β-catenin | [22] |
| miR-20a | HDAC9 | Direct targeting | [23] |
| miR-22 | HDAC6 | Direct targeting | [24] |
| miR-543 | TOB2、HDAC | Direct targeting | [25] |
| miR-17 | Smurf1 | miR-17/Smad | [65] |
| miR-24-3p | Smad | miR-24-3p/Smad | [33] |
| miR-200c | IL-6, IL-1β and CCL-5 | Direct targeting | [66] |
| miR-21 | Smad | miR-21/Smad | [30] |
| lncRNA-POIR | miR - 182 | lncRNA-POIR/miR - 182/Fox O1 | [38] |
| LINC00707 | miR-490-3p | LINC00707/miR-490-3p/FOXO1 | [39] |
| CYTOR | miR-6512-3p | CYTOR/miR-6512-3p/SOX11 | [40] |
| lncRNA-FER1L4 | miR-874-3p | FER1L4/miR-874-3p/VEGFA | [41] |
| lncRNA XIST | miR-214-3p | lncRNA XIST/miR-214-3p | [42] |
| lncRNA-DANCR | miR-758 | DANCR/miR-758/Notch2/Wnt | [67] |
| lncRNA-PCAT1 | miR-106-5p | lncRNA-PCAT1/miR-106-5p/Smad | [47] |
| LncNEAT1 | miR-214-5p | LncNEAT1/miR-214-5p/SMAD4 | [48] |
| lncRNA-MALAT1 | miR-93-5p | lncRNA-MALAT1/miR-93-5p/SMAD5 | [49] |
| lncRNA-TUG1 | miR-222-3p | lncRNA-TUG1/miR-222-3p/Smad2/7 | [50] |
| lncRNA-TUG1 | Lin28A | Direct targeting | [51] |
| GAS5 | GDF5 | GAS5/GDF5/p38/JNK | [52] |
| circCDR1as | miR-7 | circCDR1as/miR-7/GDF5/MAPK | [57] |
| circFAT1 | miR-4781-3p | circFAT1/miR-4781-3p/SMAD | [60] |
| circBIRC6 | miR-543 | circBIRC6/miR-543/PI3K / AKT / mTOR | [61] |
ncRNAs that promote osteogenic differentiation of hPDLSCs under orthodontic stress stimulation
Orthodontic treatment is more demanding for alveolar bone, and orthodontic tooth movement mainly depends on bone remodeling. Bone remodeling induced by external mechanical stress can be completed by mechanosensitive cells such as PDLSCs. It has promoted the transformation of personalized orthodontic treatment from laboratory to clinic. In addition to OTM, accelerated periodontal osteogenesis (PAOO), functional orthodontics and rapid maxillary expansion (RME) require bone tissue reconstruction in the process of orthodontic treatment, as well as alveolar bone resorption, bone defracturing and bone fenestration caused by excessive tooth movement and poor root control during the treatment. It is of great significance to maintain and promote bone remodeling during orthodontic treatment. Force-sensitive gene ncRNA combined with stem cells has a high application prospect to accelerate alveolar bone remodeling, accelerate orthodontic tooth movement, reduce complications of orthodontic diagnosis and treatment, and improve the therapeutic effect [43] (Tab. 2).
species |
Target gene/protein | Mechanism | References |
|---|---|---|---|
| miR-21 | ACVR2B | Direct targeting | [74] |
| miR-129-5p | BMP2 | miR-129-5p/Smad | [75] |
| lncRNA-SNHG8 | EZH2 | Direct targeting | [82] |
| lncRNA00638 | miRNA-424-5p | lncRNA00638/miRNA-424-5p/FGFR1 | [83] |
miRNAs that promote osteogenesis of PDLSCs under orthodontic stress conditions
The expression level of miRNA in PDLSC under mechanical force is significantly different from that in normal state [69]. miRNA can act as an important regulator under mechanical force, transforming force into biochemical signals and regulating the formation and remodeling of alveolar bone. Some scholars have listed the miRNA expression profile of PDLSCs induced by orthodontic stress, which further suggests that miRNA under orthodontic stress has a certain impact on the physiological function of stem cells. The study showed that the osteogenic differentiation of PDLSCs was significantly enhanced under dynamic tension with amplitude of 12% and frequency of 0.7 Hz. Wenfang Wang et al. used this as a guide to explore the difference of ncRNA expression in osteogenic differentiation of PDLSCs under mechanical tensile stress. 57 miRNA were found to be differentially expressed in the comparison between the tension group and the control group, and the potential ceRNA network was further delineated to construct the potential regulatory mechanism of osteogenic differentiation of PDLSCs under the effect of orthodontic force. Similarly, Wei FL et al. found that PDLSCs under mechanical stress conditions showed increased osteogenic differentiation, and 26 miRNA were up-regulated and 27 miRNA were down-regulated compared with normal. These results indicated that miRNA was involved in the osteogenic differentiation of PDLSCs under orthodontic mechanical strain [44].
It has been reported that the expression level of miR-21, as a mechanosensitive gene, is significantly increased in PDLSCs induced by mechanical force. Further studies have found that miR-21 mediates the osteogenic differentiation of stretch by directly targeting ACVR2B. Cyclic tensile stress can activate the bone morphogenetic protein 2 (BMP2)/Smad pathway by inhibiting the expression of miR-129-5p, thereby promoting the osteogenic differentiation of PDLSCs, which provides theoretical support for the clinical use of transparent elastic wire and other tensile stress systems to reconstruct alveolar bone. Notably, miRNA expressed in stretched PDLSC were involved in multiple signaling pathways related to osteogenic differentiation, such as MAPK and Wnt, but the underlying mechanisms still need to be further explored [45].
Rapid maxillary expansion (RME) can effectively enlarge the transverse dimension of the upper arch by opening the midpalatal suture in the treatment of maxillary hypoplasia. Recent studies have shown that RME can combine with a miR-21 agonist to achieve rapid bone formation. In the rat model of periodontal accelerated osteogenesis (PAOO), miR-21 agonist and miR-21 antagonist were injected into the labial, palatal and mesial alveolar mucosa of the first molar, and it was found that the OTM distance of the miR-21 agonist group was significantly increased. Studies on the treatment of bone metastasis and other bone resorption diseases with exogenous miR-34a that have entered clinical trials in phase I all indicate that miRNA has a high research value in clinical practice. Therefore, it is expected that the combination of PDLSCs and miRNA will be gradually applied to orthodontic treatment in the future, which will accelerate alveolar bone remodeling during treatment and shorten the duration of treatment [46].
lncRNAs that promote osteogenesis of PDLSCs under orthodontic stress conditions
According to the previously mentioned study, in the lncRNA expression profile of PDLSC under tension stress, 344 lncRNA were differentially expressed in the tension group and the control group. Some scholars have identified 1,339 and 1,426 differentially expressed lncrnas and mrnas under the action of mechanical force, and suggested that lncRNA SNHG12 was involved in the osteogenic differentiation pathway of PDLSCs. A total of 90 lncRNA and 519 mRNA were differentially expressed in PDLSCs under compressive stress, among which 72 lncRNA were up-regulated and 18 were down-regulated. The expression profile of lncRNA was significantly changed under compressive stress. All these evidences confirmed that lncRNA plays an indispensable role in the osteogenic differentiation of PDLSCs under external mechanical stimulation.
Under the action of mechanical force, the reduction of lncRNA small nucleolar RNA host gene 8 (SNHG8) in PDLSCs promotes osteogenic differentiation. In addition to regulating osteogenic differentiation of PDLSCs through epigenetic pathway, stress can make lncRNA adsorb miRNA to play a role. Studies have confirmed that LncRNA00638 activates the lncRNA00638/miRNA-424-5p/FGFR1 axis to regulate the osteogenic differentiation of PDLSCs by adsorptive miR-424-5p, which may provide a potential therapeutic target for bone reconstruction or improving osteogenic ability during orthodontic treatment [47].
- circRNAs that promote PDLSCs osteogenesis under orthodontic stress conditions
The study of circRNA and osteogenic differentiation of PDLSCs is in its infancy. Some researchers have found that circrnas respond to MF in PDLSC, inducing 2678 differentially expressed circrnas in stretched PDLSC. circRNA may directly or indirectly regulate miRNA-mediated osteogenic differentiation of stem cells. A number of studies have shown that specific circRNA can be used as ceRNA to promote osteogenic differentiation of PDLSC under mechanical force. The relationship between the expression of circRNA and PDLSCs osteogenesis under stress conditions may regulate OTM process and alveolar bone remodelling, which is also a field that needs further exploration in the future [48].
ncRNA vectors
Notably, although ncRNAs are powerful interventions in bone repair processes, the delivery method and delivery efficiency of ncRNA-based therapies remain to be addressed due to their vulnerability to degradation and inherent instability. At present, biological scaffolds, viral vectors, non-viral vectors, exosomes and other delivery methods have been further studied. Some studies have used a cylindrical porous ceramic hydroxyapatite material of 3mm×5mm as a scaffold carrying PDLSCs to be implanted into nude mice to verify that mir-30a promotes ectopic osteogenesis of PDLSCs in vivo. Some scholars used 40mg HA-TCP ceramic particles carrying PDLSCs to implant on the dorsal surface of 8-week-old immunocompromised mice to verify the mutual inhibition between HDAC9 and miR-17 to regulate the osteogenic differentiation of human periodontal ligament stem cells under inflammatory conditions. Exosomes derived from human bone marrow mesenchymal stem cells and macrophages have been confirmed to promote the osteogenic differentiation of hPDLSC and inhibit the development of periodontitis. In the future, it is also expected to carry ncRNA to further regulate the process of osteogenic differentiation in vivo [49].
Bovine serum albumin coated calcium-sirna Nano composites optimized by small interfering RNA (siRNA) carrier materials have been evaluated as biocompatible for PDLSCs and are expected to be used as delivery vectors in stem cell therapy in the future. An activated scaffold composed of miR-21 and Bio-oss particles can continuously and stably promote alveolar bone regeneration in rats, which is expected to be combined with stem cells for clinical use in the future. In addition, some biological materials such as nano-micro hybrid surface hydroxyapatite bioceramic mnHA, PLA/PLGA core/shell structure fiber framework, collagen /PLA/ nano-HA are considered to be efficient carriers for PDLSCs delivery. Surface modification of micro/nano materials is an effective way to further enhance the regulation of cell differentiation and cell response by scaffolds, but its combined application with gene therapy remains to be developed [50].
Summary
Gene therapy combined with stem cells provides a new direction for clinical practice, and is expected to solve the difficult problems faced by maxillofacial surgery, periodontology and orthodontics. ncRNA is a key regulator of the function of stem cells, the physiological activities of the body, and the occurrence and development of various oral diseases (such as periodontitis, cleft lip and palate, and oral cancer). miRNA, lncRNA, and circRNA can all play an important role in the osteogenic differentiation of PDLSCs. It should be noted that circRNA and lncRNA can regulate one or more miRNA/mRNA. A miRNA may interact with one or more circRNA and lncRNA, and they form a complex regulatory network ceRNA. Promoting or inhibiting the osteogenic differentiation of PDLSCs. However, the related research on ceRNA network needs a large number of experiments to verify, and the regulatory mechanism of related genes on PDLSCs osteogenesis needs to be further explored. Osteogenesis-related ncRNA of PDLSCs can be used as biomarkers for oral diagnosis and treatment. Targeted osteogenic differentiation of PDLSCs is expected to be achieved by regulating the expression level of osteogenesis-related genes, which provides candidate targets for the development of drugs for the treatment of bone defects in oral and maxillofacial surgery, periodontal disease, and orthodontic treatment complications (Fig. 2.). It has high research value and application prospect. In the future, with the improvement of delivery systems and the in-depth study of related genes, based on the ncRNA related to osteogenic differentiation of PDLSCs, the development of drugs that target one or more effector molecules in the circRNA/lncRNA-miRNA-mRNA network will bring further development to human alveolar bone repair and remodelling. It makes the clinical diagnosis and treatment of stomatology more easy (Fig. 2.).
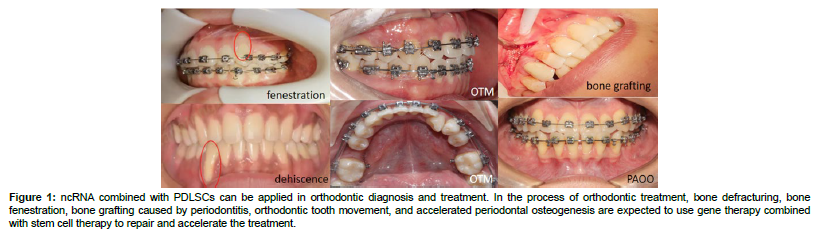
Figure 1: ncRNA combined with PDLSCs can be applied in orthodontic diagnosis and treatment. In the process of orthodontic treatment, bone defracturing, bone fenestration, bone grafting caused by periodontitis, orthodontic tooth movement, and accelerated periodontal osteogenesis are expected to use gene therapy combined with stem cell therapy to repair and accelerate the treatment.
Figure 2: Clinical application of periodontal ligament stem cell transplantation therapy based on gene modification of non-coding RNA. Genetic modification of non-coding Rnas in periodontal ligament stem cells can regulate the ability of osteogenic differentiation. Gene therapy combined with stem cell therapy is transferred to the bone defect site through a carrier to repair or remodel the bone defect. miRNAs: microRNAs. lncRNAs: long non-coding RNAs; circRNAs: circular RNAs; hPDLSCs: human periodontal ligament stem cells.
Acknowledgement
All authors confirm and have no objections.
Author Contributions
Conceptualization, Qi Su; Data curation, Qi Su; Formal analysis, Qi Su and fengqiong Huang; Funding acquisition, zhiying Zhou; Investigation, zhiying Zhou; Methodology, Qi Su and fengqiong Huang; Project administration, zhiying Zhou; Resources, Zhiying Zhou; Software, Qi Su; Supervision, Qi Su; Validation, Qi Su; Visualization, Qi Su; Writing, Qi Su.
Financial Disclosure or Funding
This research was funded by Supported by Guangzhou Science and Technology Plan Project, China (2024A03J0821).
Acknowledgments
The authors would like to thank Dr. Zhiying Zhou for helpful discussions on this work.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
ARRIVE Guidelines Statement
All methods were carried out in accordance with relevant guidelines and regulations in followed ARRIVE guidelines.
References
- Lei T, Wang J, Liu Y (2021) Proteomic profile of human stem cells from dental pulp and periodontal ligament. J Proteomics 245: 104-280.
- Moura SR, Fernandes MJ, Santos SG (2023) Circular RNAs: Promising Targets in Osteoporosis. Curr Osteoporos Rep21: 289-302.
- Feng F, Akiyama K, Liu Y (2010) Utility of PDL progenitors for in vivo tissue regeneration: a report of 3 cases. Oral Dis 16: 20‐8.
- Qiu X, Feng C, Wang W (2023) Copper-deposited diatom-biosilica enhanced osteogenic potential in periodontal ligament stem cells and rat cranium. J Biomed Mater Res B Appl Biomater 111: 1286-1298.
- Gao W, Liang Y, Wu D (2023) Graphene quantum dots enhance the osteogenic differentiation of PDLSCs in the inflammatory microenvironment. BMC Oral Health 23: 331.
- Zong C, Van Holm W, Bronckaers A (2023) Biomimetic Periodontal Ligament Transplantation Activated by Gold Nanoparticles Protects Alveolar Bone. Adv Healthc Mater 12: 230-328.
- Gu Y, Bai Y (2023) Osteogenic effect of crocin in human periodontal ligament stem cells via Wnt/β-catenin signaling. Oral Dis 30: 1429-1438.
- Wu S, Wang J, Liu L (2023) Recombinant Irisin Protects Against Alveolar Bone Destruction During Orthodontic Tooth Movement. Inflammation 46: 1106-1117.
- Zhang Y, Zhang J, Xu Z (2023) Regulation of NcRNA-protein binding in diabetic foot. Biomed Pharmacother 160: 114-361.
- Naqvi AR, Slots J (2021) Human and herpesvirus microRNAs in periodontal disease. Periodontol 87: 325-39.
- Yoshioka H, Suzuki A, Iwaya C (2022) Suppression of microRNA 124-3p and microRNA 340-5p ameliorates retinoic acid-induced cleft palate in mice. Development 149: 24-76.
- Yan F, Simon LM, Suzuki A (2022) Spatiotemporal microRNA-gene expression network related to orofacial clefts. J Dent Res 101: 13980-407.
- Liu J, Jiang X, Zou A (2021) circIGHG-Induced epithelial-to-mesenchymal transition promotes oral squamous cell carcinoma progression via miR-142-5p/IGF2BP3 signaling. Cancer Res 81: 344-55.
- Li YY, Tao YW, Gao S (2018) Cancer-associated fibroblasts contribute to oral cancer cells proliferation and metastasis via exosome-mediated paracrine miR-34a-5p. EBioMedicine 36: 209-20.
- Iaquinta MR, Lanzillotti C, Mazziotta C (2021) The role of microRNAs in the osteogenic and chondrogenic differentiation of mesenchymal stem cells and bone pathologies. Theranostics 11: 6573-6591.
- Yang Y, Yujiao W, Fang W (2020) The roles of mirna, lncrna and circrna in the development of osteoporosis. Biol res 53: 40.
- Li Z, Xue H, Tan G (2021) Effects of miRNAs, lncRNAs and circRNAs on osteoporosis as regulatory factors of bone homeostasis (Review). Mol Med Rep 24: 788.
- Li J, Jin F, Cai M (2022) LncRNA Nron Inhibits Bone Resorption in Periodontitis [J]. J Dental Res 101: 187-195.
- Subramaniam R, Vijakumaran U, Shanmuganantha L (2023) The Role and Mechanism of MicroRNA 21 in Osteogenesis: An Update. Int J Mol Sci 24: 13-30.
- Y. Hao, Y. Ge, J. Li (2017) Identification of microRNAs by microarray analysis and prediction of target genes involved in the osteogenic differentiation of human periodontal ligament stem cells. J Periodontol 88: 1105-1113.
- Shi F, He R, Zhu J (2022) miR-589-3p promoted osteogenic differentiation of periodontal ligament stem cells through targeting ATF1. J Orthop Surg Res 17: 221.
- Liu F, Zhou Z, Xue Y (2021) Activation of mir-30a-wnt/β-catenin signaling pathway upregulates cathepsin K expression to promote cementogenic differentiation of periodontal ligament stem cells. Nan Fang Yi Ke Da Xue Xue Bao 41: 1439-1447.
- Wei X, Liu Q, Guo S (2021) Role of Wnt5a in periodontal tissue development, maintenance, and periodontitis: Implications for periodontal regeneration. Mol Med Rep 23: 167.
- Liu W, Liu Y, Guo T (2013) TCF3, a novel positive regulator of osteogenesis, plays a crucial role in miR-17 modulating the diverse effect of canonical Wnt signaling in different microenvironments. Cell Death Dis 4: 539.
- Cao F, Zhan J, Chen X (2017) miR-214 promotes periodontal ligament stem cell osteoblastic differentiation by modulating Wnt/β catenin signaling. Mol Med Rep 16: 9301-9308.
- Mazziotta C, Lanzillotti C, Iaquinta MR (2021) MicroRNAs Modulate Signaling Pathways in Osteogenic Differentiation of Mesenchymal Stem Cells. Int J Mol Sci 27: 2362.
- Yang QJ, Liu WJ, Chang WY (2016) Effects of miR20a on the Osteogenic Differentiation potential of the inflammatory PDLSCs [J]. J Pract Stomatol 32: 186-189.
- Yan G, Wang X, Yang F (2017) MicroRNA-22 promoted osteogenic differentiation of human periodontal ligament stem cells by targeting HDAC6. J Cell Biochem 118: 1653-1658.
- Ge Y, Li J, Hao Y (2018) MicroRNA-543 functions as an osteogenesis promoter in human periodontal ligament-derived stem cells by inhibiting transducer of ERBB2, 2. J Periodontal Res 53: 832-841.
- Iaculli F, Di Filippo ES, Piattelli A (2017) Dental Pulp Stem Cells Grown on Dental Implant Titanium Surfaces: An in Vitro Evaluation of Differentiation and MicroRNAs Expression: MicroRNAS Expression during Osteoblasts Differentiation. J Biomed Mater Res B Appl Biomater 105: 953-965.
- Wei F, Yang S, Guo Q (2017) MicroRNA-21 regulates osteogenic differentiation of periodontal ligament stem cells by targeting Smad5. Sci Rep 7: 16608.
- Fang T, Wu Q, Zhou L (2016) miR-106b-5p and miR-17-5p suppress osteogenic differentiation by targeting Smad5 and inhibit bone formation. Exp Cell Res 1: 74-82.
- Li Z, Sun Y, Cao S (2190) Downregulation of miR-24-3p promotes osteogenic differentiation of human periodontal ligament stem cells by targeting SMAD family member 5. J Cell Physiol 234: 7411-7419.
- Zhang Q, Chen L, Cui S (2017) Expression and regulation of long noncoding RNAs during the osteogenic differentiation of periodontal ligament stem cells in the inflammatory microenvironment. Sci Rep 7: 13991.
- Feng Y, Wan P, Yin L (2020) Long noncoding RNA X-inactive specific transcript (XIST) promotes osteogenic differentiation of periodontal ligament stem cells by sponging microRNA-214-3p. Med Sci Monit 26: 918-932.
- Tong X, Gu PC, Xu SZ (2015) Long non-coding RNA-DANCR in human circulating monocytes: a potential biomarker associated with postmenopausal osteoporosis. Biosci Biotechnol Biochem. 79: 732-137.
- Jia Q, Jiang W, Ni L (2015) Down-regulated non-coding RNA (lncRNA-ANCR) promotes osteogenic differentiation of periodontal ligament stem cells. Arch Oral Biol 60: 234-41.
- Wang L, Wu F, Song Y (2016) Long noncoding RNA related to periodontitis interacts with miR-182 to upregulated osteogenic differentiation in periodontal mesenchymal stem cells of periodontitis patients. Cell Death Dis 7: 23-27.
- Guo J, Zheng M (2022) The regulation mechanism of LINC00707 on the osteogenic differentiation of human periodontal ligament stem cells. J Mol Histol 53: 13-26.
- Tu S, Chen Y, Feng Y (2023) lncRNA CYTOR Facilitates Osteogenic Differentiation of Human Periodontal Ligament Stem Cells by Modulating SOX11 via sponging miR-6512-3p. Stem Cells Int 3: 56-71.
- Huang Y, Han Y, Guo R (2020) Long non-coding RNA FER1L4 promotes osteogenic differentiation of human periodontal ligament stromal cells via miR-874-3p and vascular endothelial growth factor A. Stem Cell Res Ther 11: 5.
- Feng Y, Wan P, Yin L (2020) Long Noncoding RNA X-Inactive Specific Transcript (XIST) Promotes Osteogenic Differentiation of Periodontal Ligament Stem Cells by Sponging MicroRNA-214-3p. Med Sci Monit 14: 91-93.
- He Q, Yang S, Gu X (2018) Long noncoding RNA TUG1 facilitates osteogenic differentiation of periodontal ligament stem cells via interacting with Lin28A. Cell Death Dis 9: 455.
- Yang Q, Han Y, Liu P (2020) Long Noncoding RNA GAS5 Promotes Osteogenic Differentiation of Human Periodontal Ligament Stem Cells by Regulating GDF5 and p38/JNK Signaling Pathway. Front Pharmacol 20: 701.
- Gong YY, Peng MY, Yin DQ (2018) Long non‐coding RNA H19 promotes the osteogenic differentiation of rat ectomesenchymal stem cells via Wnt/β‐catenin signaling pathway. Eur Rev Med Pharmacol Sci 22: 8805-13.
- Wang L, Wu F, Song Y (2016) Long noncoding RNA related to periodontitis interacts with miR-182 to upregulated osteogenic differentiation in periodontal mesenchymal stem cells of periodontitis patients. Cell Death Dis 7: 23-27.
- Jia B (2018) A study on mechanism of LncRNA-PCAT1 in regulating osteogenic differentiation of human periodontal ligament stem cells [D]. Guangzhou: Southern Medical University.
- Seo BM, Miura M, Gronthos S (2004) Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 364: 149-155.
- Gu Y, Bai Y (2023) LncRNA MALAT1 promotes osteogenic differentiation through the miR-93-5p/SMAD5 axis. Oral Dis.
- Wu D (2020) Long noncoding RNA TUG1 promotes osteogenic differentiation of human periodontal ligament stem cell through sponging microRNA-222-3p to negatively regulate Smad2/7. Arch Oral Biol 117: 104-814.
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Citation: Zhiying Z (2024) Progress in the Study of the Role of ncRNA onOsteogenic Differentiation of Human Periodontal Stem Cells. Cell Mol Biol, 70: 323.
Copyright: © 2024 Zhiying Z. This is an open-access article distributed under theterms of the Creative Commons Attribution License, which permits unrestricteduse, distribution, and reproduction in any medium, provided the original author andsource are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 382
- [From(publication date): 0-2024 - Apr 04, 2025]
- Breakdown by view type
- HTML page views: 210
- PDF downloads: 172