Prognostic Value of Liver Histology at the Time of Kasai Procedure in Children with Biliary Atresia
Received: 26-Oct-2020 / Accepted Date: 09-Nov-2020 / Published Date: 16-Nov-2020 DOI: 10.4172/2572-4983.1000196
Abstract
Objectives: Biliary atresia is a chronic cholestatic disease resulting from irreversible extrahepatic bile duct occlusions, which leads to liver transplantation in childhood. Solid prognostic values are lacking.
Methods: We investigated the prognostic validity of the PELD score and other laboratory parameters that concern the transplantation-free survival of children with biliary atresia. We correlated the degree of liver fibrosis and neoproliferation of bile ducts at the time of Kasai hepatic portoenterostomy (KPE) and liver transplantation with serological and clinical parameters. Liver biopsies were obtained at the time of KPE in 26 children and directly before the liver transplantation (LTx) of 22 children. The ISHAK score was used to calculate liver fibrosis. Neo-proliferation of the biliary tract was shown by expression of cytokeratin 7.
Results:The survival rate with their own liver was significantly high in children who underwent KPE in less than 28 days of age (p<0.01, cut off 30 days, sensitivity 95.5%, specificity 100%, negative predictive value 80%). Patients with liver enzyme values AST < 83 U/l before surgery also pointed to a significantly better result than patients with higher values (p<0.01, sensitivity 100%, specificity 100%). The bilirubin clearance in children in the group without LTx three months after KPE was significantly better (0.6 (0.1-1.2) vs. 10.18 (0.3-21.9) mg/dl, p<0.05). PELD score and histology had no predictive value.
Conclusions: This study confirms that histological findings do not correlate with serological markers at KPE. However, age and serological markers at the time of KPE appear to be a predictive factor for a positive outcome.
Keywords: ISHAK score, Liver fibrosis, Prognostic markers, Pediatric liver transplantation, PELD score
Keywords
ISHAK score, Liver fibrosis, Prognostic markers, Pediatric liver transplantation, PELD score
Introduction
Biliary atresia is the most common cause of LTx in children [1,2]. Although several causes have been hypothesized — including genetic, embryological, infectious, and immunological factors [3-6] the etiology of this infantile obstructive cholangiopathy remains unknown. An untreated extrahepatic obstruction of the bile ducts leads to progressive inflammatory destruction of the extrahepatic and intrahepatic bile duct system, to cholestasis and secondary liver damage with consecutive fibrosis, cirrhosis, and portal hypertension and subsequently to death [6,7].
The focal point of treatment is to restore bile flow to the intestine, which can be achieved with KPE. In most cases, this involves a bridging therapy to gain time for a later LTx, which is necessary in about 80% of cases [8,9]. One of the major risks in managing this disease is the timing of diagnosis as delayed referral may lead to a late diagnosis which, in turn, may result in an urgent need for LTx [10]. Abnormal prognostic parameters at the time of diagnosis indicate the progress of the disease and consequently, the consideration of adequate treatment.
The correlation of KPE timing and survival with the patient's own liver has been widely discussed in various literature and the cut-off has been reduced from 12 weeks to 30 days, as reported by Nio et al. 2003 [11-16]. Bilirubin clearance after KPE also appears to have an influence on transplantation-free survival [17-20]: children with normal bilirubin serum levels three to six months after KPE had significantly higher survival rates without LTx. In addition to bilirubin, Goda et al. found a clear correlation between AST and ALT levels and transplantation-free survival [21].
There are various theories in literature on the correlation between fibrosis at the time of KPE and the clinical course of the children with the disease. Certain studies showed no correlation exists between the two [22], while others showed a remarkable link between the degree of fibrosis and long-term outcome [23,24]. In this study, fibrosis was measured using the Pico-Sirius Red Staining, which is a specific method for identifying collagen I, collagen II, and collagen III. It was used in renal fibrosis measurements and has shown informative correlations between function and histology [25-27]. In the 1990s, this method was used by Moragas et al. to identify liver fibrosis in children who will undergo LTx. Another change in liver histology in children with BA is neoproliferation of the bile ducts, which can be measured through cytokeratin-7 staining, indicating an increased presence of neo-bile ducts as a reaction of the liver to cholestasis and as an indicator of liver damage [28,29].
In this study, we have thus investigated whether the histological findings at the time of KPE and LTx correlated with the clinical course measured by biochemical variables in order to be used as prognostic parameters. Therefore, we also used the pediatric end-stage liver disease score (PELD) as an objective tool to prioritize children waiting for LTx. A higher PELD score in children is associated with increased mortality before LTx [30,31].
Patients and Methods
51 patients in a period of 6 years with suspected biliary atresia were included in this study. They all underwent the same diagnostic procedure at the KUNO University Children's Hospital, Regensburg. They were first subjected to endoscopic retrograde cholangiopancreatography (ERCP) and, if the bile duct was present, a biopsy was performed for further diagnosis. In the absence of a biliary tract, KPE was performed. In this case, the diagnosis was confirmed by the operative findings (biopsy, cholangiography). Patients' regular follow-ups in our pediatric clinic were done by ultrasound and routine laboratory tests. For this study, the clinical course from KPE for up to 3 months post-surgery was used to assess prognosis markers. The median observation period is 5.1 months (0.9-80.6). The indication for liver transplantation was synthetic liver dysfunction including portal hypertension.
Histopathology
Needle biopsies were taken from the explanted livers during the KPE and directly before liver transplantation. They were immediately fixed in formalin, alcohol, and acetic acid before being embedded in paraffin wax. Fibrosis was measured by a single senior pathologist and evaluated semi-quantitatively according to the ISHAK Fibrosis Score [17,32]. To measure the neoproliferation of the bile ducts, we used cytokeratin-7 immunohistochemistry.
Pico-Sirius Red (PSR) staining
The PSR staining was performed according to standard procedures [17]. Fibrosis was then assessed using the ISHAK score [32].
CK7 staining
The CK 7 staining was performed automatically by Roche's BenchMark ULTRA IHC/ISH staining module. The histological scoring was adjusted according to Yabushita et al. [26,27].
PELD score
The PELD score was evaluated immediately before and after the KPE [31]. Influencing factors are age, bilirubin levels, INR, albumin, and growth failure.
Statistical analysis and ethical tuning
Statistical analyses were performed with SPSS, Version 23.0 (SPSS, Inc., Chicago, IL). The data are displayed with a median and range. Overall patient survival was analyzed using Kaplan-Meier analysis, wherein p
Results
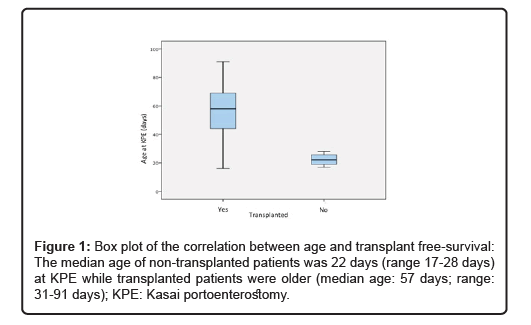
For this study, 51 patients with neonatal cholestasis and suspected biliary atresia were included (Table 1). The patients were diagnosed by ERCP to visualize the extra- and intrahepatic bile ducts. 18 patients had successful visualization of the bile ducts and underwent liver biopsy for differential diagnosis, while 33 children showed no contrast enhancement of the bile system up to the left and right hepatic duct. 7 patients showed an advanced stage of the disease with evidence of synthetic liver dysfunction (including portal hypertension) and were listed for LTx without KPE. 26 children underwent Kasai surgery (13 male and 13 female patients; mean age: 52 days, range: 17-91 days). At the end of the observation period 4 of them survived with their own liver while 22 had to undergo liver transplantation. Reason for transplantation were synthetic liver dysfunction including portal hypertension. The median age of the non-transplant patients was 22 days (range: 17-28 days) for the KPE, while the transplant patients were older (median age: 57 days; range: 31-91 days). The median age during liver transplantation was 189 days (range 103-389 days). 3 patients died after the transplantation due to sepsis (n=2) or organ failure (n=1). Patients who survived with their own liver had an observation period of at least two years.
| Demographic data | N |
|---|---|
| Patients | 51 |
| Female/Male | 20/31 |
| Biliary tract present/absent in ERCP | 18/33 |
| Performed Kasai procedure | 26 |
| Female/male KPE patients | 13/13 |
| Other diagnosis | 18 |
| Primary LTX | 7 |
| W/o LTX | 4 |
| With LTX | 22 |
| LTX and deceased | 3 |
| Survival (2 years) KPE group | 88.50% |
| Median (Range) | |
| Age at KPE (days) all patients | 52 (17-91) |
| Age at LTX after KPE | 189 (103-389) |
| Age at KPE (w/o LTX) | 22 (17-28) |
| Age at KPE (with LTX) | 57 (31-91) |
| Time (months) until LTX | 5.1 (0.9-80.6) |
Table 1: Demographic data of study patients
Non-transplant patients underwent KPE significantly earlier than transplant patients (exact Mann-Whitney-U-Test: z=-2.8, p=0.002). BA patients who underwent KPE after 30 days of age have a negative predictive value of 0.80 (sensitivity 95.5%, specificity 100%; Figure 1). Liver enzyme levels were also different between the two groups at KPE: AST and ALT levels were significantly lower in patients who survived the observation period without LTx (p= 0.000**; Table 2). The LTx cut- off in our study population was >83 U/l for AST and >48 U/l for ALT with a sensitivity level of 100% and specificity of 100%). Bilirubin levels in both groups showed no significant differences before KPE.
Three months after KPE, patients who survived with their own liver showed significantly lower AST and ALT levels than patients who needed LTx (p<0.05). Bilirubin clearance was also significantly better (without LTx: 0.6 (0.1-1.2); with LTx: 10.1 (0.3-21.9); p<0.05). The deRitis quotient was significantly higher in the LTx group (0.96 vs. 1.64, p<0.05).
The PELD score at the time of KPE was significantly lower in the non-transplant group (without LTx: 5 (3-5) vs. LTx: 7 (4-28); p<0.05). This was confirmed after three months when the non-transplant group showed a significantly lower PELD score (without LTx -4 (-6 - -3); with LTx 11 (0-25); p=0.005).
The histological findings involving the ISHAK fibrosis score and the CK 7 staining did not show a clear correlation between the degree of fibrosis or neoproliferation of the bile ducts in both groups at the time of KPE and even three months after surgery (Table 2). From the histological findings, the clinical course of the patients could be inferred (Table 2, Figure 2). No correlation between histology and PELD score could be established (p>0.05).
| w/o LTX | With LTX | P value | |
|---|---|---|---|
| (n= 4) | (n=22) | ||
| Age at KPE (days) | 22 (17-28) | 57 (16-91) | p=0.002** |
| Age at LTX (days) | - | 189 (103-389) | - |
| AST level at KPE (U/l) | 69(48-83) | 168 (68-604) | p=0.000** |
| AST after 3 months (U/l) | 42 (35-109) | 298 (62-553) | p<0.05* |
| AST level at LTX (U/l) | - | 187.5 (69-697) | - |
| ALT level at KPE (U/l) | 40 (26-48) | 117 (53-401) | p=0.000** |
| ALT after 3 months (U/l) | 39.5 (34-122) | 170.5 (30-436) | P<0.05* |
| ALT level at LTX (U/l) | - | 101 (21-417) | - |
| Bilirubin at KPE (mg/dl) | 7.55 (3.9-12.3) | 7.6 (4.3-9.8) | n.s. |
| Bilirubin after 3 months (mg/dl) | 0.6 (0.1-1.2) | 10.18 (0.3-21.9) | p<0.05* |
| Bilirubin at LTX | - | 14.85 (0.3-24.9) | - |
| DeRitis (AST/AALT) at KPE | 1.89 (1.35-1.97) | 1.52 (1.04-2.45) | n.s. |
| DeRitis (AST/ALT) after 3 months | 0.96 (0.83-1.35) | 1.64 (0.68-2.2) | P<0.05* |
| DeRitis (AST/ALT) at LTX | - | 1,83 (1.12-3.29) | - |
| PELD at KPE | 5 (3-5) | 7 (4-28) | n.s. |
| PELD after 3 months | -4 (-6 - -3) | 11 (0-25) | P=0.005** |
| PELD at LTX | - | 17 (-10-26) | - |
| ISHAK at KPE | 3.25 (1-5) | 3.52 (2-6) | n.s. |
| IHSAK at LTX | - | 5.62 (4-6) | - |
| CK7 at KPE | 1.0 (0-2) | 1.0 (0-2) | n.s. |
| CK7 at LTX | - | 2.0 (2-3) | - |
ALT: Alanine Aminotransferase; AST: Aspartate Aminotransferase; CK7: Cytokeratin 7; ERCP: Endoscopic retrograde cholangiopancreatography: KPE: Kasai Hepatic Portoenterostomy; LTX: Liver transplantation: PELD: Pediatric end-stage liver disease; * : Statistical Differention <0.05
Table 2: Transplanted and non-transplanted patients: serum levels and histological findings
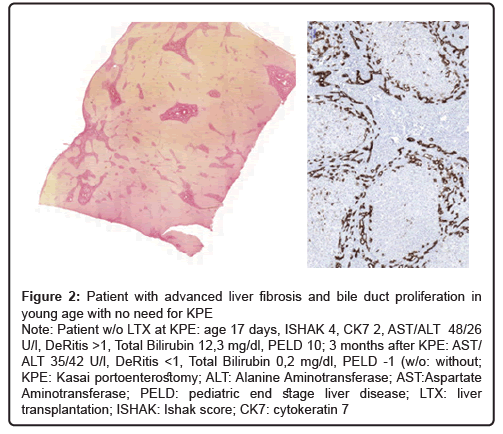
Figure 2: Patient with advanced liver fibrosis and bile duct proliferation in young age with no need for KPE
Note: Patient w/o LTX at KPE: age 17 days, ISHAK 4, CK7 2, AST/ALT 48/26 U/l, DeRitis >1, Total Bilirubin 12,3 mg/dl, PELD 10; 3 months after KPE: AST/ ALT 35/42 U/l, DeRitis <1, Total Bilirubin 0,2 mg/dl, PELD -1 (w/o: without;KPE: Kasai portoenterostomy; ALT: Alanine Aminotransferase; AST:Aspartate Aminotransferase; PELD: pediatric end stage liver disease; LTX: liver transplantation; ISHAK: Ishak score; CK7: cytokeratin 7
Discussion
Prognostic value of fibrosis and neoproliferation of the bile ducts at the time of KPE for transplantation-free survival in biliary atresia and correlation of histological findings with clinical parameters could not be found in this study. However, serum levels of liver enzymes and bilirubin levels after three months are of significant predictive value for transplantation-free survival. Furthermore, the PELD score was clearly correlated with the clinical course but is not suitable as a prognostic method for determining transplantation-free survival. In addition, the time of KPE had a clear influence on survival with the patient's own liver.
Prognostic parameters are needed to predict the clinical course of children with BA. Different aspects, such as serum parameters, age at the time of KPE, and postoperative steroid therapy have been investigated [11-21,33-37] in this study, we were able to evaluate the histological findings at the time of KPE, after LTx and the clinical course in comparison to patients without LTx. We were thus able to confirm the findings of Pape et al. [17] that liver fibrosis as measured by the PicoSirius Red staining, evaluated by the ISHAK Fibrosis Score, shows no correlation with the outcome in children with BA. In addition, the evaluation of neoproliferation of bile products by CK7 staining showed no correlation with disease progression in either group at KPE or LTx. A correlation between both staining methods and serum parameters of liver enzymes and therefore to the PELD score was also not found (p>0.05).
However, the serum values of the liver enzymes as well as the bilirubin values allowed prognostic conclusions to predict a clinical course. We found a cut-off value for transplantation-free survival at the time of KPE for AST levels <83 U/l and for ALT <48 U/l with a sensitivity level of 100% and a specificity of 100%. These observations were confirmed three months after KPE with an equally significant difference in both groups. We have similar results to those published by Goda et al. in 2013 [21], which showed a prognostic AST value two months after KPE as a reliable indicator of long-term outcomes. Moreover, this was confirmed by the fact that the DeRits-quotient had improved significantly in the non-transplant group.
As for the development of bilirubin levels, we have identical findings to those found in existing literature [17-20]: Children without LTx had significantly lower bilirubin levels (0.6 mg/dl vs. 10.18 mg/dl) three months after KPE. We were thus able to confirm bilirubin as a practical prognostic parameter for long-term outcomes.
A frequently discussed issue in children with BA is the time of diagnosis and the subsequent start of treatment. Once again, we were able to show that early KPE is an important prognostic factor since all patients for whom LTx was not necessary were operated on before 30 days of age. This cut-off is the same as that published by Matsui and Kudo in 2006 from the Japanese biliary atresia registry and much earlier than indicated in other studies, e.g. by Lin et al., who set a value of 60 days of age [16,38]. Patients undergoing LTx had a median age at KPE of 52 days, and survival with their own liver was 6 months on average. A conceivable explanation for this finding that contrasts those in existing literature [10] is the high patient selection filtered through ERCP diagnosis to exclude other reasons for cholestasis with generally better outcomes [39]. This procedure leads to a very select patient population where of the 51 patients suspected to have biliary atresia, 18 could be excluded by ERCP. Of the remaining 26 patients with confirmed biliary atresia who underwent KPE in our facility, 15% still had their own liver at the end of the observation period. This contradicts the results of other study groups in Europe [40,41] with better results. In our opinion, this is due to the highly specific diagnostics leading to the highly select patient population. In this manner, other underlying diseases with similar appearance but with better outcomes (such as Alagille syndrome) are excluded.
Conclusion
The histological findings at the time of KPE had no predictive value for the long-term outcome of patients with KPE. We also found no correlation of histology with clinical course. However, serological markers like AST, ALT, and bilirubin, as well as the time of KPE, do appear to have predictive value. The PELD score is a useful marker for visualizing the clinical progress of the disease.
Limitations
This study is limited because of the small number of patients available, which is something that is attributed to the rarity of the disease in question. However, there are clear trends that are deemed worthy of being looked into in larger studies. In addition, the possibility of making a sampling error when the area of biopsy is not representative of the degree of fibrosis is reported. The Ishak Score was also originally developed for the classification and staging of chronic hepatitis B and not for fibrosis resulting from BA.
References
- Hackl C (2015) Current developments in pediatric liver transplantation. World J Hepatol 7:1509
- Fabris L, Spirli C, Cadamuro M, Fiorotto R, Strazzabosco M (2017) Emerging concepts in biliary repair and fibrosis. Am J Physiol Gastrointest Liver Physiol 313: G102-G116
- Alagille D (1984) Extrahepatic Biliary Atresia. Hepatology 4: 7S – 10S
- Wildhaber BE (2012) Biliary Atresia: 50 Years after the First Kasai. ISRN Surgery 2012:1–15
- Hartley JL, Davenport M, Kelly DA (2009) Biliary atresia. The Lancet 374:1704–1713
- Petersen C, Davenport M (2013) Aetiology of biliary atresia: what is actually known? Orphanet J Rare Dis 8:128
- Karrer FM, Lilly JR, Stewart BA, Hall RJ (1990) Biliary atresia registry, 1976 to 1989. J Pediatr Surg 25:1076–1080
- Karrer FM, Price MR, Bensard DD, Sokol RJ, Narkewicz MR, et al. (1996) Long-term results with the Kasai operation for biliary atresia. Arch Surg 131:493-496
- Ohi R. Biliary atresia. A surgical perspective. Clin Liver Dis 2000; 4:779-804
- Petersen C, Harder D, Melter M, Becker T, Wasielewski RV, et al. (2008) Postoperative high-dose steroids do not improve mid-term survival with native liver in biliary atresia. Am J Gastroenterol 103:712-719.
- Hitch DC, Shikes RH, Lilly JR (1979) Determinants of survival after Kasai’s operation for biliary atresia using actuarial analysis. J Pediatr Surg 14: 310–314
- Mieli-Vergani G, Howard ER, Portman B (1989) Late referral for biliary atresia--missed opportunities for effective surgery. Lancet 25;1: 421–423
- Chardot C, Carton M, Spire-Bendelac N, Pommelet C Le, Golmard JL, et al. (1999) Prognosis of biliary atresia in the era of liver transplantation: French national study from 1986 to 1996. Hepatology 30: 606–611
- Lally KP, Kanegaye J, Matsumura M, Rosenthal P, Sinatra F, et al. (1989) Perioperative factors affecting the outcome following repair of biliary atresia. Pediatrics 83:723–726.
- Nio M, Ohi R, Miyano T, Saeki M, Shiraki K, et al. (2003) Five- and 10-year survival rates after surgery for biliary atresia: a report from the Japanese Biliary Atresia Registry. J Pediatr Surg 38: 997–1000
- Lin JS, Chen SCC, Lu CL, Lee HC, Yeung CY, et al. (2015) Reduction of the ages at diagnosis and operation of biliary atresia in Taiwan: A 15-year population-based cohort study. World J Gastroenterol 14; 21:13080–130806
- Pape L, Olsson K, Petersen C, Wasilewski RV, Melter M, et al. (2009) Prognostic value of computerized quantification of liver fibrosis in children with biliary atresia. Liver Transpl 15: 876–82
- Shneider BL, Magee JC, Karpen SJ, Rand EB, Narkewicz MR, et al. (2016) Total Serum Bilirubin within 3 Months of Hepatoportoenterostomy Predicts Short-Term Outcomes in Biliary Atresia. J Pediatr 170: 211–217
- Utterson EC, Shepherd RW, Sokol RJ, Bucuvalas J, Magee JC, et al. (2005) Biliary atresia: clinical profiles, risk factors, and outcomes of 755 patients listed for liver transplantation. J Pediatr 147:180–5.
- Chusilp S, Sookpotarom P, Tepmalai K, Rajatapiti P, Chongsrisawat V, et al. (2016) Prognostic values of serum bilirubin at 7th day post-Kasai for survival with native livers in patients with biliary atresia. Pediatr Surg Int 32: 927–931
- Goda T, Kawahara H, Kubota A, Hirano K, Umeda S, et al. (2013) The most reliable early predictors of outcome in patients with biliary atresia after Kasai’s operation. J Pediatr Surg 48: 2373–2377
- Shteyer E, Ramm GA, Xu C, White FV, Shepherd RW (2006) Outcome after portoenterostomy in biliary atresia: pivotal role of degree of liver fibrosis and intensity of stellate cell activation. J Pediatr Gastroenterol Nutr 42: 93-99
- Kang N, Davenport M, Driver M, Howard ER (1993) Hepatic histology and the development of esophageal varices in biliary atresia. J Pediatr Surg 28: 63-66
- Carceller A, Blanchard H, Alvarez F, Vil DS, Bensoussan AL, et al. (2000) Past and future of biliary atresia. J Pediatr Surg 35: 717-720.
- Junqueira LC, Bignolas G, Brentani RR (1979) Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem J 11: 447-455
- Flores O, Arevalo M, Gallego B, Hidalgo F, Vidal S, et al. (1998) Beneficial effect of the long-term treatment with the combination of an ACE inhibitor and a calcium hannel blocker on renal injury in rats with 5/6 nephrec- tomy. Exp Nephrol 6: 39-49
- Moreso F, Seron D, Vitria J, Grinyó JM, Françoise M.Colomé-Serra et al. (1994) Quantification of interstitial chronic renal damage by means of texture analysis. Kidney Int; 46: 1721-1727.
- Suriawinata AA, Thung SN. Liver pathology: an atlas and concise guide. New York: Demos Medical; 2011: 9
- Yabushita K, Yamamoto K, Ibuki N, Okano N, Matsumura S, et al. (2001) Aberrant expression of cytokeratin 7 as a histological marker of progression in primary biliary cirrhosis. Liver 21: 50–55
- Shinkai M, Ohhama Y, Take H, Fukuzato Y, Fujita S, et al (2003)  Evaluation of the PELD risk score as a severity index of biliary atresia. J Pediatr Surg 38: 1001–1004
- Rhu J, Jung S-M, Choe YH, Seo JM, Lee SK (2012) PELD score and age as a prognostic index of biliary atresia patients undergoing Kasai portoenterostomy. Pediatr Surg Int 28: 385–391
- Ishak K, Baptista A, Bianchi L, Callea F, Groote JD, et al. (1995) Histological grading and staging of chronic hepatitis. J Hepatol 22: 696-699
- Dong R, Song Z, Chen G, Zheng S, Xiao XM (2013) Improved outcome of biliary atresia with postoperative high-dose steroid. Gastroenterol Res Pract 2013:902431.
- Petersen C, Harder D, Melter M, Becker T, Wasielewski RV, et al. (2008) Postoperative high-dose steroids do not improve mid-term survival with native liver in biliary atresia. Am J Gastroenterol 103: 712–719.
- Tyraskis A, Parsons C, Davenport M. Glucocorticosteroids for infants with biliary atresia following Kasai portoenterostomy. Cochrane Database Syst Rev 2018; 5: CD008735.
- Wiesner RH, McDiarmid SV, Kamath PS, Edwards EB, Malinchoc M, et al. (2001) MELD and PELD: application of survival models to liver allocation. Liver Transpl 7: 567–580.
- Tang N, Zhang Y, Liu Z, Fu T, Liang Q, et al. (2016) Correlation analysis between four serum biomarkers of liver fibrosis and liver function in infants with cholestasis Biomed Rep 5:107-112.
- Matsui, A, Kudo T (2006) Evidence for medical benefit from early diagnosis and bile drainage to the patients with biliary atresia – Japanese biliary registry 1989-2004. J Pediatric Gastroenterol Nutrit 42; 5.
- Negm AA, Petersen C, Markowski A, Luettig B, Ringe KI, et al. (2018) The Role of Endoscopic Retrograde Cholangiopancreatography in the Diagnosis of Biliary Atresia: 14 Years' Experience. Eur J Pediatr Surg 28: 261-2.
- Fanna M, Masson G, Capito C, Girard M, Guerin F, et al. (2019) Management of Biliary Atresia in France 1986 to 2015: Long-term Results. J Pediatr Gastroenterol Nutr 69: 416-424.
- Pakarinen MP, Johansen LS, Svensson JF, Bjørnland K, Gatzinsky V et al. (2018) Outcomes of biliary atresia in the Nordic countries: A multicenter study of 158 patients during 2005-2016. J Pediatr Surg 53: 1509-1515.
Citation: Loose O, Hofstetter L, Matar G, Utpatel K, Lang T, et al. (2020) Prognostic Value of Liver Histology at the Time of Kasai Procedure in Children with Biliary Atresia. Neonat Pediatr Med 7: 196. DOI: 10.4172/2572-4983.1000196
Copyright: © 2020 Loose O. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Conferences
42nd Global Conference on Nursing Care & Patient Safety
Toronto, CanadaRecommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 2175
- [From(publication date): 0-2021 - Apr 03, 2025]
- Breakdown by view type
- HTML page views: 1352
- PDF downloads: 823