Prognostic Role of H3K9 Methyltransferase G9a and Sprouty 1 in Colorectal Carcinoma
Received: 19-Dec-2016 / Accepted Date: 28-Dec-2016 / Published Date: 02-Jan-2017
Abstract
Background: Despite recent advances in the treatment of both early and advanced colorectal cancer, it remains the second leading cause of cancer deaths in the western world. G9a-dependent H3K9 methylations (G9a) have been shown to mediate epigenetic silencing of several tumours suppressor genes including DSC3, MASPIN, and CDH1. Sprouty1 (SPRY1) appears to act as a tumour suppressor in cancer, whereas we demonstrated that SPRY2 functions as a putative oncogene in colorectal cancer.
Methods: The clinic pathological correlation of G9a and S SPRY 1 Immuno-histochemical expression was assessed in tumour specimens of paraffin blocks retrieved from 50 colorectal carcinoma patients.
Results: Our patients included 30 men and 20 women, with a median age of years 57 (range, 29 80 years). Adenocarcinoma represented in 45 (90%) patients. Of the 50 patients, G9a was highly expressed in 56% (28/50) patients and 54% (27/50) patients had high expression of SPRY 1. The high expression of G9a in CRC was significantly correlated with the tumour size, grade, stage, presence of lymph node, distant metastases (p<0.001 for all), and tumour site (p=0.002), No significant correlation was found between G9a expression and age or sex of our patients. For SPRY1, high expression was negatively correlated with the tumour size, presence of lymph node metastases (p<0.001 for both), stage (p=0.002), and presence of distant metastases (p=0.03). No significant correlation was found between SPRY1 expression, age, sex, grade, and size or tumour site. After a median follow up of 32 (range; 8-35) months, patients with low G9a expression had significant higher median TDP than those with high expression (p<0.001). In controversy, patients with high SPRY1 expression had significant higher TDP (p<0.001). 2-Year OS of patients with low G9a expression was significantly higher than those with high expression (100% vs. 49.5%, respectively; p<0.001). For SPRY 1 expression; patients with high expression had significant higher 2-year OS rate (100% vs. 38.3%; respectively; p<0.001). Patients with combined low G9a and high SPRY 1 1expression had the highest TDP and OS rate.
Conclusion: Assessment of Sprouty-1 and G9a expression markers can be considered as useful promising prognostic markers for CRC patients. Value of combination of both markers could be considered in future studies.
Keywords: Colorectal carcinoma; H3K9 Methyltransferase G9a; Sprouty 1; Prognosis
77297Introduction
Colorectal cancer (CRC) is a commonly diagnosed malignant tumour all over the world and the third among causes of cancerrelated death in both males and females [1]. Although the advanced precancerous screening and surgical techniques; the prognosis of patients with CRC still poor, this is due to its recurrence and occurrence of lymphatic and blood metastases [2]. The clinicopathological parameters, such as Tumour-Node-Metastasis (TNM) staging has been used for expecting prognosis of cancer patients, but most of them found to be inaccurate for individual prognosis [3]. So, novel molecular markers should be discovered and used in expecting patient outcome. Several mechanisms have been studied to explain the occurrence of metastases. The epithelial-mesenchymal transition (EMT) phenomenon has been the most recent mechanism that can explain the occurrence of carcinoma distant metastases by disruption of intercellular junctions and loss of cell-cell contact, which results in the loss of epithelial criteria and the gain of mesenchymal features which enhance cell motility, resulting in the ability to form new malignant growths at distant sites [4]. G9a, a histone methyltransferase for lysine 9 of histone 3 (H3K9) is a histone methyltransferase (HMT) that mediates euchromatin gene silencing and is vital for embryogenesis through regulating developmental gene expression [5]. G9a-dependent H3K9 methylations have been shown to mediate epigenetic silencing of several tumour suppressor genes [6], and were required to sustain cancer cell behaviours like hypoxia response, cell proliferation, autophagy, cancer stemness, and is involved in the epigenetic control of some steps during EMT [7,8]. Previous studies have proved that H3K9 methyltransferase G9a is involved in the epigenetic control of phenotypic and cellular plasticity during EMT [6]. In different tumour types, the molecular relation between H3K9 methylations and cancer progression is still an important point of research, the clinical significance and functional role of H3K9 methyltransferase G9a in colon cancer is still an area of controversy [9].
Sprouty (SPRY) is an intracellular regulator of receptor tyrosine kinase signalling involved in growth, differentiation and tumour genesis. Four family members of SPRY (SPRY1–4) have been identified [10,11]. Sprouty proteins have been implicated in the regulation of the biological processes central to tumour growth, development and metastasis, including cell proliferation, migration, invasion and survival [12]. Up- or down-regulation of Sprouty 1 in a variety of neoplasms consider it as a possible tumour marker and a promising target for drug intervention [13].
Therefore, our study aimed to evaluate the significance of H3K9 methyltransferase G9a and Sprouty 1expression in colorectal carcinoma (CRC), detecting their prognosis role in patients with CRC.
Patients and Methods
Immunohistochemical expressions of H3K9 methyltransferase G9a and Sprouty 1 were evaluated in sections from 50 paraffin blocks retrieved from 50 cases of colorectal carcinoma (CRC) that were collected from archives of Pathology and Oncology Departments, Faculty of Medicine, Zagazig, Beni-Suif Universities and National Cancer Institute, Cairo university during the period from April 2013 to April 2016. The seventh edition of the American Joint Committee on Cancer staging system (AJCC-7) classification and the World Health Organization (WHO) classification were used for pathologic staging and a pathologic grading respectively [14,15].
Clinical criteria as sex, age, tumour size, grade and stage have been identified by retrospective examination of the slides and the patient’s files.
Approval for the study was obtained from the ethics committee of the contributing centres. Patients with stages I-III undergone surgery and adjuvant chemotherapy for stage III and high risk stage II, while chemotherapy was the first line for metastatic CRC. None of the patients had received preoperative chemotherapy or radiation therapy.
Immunohistochemical staining
Formalin-fixed, paraffin-embedded tissue sec¬tions (5 μmthicks) were deparaffinized with xylene and rehydrated. For antigen retrieval, sec-tions were placed in either 10 mM Tris base, 1 mM EDTA solution at pH 9.0 10 mM sodium citrate buffer at pH 6.0 and exposed to repeated (twice) microwave heating of 10 min at 750W. After 10 min incubation with 3% hydrogen peroxide for inactivation of endogenous peroxidase activity, sections were blocked with DAKO blocking buffer followed by incubation at 4°C overnight with primary anti Sprouty1 mouse monoclonal antibody (1:300) (ab56670 Abcam, Cambridge, MA, USA) and rabbit monoclonal anti-G9a antibody (1:500 dilution; ab185050, Abcam, Cambridge, MA, USA). Sections of heart tissue and lymph node were used as positive controls for Sprouty1 and G9a respectively. Specimens were then incubated with secondary antibody using EnVision Plus kit (DAKO) for 30 min and then with diaminobenzidine chromogen for 5 min. All slides were counterstained with hematoxylin. For negative controls, the primary antibodies were omitted and replaced with normal saline. Under light microscope, staining of the epithelial cells was evaluated and scored by three pathologists. Representative slides were photographed.
Determination of Sprouty 1 Expression by Immunohistochemical Assay
The positive Sprouty 1 expression cells have brownish-yellow granules in their cytoplasm. Semi-quantitative scoring based on the intensity and the percentage of immunoreactive cells. A four-value intensity score (0, no immunoreactivity; 1, weak; 2, moderate and 3, strong) was used as well as a four-value extent score as follows; (0, none; 1, 1-33%; 2, 34-66%; and 3, 67-100%) [16], then the average intensity of the stain and extent of stained tumor cells scores were multiplied resulting in a 9-point score ranging from 0 (no staining) to 9 (extensive, strong staining) for each case.
We consider the cut off value 4 above which is considered high total score and below which low total score.
Determination of H3K9 methyltransferase G9a expression by immunohistochemical assay
The positive H3K9 methyltransferase G9a expression cells exhibited to have brownish granules in their nuclei. Its expression was graded on the basis of the following standards: cancer cells that were unstained or that were stained less than10% classified as negative and cancer cells that were stained more than10% identified as positive [17].
The intensity score (0, no immunoreactivity; 1, weak; 2, strong) was used as well as the extent score (1, 1-10%; 2, 10-50%; and 3, 50-100%). The average intensity of the stain and extent of stained tumor cells were then multiplied yielding a 6-point score ranging from 0 (no staining) to 6 (extensive, strong staining) for each case.
High expression was defined as either positive staining present in 10%–50% of the cells (staining intensity score=2) or more than 50% of the cells (staining intensity score=3) [18].
Statistics Analysis
Continuous variables were expressed as the mean ± SD and median (range), and the categorical variables were expressed as a number (percentage). Mann Whitney U test was used to compare between two groups of non-normally distributed variables. Relapse Free Survival (RFS) was calculated as the time from end of treatment to date at which recurrence was detected. Stratification of OS and RFS was done according to all clinic-pathological features and immunehistochemical markers. These time-to-death distributions were estimated using the method of Kaplan-Meier plot. A p-value <0.05 was considered significant. All statistics were performed using SPSS 22.0 for windows (SPSS Inc., Chicago, IL, USA) and MedCalc windows (MedCalc Software bvba 13, Ostend, Belgium).
Results
Patients characteristics
The clinical characteristics of the 50 patients with colorectal carcinoma (CRC) that were included in the study are summarized in Table 1.
| Characteristics | All (N=50) No. (%) |
SPRY1 | p-value | H3K9 methyl-transferase G9a | p-value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Low (N=23) No. (%) |
High (N=27) No. (%) |
Low (N=22) No. (%) |
High (N=28) No. (%) |
|||||||||
| Age (years) | ||||||||||||
| Mean ± SD | 58.12 | ± 12.02 | 58.52 | ± 13.45 | 57.78 | ± 10.91 | 0.830* | 55.36 | ± 10.03 | 60.29 | ± 13.15 | 0.153* |
| Median (Range) | 57 | (29-80) | 60 | (29-80) | 55 | (30-75) | 55 | (30-75) | 62 | (29-80) | ||
| 26-52%11-47.80%15-55.60%0.586‡14-63.60%12-42.90%0.144‡ | ||||||||||||
| ≥60 years | 24 | -48% | 12 | -52.20% | 12 | -44.40% | 8 | -36.40% | 16 | -57.10% | ||
| Sex | ||||||||||||
| Male | 30 | -60% | 16 | -69.60% | 14 | -51.90% | 0.203‡ | 12 | -54.50% | 18 | -64.30% | 0.485‡ |
| Female | 20 | -40% | 7 | -30.40% | 13 | -48.10% | 10 | -45.50% | 10 | -35.70% | ||
| Location | ||||||||||||
| Ascending colon | 15 | -30% | 9 | -39.10% | 6 | -22.20% | 0.265‡ | 1 | -4.50% | 14 | -50% | 0.002‡ |
| Transverse colon | 4 | -8% | 3 | -13% | 1 | -3.70% | 1 | -4.50% | 3 | -10.70% | ||
| Descending colon | 5 | -10% | 2 | -8.70% | 3 | -11.10% | 3 | -13.60% | 2 | -7.10% | ||
| Rectosigmoid | 26 | -52% | 9 | -39.10% | 17 | -63% | 17 | -77.30% | 9 | -32.10% | ||
| Size (cm) | ||||||||||||
| ≤ 5 cm | 22 | -44% | 4 | -17.40% | 18 | -66.70% | <0.001‡ | 18 | -81.80% | 4 | -14.30% | <0.001‡ |
| > 5 cm | 28 | -56% | 19 | -82.60% | 9 | -33.30% | 4 | -18.20% | 24 | -85.70% | ||
| Pathological Type | ||||||||||||
| Adenocarcinoma | 45 | -90% | 19 | -82.60% | 26 | -96.30% | 0.167‡ | 22 | -100% | 23 | -82.10% | 0.059‡ |
| Mucinous | 5 | -10% | 4 | -17.40% | 1 | -3.70% | 0 | 0% | 5 | -17.90% | ||
| Grade | ||||||||||||
| Grade I | 7 | -14% | 2 | -8.70% | 5 | -18.50% | 0.069• | 7 | -31.80% | 0 | 0% | <0.001• |
| Grade II | 35 | -70% | 15 | -65.20% | 20 | -74.10% | 15 | -68.20% | 20 | -71.40% | ||
| Grade III | 8 | -16% | 6 | -26.10% | 2 | -7.40% | 0 | 0% | 8 | -28.60% | ||
| Lymph node | ||||||||||||
| Negative | 21 | -42% | 4 | -17.40% | 17 | -63% | 0.001‡ | 17 | -77.30% | 4 | -14.30% | <0.001‡ |
| Positive | 29 | -58% | 19 | -82.60% | 10 | -37% | 5 | -22.70% | 24 | -85.70% | ||
| DM | ||||||||||||
| Absent | 40 | -80% | 15 | -65.20% | 25 | -92.60% | 0.030‡ | 22 | -100% | 18 | -64.30% | 0.001‡ |
| Present | 10 | -20% | 8 | -34.80% | 2 | -7.40% | 0 | 0% | 10 | -35.70% | ||
| Stage | ||||||||||||
| Stage I | 8 | -16% | 2 | -8.70% | 6 | -22.20% | 0.002• | 8 | -36.40% | 0 | 0% | <0.001• |
| Stage II | 13 | -26% | 2 | -8.70% | 11 | -40.70% | 9 | -40.90% | 4 | -14.30% | ||
| Stage III | 19 | -38% | 11 | -47.80% | 8 | -29.60% | 5 | -22.70% | 14 | -50% | ||
| Stage IV | 10 | -20% | 8 | -34.80% | 2 | -7.40% | 0 | 0% | 10 | -35.70% | ||
| SPRY1 | ||||||||||||
| Low | 23 | -46% | 2 | -9.10% | 21 | -75% | <0.001‡ | |||||
| High | 27 | -54% | 20 | -90.90% | 7 | -25% | ||||||
| H3K9 methyl-transferase G9a | ||||||||||||
| Low | 22 | -44% | 2 | -8.70% | 20 | -74.10% | <0.001‡ | |||||
| High | 28 | -56% | 21 | -91.30% | 7 | -25.90% | ||||||
Categorical variables were expressed as number (percentage), continuous variables were expressed as mean ± SD and median (range)
* Independent samples Student's t-test; ‡Chi-square test; •Chi-square test for trend; p
Table 1: correlation between clinicopathological features, SPRY1 and H3K9 methyl-transferase G9a expression in colorectal carcinoma.
30 (60%) males and 20 (40%) females with age ranged from (29-80) years (Mean: 58.12 ± 12.02 years), 45 (90%) cases were conventional adenocarcinoma.
H3K9 methyltransferase G9a immunoexpression and its correlation clinicopathological features of CRC patients
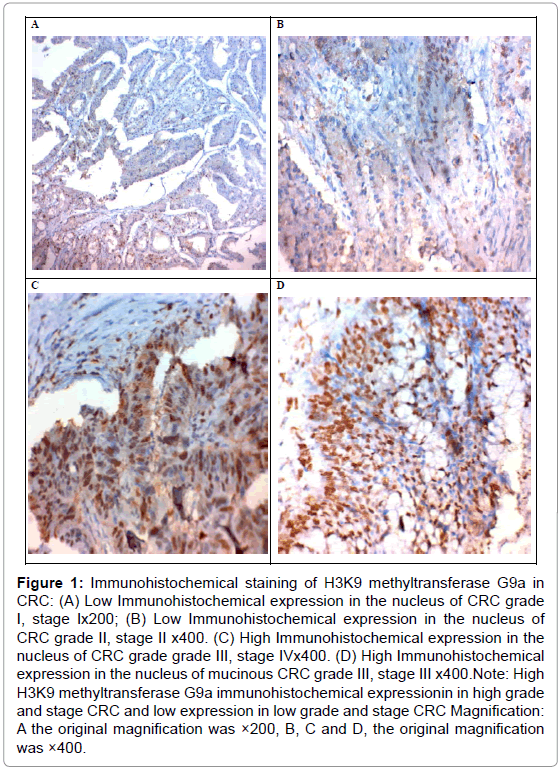
Of the 50 patients, G9a had nuclear expression in 56% (28/50) patients. The high expression of G9a in CRC was signifi cantly correlated with the tumor size, grade, stage, presence of lymph node, distant metastases (p<0.001 for all), and tumor site (p=0.002), No significant correlation was found between G9a expression and age or sex of our patients (Table 1 and Figure 1).
Figure 1: Immunohistochemical staining of H3K9 methyltransferase G9a in CRC: (A) Low Immunohistochemical expression in the nucleus of CRC grade I, stage Ix200; (B) Low Immunohistochemical expression in the nucleus of CRC grade II, stage II x400. (C) High Immunohistochemical expression in the nucleus of CRC grade grade III, stage IVx400. (D) High Immunohistochemical expression in the nucleus of mucinous CRC grade III, stage III x400.Note: High H3K9 methyltransferase G9a immunohistochemical expressionin in high grade and stage CRC and low expression in low grade and stage CRC Magnification: A the original magnification was ×200, B, C and D, the original magnification was ×400.
Sprouty 1 immunoexpression and their correlation clinicopathological features of the patients
High cytoplasmic expression of Sprouty 1 was detected in 27 out of 50 (54%) cases of CRC.
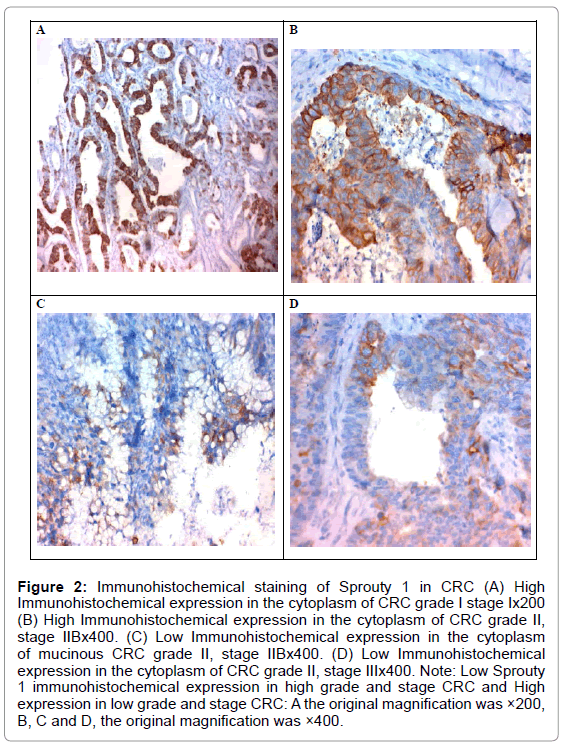
The high expression of SPRY1 was negatively correlated with the tumour size, presence of lymph node metastases (p<0.001 for both), stage (p=0.002), and presence of distant metastases (p=0.03). No significant correlation was found between SPRY1 expression, age, sex, grade, and size or tumour site (Table 1 and Figure 2)
Figure 2: Immunohistochemical staining of Sprouty 1 in CRC (A) High Immunohistochemical expression in the cytoplasm of CRC grade I stage Ix200 (B) High Immunohistochemical expression in the cytoplasm of CRC grade II, stage IIBx400. (C) Low Immunohistochemical expression in the cytoplasm of mucinous CRC grade II, stage IIBx400. (D) Low Immunohistochemical expression in the cytoplasm of CRC grade II, stage IIIx400. Note: Low Sprouty 1 immunohistochemical expression in high grade and stage CRC and High expression in low grade and stage CRC: A the original magnification was ×200, B, C and D, the original magnification was ×400.
Survival analysis
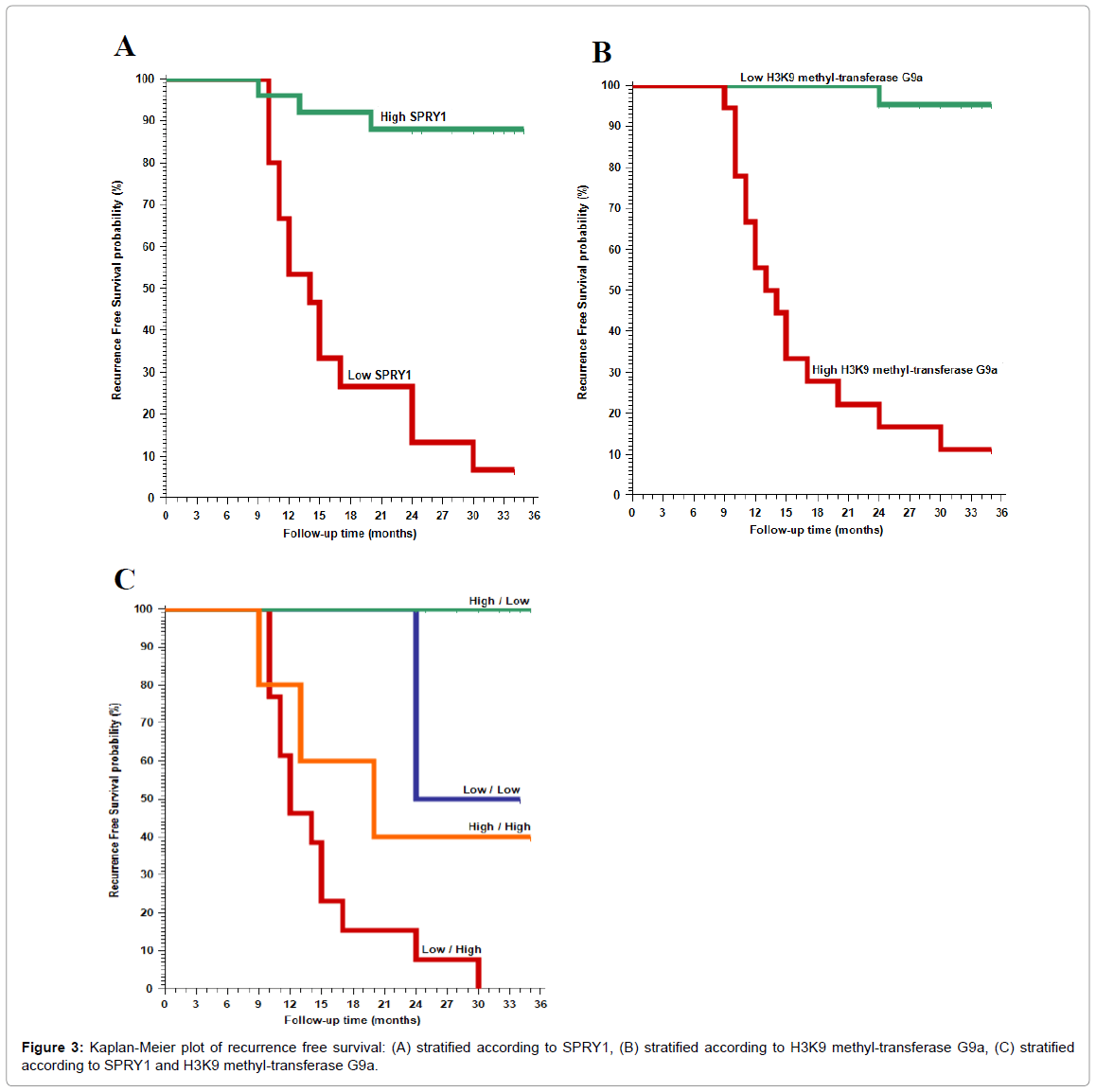
After a median follow up of 32 (range; 8-35) months, seventeen cases had recurrence of the tumor after treatment, 16 of them had high H3K9 methyltransferase G9a expression and 14 of them had low sprouty 1 expression.
The 2-year recurrence free survival rate of our patients was 95.5% and 16.7% in patients with low and high methyltransferase G9a expression respectively, and 13.3% and 88% in patients with low and high SPRY1 1expression respectively (p<0.001) (Table 2).
| Characteristics | All (N=40) No. (%) |
Recurrence | p-value | Recurrence Free Survival (RFS) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No (N=23) No. (%) |
Yes (N=17) No. (%) |
Median RFS (months) | 2 years RFS (%) | |||||||
| p-value§ | ||||||||||
| Age (years) | ||||||||||
| Mean ± SD | 55.78 | ± 11.81 | 55.3 | ± 9.81 | 56.41 | ± 14.39 | 0.774* | |||
| Median (Range) | 55 | (29-80) | 55 | (30-75) | 55 | (29-80) | ||||
| 25-62.50%15-60%10-40%0.680‡NR60%0.703 | ||||||||||
| ≥ 60 years | 15 | -37.50% | 8 | -53.30% | 7 | -46.70% | NR | 60% | ||
| Sex | ||||||||||
| Male | 24 | -60% | 13 | -54.20% | 11 | -45.80% | 0.601‡ | NR | 58.30% | 0.831 |
| Female | 16 | -40% | 10 | -62.50% | 6 | -37.50% | NR | 62.50% | ||
| Location | ||||||||||
| Ascending colon | 9 | -22.50% | 1 | -11.10% | 8 | (88.9% | 0.007‡ | 14 months | 11.10% | 0.001 |
| Transverse colon | 3 | -7.50% | 1 | -33.30% | 2 | -66.70% | NR | 82.60% | ||
| Descending colon | 5 | -12.50% | 4 | -80% | 1 | -20% | NR | 100% | ||
| Rectosigmoid | 23 | -57.50% | 17 | -73.90% | 6 | -26.10% | 15 months | 33.30% | ||
| Size (cm) | ||||||||||
| ≤ 5 cm | 22 | -55% | 19 | -86.40% | 3 | -13.60% | <0.001‡ | NR | 90.90% | <0.001 |
| > 5 cm | 18 | -45% | 4 | -22.20% | 14 | -77.80% | 13.5 months | 22.20% | ||
| Pathological Type | ||||||||||
| Adenocarcinoma | 40 | -100% | 23 | -57.50% | 17 | -42.50% | ---- | NR | 60% | ---- |
| Mucinous | --- | --- | --- | --- | --- | --- | ---- | ---- | ||
| Grade | ||||||||||
| Grade I | 7 | -17.50% | 6 | -85.70% | 1 | -14.30% | 0.205‡ | NR | 85.70% | 0.101 |
| Grade II | 33 | -82.50% | 17 | -51.50% | 16 | -48.50% | NR | 54.50% | ||
| Grade III | --- | --- | --- | --- | --- | --- | ---- | ---- | ||
| Lymph node | ||||||||||
| Negative | 21 | -52.50% | 18 | -85.70% | 3 | -14.30% | <0.001‡ | NR | 90.50% | <0.001 |
| Positive | 19 | -47.50% | 5 | -26.30% | 14 | -73.70% | 14 months | 26.30% | ||
| Stage | ||||||||||
| Stage I | 8 | -50% | 7 | -87.50% | 1 | -12.50% | 0.001• | NR | 87.50% | <0.001 |
| Stage II | 13 | -32.50% | 11 | -84.60% | 2 | -15.40% | NR | 92.30% | ||
| Stage III | 19 | -47.50% | 5 | -26.30% | 14 | -73.70% | 14 months | 26.30% | ||
| Stage IV | --- | --- | --- | --- | --- | --- | ---- | ---- | ||
| SPRY1 | ||||||||||
| Low | 15 | -37.50% | 1 | -6.70% | 14 | -93.30% | <0.001‡ | 14 months | 13.30% | <0.001 |
| High | 25 | -62.50% | 22 | -88% | 3 | -12% | NR | 88% | ||
| H3K9 methyl-transferase G9a | ||||||||||
| Low | 22 | -55% | 21 | -95.50% | 1 | -4.50% | <0.001‡ | NR | 95.50% | <0.001 |
| High | 18 | -45% | 2 | -11.10% | 16 | -88.90% | 13.5 months | 16.70% | ||
| SPRY1/H3K9 MT G9a | ||||||||||
| Low/Low | 2 | -5% | 1 | -50% | 1 | -50% | <0.001‡ | NR | 50% | <0.001 |
| Low/High | 13 | -32.50% | 0 | 0% | 13 | -100% | 12 months | 76.90% | ||
| High/Low | 20 | -50% | 20 | -100% | 0 | 0% | NR | 100% | ||
| High/High | 5 | -12.50% | 2 | -40% | 3 | -60% | 20 months | 40% | ||
Categorical variables were expressed as number (percentage), continuous variables were expressed as mean ± SD and median (range)
* Independent samples Student's t-test; ‡Chi-square test; •Chi-square test for trend; NR denote not reached yet; §Log rank test; p
Table 2: correlation between clinicopathological features, SPRY1 and H3K9 methyl-transferase G9a expression and recurrence of colorectal carcinoma.
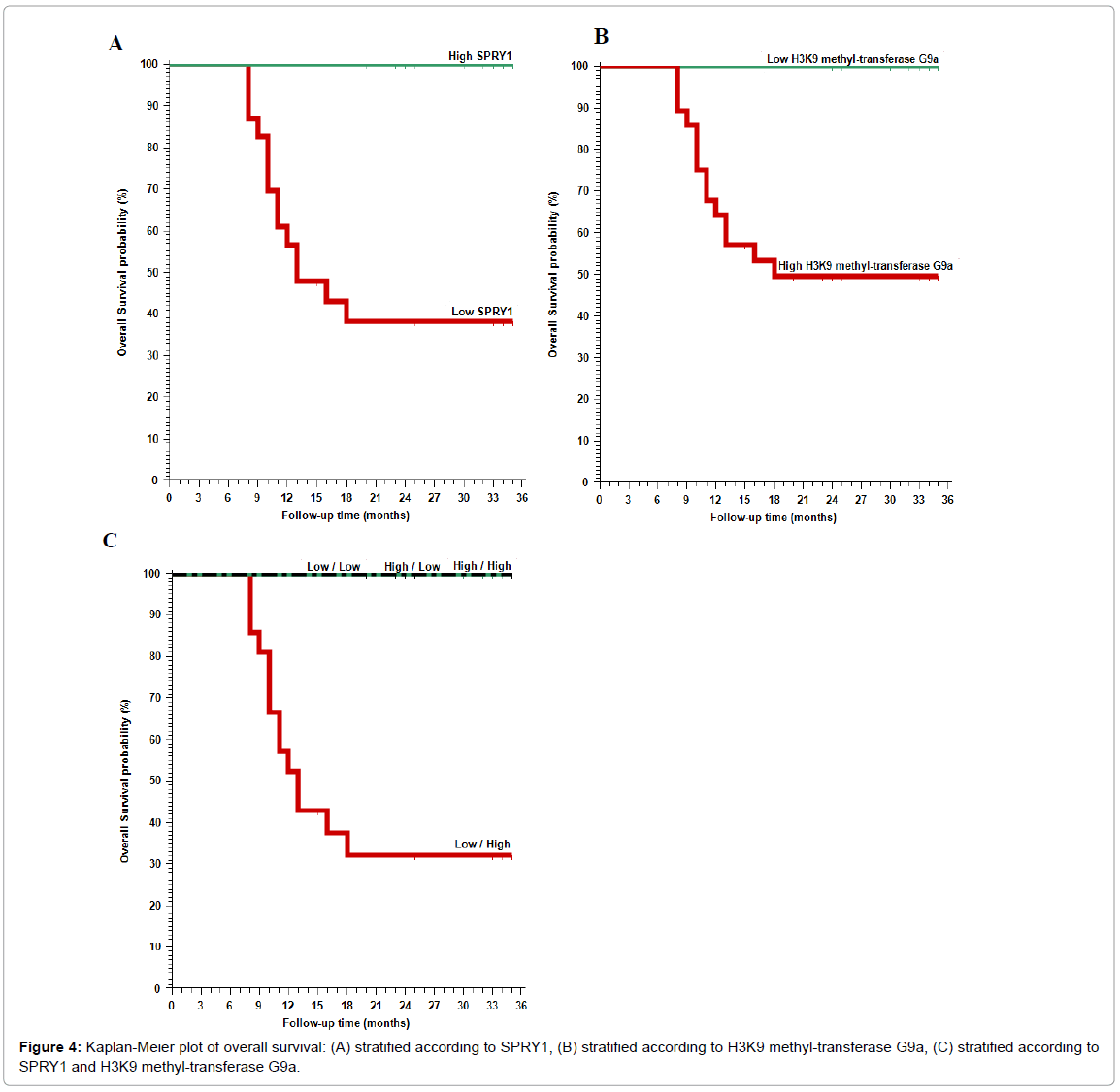
The 2-year overall survival (OS) rate was 38.3% in patients with low SPRY1 expression (p=0.008), and 49.5% in patients with high methyltransferase G9a (p<0.001).
High H3K9 methyltransferase G9a expression was significantly positively correlated with high incidence of recurrence of the cancer, worse recurrence free survival (RFS) rate and worse 2-year overall survival (OS) rate (p<0.001).
SPRY1 expression was significantly negatively correlated with high incidence of recurrence of the cancer, worse recurrence free survival (RFS) rate and worse 2-year overall survival (OS) rate (p<0.001).
Regarding expecting recurrence of CRC in our patients; the sensitivity of only high H3K9 methyltransferase G9a expression was 82.4%, sensitivity of only low SPRY1 expression while the sensitivity of combination of high H3K9 methyltransferase G9a expression and low sprouty 1 expression was 100% and the specificity was 87%.
Cases of colorectal cancer with unfavourable prognosis had low expression of SPRY1 and high expression G9a.
Both markers were negatively correlated to each other (p<0.001; Tables 1-3, Figures 3 and 4).
| Characteristics | All (N=50) No. (%) |
Survival | p-value | Overall Survival (OS) | p-value§ | ||||
|---|---|---|---|---|---|---|---|---|---|
| Alive (N=36) No. (%) |
Died (N=14) No. (%) |
Median OS (months) |
2 years OS (%) |
||||||
| Age (years) | |||||||||
| Mean ± SD | ±12.02 | 57.78 | ±10.26 | 59 | ±16.12 | 0.795* | |||
| Median (Range) | (29-80) | 55 | (30-75) | 62 | (29-80) | ||||
| -52%21-80.80%5-19.20%0.151‡NR80.80%0.119 | |||||||||
| ≥60 years | -48% | 15 | -62.50% | 9 | -37.50% | NR | 62.20% | ||
| Sex | |||||||||
| Male | -60% | 19 | -63.30% | 11 | -36.70% | 0.095‡ | NR | 63.30% | 0.094 |
| Female | -40% | 17 | -85% | 3 | -15% | NR | 85% | ||
| Location | |||||||||
| Ascending colon | -30% | 8 | -53.30% | 7 | -46.70% | 0.090‡ | NR | 51.90% | 0.111 |
| Transverse colon | -8% | 2 | -50% | 2 | -50% | NR | 80.80% | ||
| Descending colon | -10% | 5 | -100% | 0 | 0% | NR | 100% | ||
| Rectosigmoid | -52% | 21 | -80.80% | 5 | -19.20% | NR | 50% | ||
| Size (cm) | |||||||||
| ≤ 5 cm | -44% | 22 | -100% | 0 | 0% | <0.001‡ | NR | 100% | <0.001 |
| > 5 cm | -56% | 14 | -50% | 14 | -50% | 18 months | 49.50% | ||
| Pathological Type | |||||||||
| Adenocarcinoma | -90% | 34 | -75.60% | 11 | -24.40% | 0.126‡ | NR | 75.60% | 0.007 |
| Mucinous | -10% | 2 | -40% | 3 | -60% | 8 months | 40% | ||
| Grade | |||||||||
| Grade I | -14% | 7 | -100% | 0 | 0% | 0.007• | NR | 100% | 0.004 |
| Grade II | -70% | 26 | -74.30% | 9 | -25.70% | NR | 74.30% | ||
| Grade III | -16% | 3 | -37.50% | 5 | -62.50% | 10 months | 37.30% | ||
| Lymph node | |||||||||
| Negative | -42% | 21 | -100% | 0 | 0% | <0.001‡ | NR | 100% | <0.001 |
| Positive | -58% | 15 | -51.70% | 14 | -48.30% | NR | 51.30% | ||
| DM | |||||||||
| Absent | -80% | 33 | -82.50% | 7 | -17.50% | 0.003‡ | NR | 82.50% | <0.001 |
| Present | -20% | 3 | -30% | 7 | -70% | 10 months | 30% | ||
| Stage | |||||||||
| Stage I | -16% | 8 | -100% | 0 | 0% | <0.001• | NR | 100% | <0.001 |
| Stage II | -26% | 13 | -100% | 0 | 0% | NR | 100% | ||
| Stage III | -38% | 12 | -63.20% | 7 | -36.80% | NR | 63.20% | ||
| Stage IV | -20% | 3 | -30% | 7 | -70% | 10 months | 30% | ||
| SPRY1 | |||||||||
| Low | -46% | 9 | -39.10% | 14 | -60.90% | <0.001‡ | 13 months | 38.30% | <0.001 |
| High | -54% | 27 | -100% | 0 | -100% | NR | 100% | ||
| H3K9 methyl-transferase G9a | |||||||||
| Low | -44% | 22 | -100% | 0 | 0% | <0.001‡ | NR | 100% | <0.001 |
| High | -56% | 14 | -50% | 14 | -50% | 18 months | 49.50% | ||
| SPRY1/H3K9 MT G9a | |||||||||
| Low/Low | 2 | -100% | 0 | 0% | <0.001‡ | NR | 100% | <0.001 | |
| Low/High | 7 | -33.30% | 14 | -66.70% | 13 months | 32.10% | |||
| High/Low | 20 | -100% | 0 | 0% | NR | 100% | |||
| High/High | 7 | -100% | 0 | 0% | NR | 100% | |||
Categorical variables were expressed as number (percentage), continuous variables were expressed as mean ± SD and median (range)
*Independent samples Student's t-test; ‡Chi-square test; •Chi-square test for trend; NR denote not reached yet; §Log rank test; p
Table 3: correlation between clinicopathological features, SPRY1 and H3K9 methyl-transferase G9a expression and survival of patients with colorectal carcinoma.
Discussion
Here, we observed that the expression of H3K9 methyltransferase G9a in CRC was significantly positively correlated with size of the tumour, grade, stage, presence of lymph node and distant metastases (p<0.001), and location of the tumour within the colon (p=0.002) but there was none significant correlation between its expression and age or sex of our patients.
Also, patients with high H3K9 methyltransferase G9a expression had significantly poorer 2-year OS rate (p<0.001) and poorer 2-year local recurrence free survival rate (p<0.001).
So, H3K9 methyltransferase G9a found to be a recent marker for prognosis of CRC, plays an important role in its progression and metastasis and may be a novel therapeutic target for such type of cancer. These results were similar to previous reports in the CRC, serous ovarian carcinoma and endometrial cancer progression and poor prognosis [18-20].
The epigenetic changes and histone modification are important for occurrence and progression of cancer [21,22], H3K9 methyltransferase G9a plays an important role in such epigenetic changes as DNA methylation of embryonic and germ-line genes [23], necessary for the maintenance of the DNA methylation profile of mammalian cells [24], and its deletion in prostate cancer cells inhibited cell growth and led to senescence of malignant cells with telomere abnormalities [25], but the molecular link between H3K9 methylation and cancer progression remains uncertain, although there is a clear correlation among the global H3K9 methylation patterns, clinical outcome, tumour phenotypes and prognostic factors, in cancers of many organs [9]. H3K9 methyltransferase G9a can cooperate with other transcription factors to regulate gene expression [26], and is involved with important cancer sustaining cellular activities such as proliferation, autophagy, EMT, metabolic changes, specific responses to hypoxia and cancer stemness [6-8,25]. Previous researches demonstrated that H3K9 methyltransferase G9a is essential for Snail-mediated EMT [6], and required for progression and spread of cancer cells.
The epigenetic changes, unlike genetic mutations, can be reversed to their parent non mutated state by epigenetic therapy [27], which includes anticancer drugs targeting histone deacetylases and histone methyltransferase [28]. The analysis of the mechanisms underlying the roles of epigenetic alteration in tumour progression is still an early stage and the functional roles of H3K9 methyltransferase G9a in cancer remain obscure. However, based on our results data, inhibition of H3K9 methylation is a rational target for cancer therapy.
We found that expression of Spourty 1 was inversely correlated with aggressive clinic pathological features, including size of the tumour and presence of lymph node metastases (p<0.001), stage (p=0.002), and presence of distant metastases (p=0.03). No significant correlation was found between Spourty 1 expression, age or sex of our patients, grade, size and location of the tumour, also we found that Spourty 1 low-expressing patients had significantly poorer 2-year OS (p<0.001) and 2-year local recurrence free survival (p<0.001) than those with high expression of Spourty 1. There is an inverse correlation between the expression of Spourty 1 protein and growth, proliferation, migration and invasion of colorectal cancer cells, also our results identified Spourty 1 as an independent predictor of overall survival and recurrence in CRC. Many previous studies found results that were similar to ours in epithelial ovarian carcinoma (EOC), medullary thyroid carcinoma, breast cancer and osteosarcoma cells that decreased expression of Spourty 1 is a marker of poor prognosis and increasing aggressiveness of that types of cancer [29-31].
The expression of Spourty 1 in human CRC has been explored extensively but contradictory results to that of ours have been reached, Zhang et al. [32] demonstrated that Spourty suppression alters cancer cell morphology from mesenchymal to epithelial type and inhibits spread of cancer with increase in E-cadherin expression and that over expression of Spourty 1 may increase EMT markers or EMT-inducing transcription factors and increasing the incidence of metastases and associated with poor prognosis.
These opposing results were explained by that different cell lines showed different epithelial and EMT markers changed following Spourty expression. Colon cancer cell lines differ in many genetic alterations. This is evidenced by their different growth rates and response to various drugs that leads to different response to changes in Spourty 1 expression as these cells also differ in Spourty substrates and Spourty regulators [33].
The pattern of alteration in Spourty 1 expression is not similar across the whole range of CRC studied and some studies found that diminished expression of some of Spourty in colon cancer reporting elevated expression of Spourty 1 in hepatocellular carcinoma (HCC), but it proved that Spourty 1 mRNA was found insignificant when the Spry1 expression in HCC tissues was compared with its expression in cirrhotic tissue, thereby implicating other causes, including aberrant hepatocyte function, in up regulation of Spry [34].
In conclusion, we found an inverse relationship between the expression of H3K9 methyl-transferase G9a and sprouty 1in CRC and that the combination of both markers give highly significant benefit in expecting its prognosis, as cases of colorectal cancer with unfavourable prognosis had low expression of Spourty 1 and high expression of H3K9 methyl-transferase G9a that may help to find new therapeutic agents aiming to down regulating H3K9 methyl-transferase G9a and/ or stimulation of sprouty 1 in such type of cancer to decrease spread and metastases of CRC and improving its prognosis.
Other studies regarding the expression of both markers in large number of cases of CRC and different types of cancers to prove and highlight our results.
References
- Siegel R, Desantis C, Jemal A (2014) Colorectal cancer statistics, CA. Cancer J Clin 64: 104-117.
- DeSantis CE, Lin CC, Mariotto AB, Siegel RL, Stein KD, Kramer JL, et al. (2014) Cancer treatment and survivorship statistics, CA. Cancer J Clin 64: 252-271.
- Ribero D, Vigano L, Amisano M, Capussotti L (2013) Prognostic factors after resection of colorecÂtal liver metastases: from morphology to biology. Future Oncol 9: 45-57.
- Christofori G, Bill R (2015) The relevance of EMT in breast cancer metastasis: Correlation or causality. FEBS 589: 1577-1587.
- Tachibana M, Sugimoto K, Fukushima T, Shinkai Y (2001) Set domain-containing protein, G9a, is a novel lysine-preferring mammalian histone methyltransferase with hyperactivity and specific selectivity to lysines 9 and 27 of histone H3. J Biol Chem 276: 25309-25317.
- Dong C, Wu Y, Yao J, Wang Y, Yu Y, et al. (2012) G9a interacts with Snail and is critical for Snail-mediated E-cadherin repression in human breast cancer. J Clin Invest 122: 1469-1486.
- Lehnertz B, Lehnertz B, Pabst C, Su L, Miller M, et al. (2014) The methyltransferase G9a regulates HoxA9- dependent transcription in AML. Genes Dev 28: 317-327.
- Ding J, Li T, Wang X, Zhao E, Choi JH, et al. (2013) The histone H3 methyltransferase G9A epigenetically activates the serine-glycine synthesis pathway to sustain cancer cell survival and proliferation. Cell Metab 18: 896-907.
- Nakazawa T, Kondo T, Ma D, Niu D, Mochizuki K, et al. (2012) Global histone modification of histone H3 in colorectal cancer and its precursor lesions. Hum Pathol 43: 834-842.
- Hacohen N, Kramer S, Sutherland D, Hiromi Y, Krasnow MA (1998) sprouty encodes a novel antagonist of FGF signaling that patterns apical branching of the Drosophila airways. Cell 92: 253-263.
- Cabrita MA, Christofori G (2008) Sprouty proteins, masterminds of receptor tyrosine kinase signaling. Angiogenesis 11: 53-62.
- Lo TL, Fong CW, Yusoff P, McKie AB, Chua MS, et al. (2006) Sprouty and cancer: the first terms report. Cancer Lett 242: 141-150.
- Sirivatanauksorn Y, Sirivatanauksorn V, Srisawat C, Khongmanee A, Tongkham C (2012) Differential expression of sprouty genes in hepatocellular carcinoma. Journal of Surgical Oncology 105: 273-276.
- Edge SB, Compton CC (2010) AJCC Cancer Staging Manual. (7thedn) New York (NY). pp: 143-164.
- Ueno H, Kajiwara Y, Shimazaki H, Shinto E, Hashiguchi Y, et al. (2012) New Criteria for Histologic Grading of Colorectal Cancer. American Journal of Surgical Pathology 36: 193-201.
- Kwabi-Addo B, Wang J, Erdem H, Vaid A, Castro P, et al. (2004) The expression of Sprouty1, an inhibitor of fibroblast growth factor signal transduction, is decreased in human prostate cancer. Cancer Res 64: 4728-4735.
- Bai K, Cao Y, Huang C, Chen J, Zhang X, et al. (2016) Association of Histone Methyltransferase G9a and Overall Survival after Liver Resection of Patients with Hepatocellular Carcinoma with a Median Observation of 40 Months Medicine 95: e2493.
- Hua K, Wang M, Chen M, Wei L, Chen C, et al. (2014) The H3K9 methyltransferase G9a is a marker of aggressive ovarian cancer that promotes peritoneal metastasis. Molecular Cancer 13: 189.
- Zhang j, He P, Xi Y, Geng M, Chen Y, et al. (2014) Down-regulation of G9a triggers DNA damage response and inhibits colorectal cancer cells proliferation 6: 2917-2927.
- Hsiao SM, Chen MW, Chen CA, Chien MH, Hua KT, et al. (2015) The H3K9 Methyltransferase G9a Represses E-cadherin and is Associated with Myometrial Invasion in Endometrial Cancer. Ann Surg Oncol 22: 1556-1565.
- Feullgrabe J, Kavanagh E, Joseph B (2011) Histone onco-modifications. Oncogene 30: 3391-403.
- Greer EL, Shi Y (2012) Histone methylation: a dynamic mark in health, disease and inheritance. Nat Rev Genet 13: 343-57.
- Dong KB, Maksakova IA, Mohn F, Leung D, Appanah R, et al. (2008) DNA methylation in ES cells requires the lysine methyltransferase G9a but not its catalytic activity. EMBO J 27: 2691-2701.
- Ikegami K, Iwatani M, Suzuki M, Tachibana M, Shinkai Y, et al. (2007) Genome-wide and locusspecific DNA hypomethylation in G9a deficient mouse embryonic stem cells. Genes Cells 12: 1-11.
- Kondo Y, Shen L, Ahmed S, Boumber Y, Sekido Y, et al. (2008) Downregulation of histone H3 lysine 9 methyltransferase G9a induces centrosome disruption and chromosome instability in cancer cells. PLoS One 3: e2037.
- Shankar SR, Bahirvani AG, Rao VK, Bharathy N, Ow JR, et al. (2013) G9a, a multipotent regulator of gene expression. Epigenetics official journal of the DNA Methylation Society 8: 16-22.
- Kelly TK, De Carvalho DD, Jones PA (2010) Epigenetic modifications as therapeutic targets. Nat Biotechnol 28: 1069-78.
- Wagner T, Jung M (2012) New lysine methyltransferase drug targets in cancer. Nat Biotechnol 30: 622-3.
- Masoumi-Moghaddam S, Amini A, Ehteda A, Wei AQ (2015) Robertson3 G and Morris D1Sprouty 1 predicts prognosis in human epithelial ovarian cancer. Am J Cancer Res 5: 1531-1541.
- Macia A, Gallel P, Vaquero M, Gou-Fabregas M, Santacana M, et al. (2012) Sprouty1 is a candidate tumor-supÂpressor gene in medullary thyroid carcinoma. Oncogene 31: 3961-3972.
- Mekkawy AH, Morris DL (2013) Human Sprouty1 Suppresses Urokinase Receptor-Stimulated Cell Migration and Invasion. ISRN Biochem.
- Zhang Q, Wei T, Shim K, Wright K, Xu K, et al. (2016) A typical role of sprouty in colorectal cancer: sprout repression inhibits epithelial–mesenchymal transition. Oncogene 35: 3151-3162.
- Barretina J, Caponigro G, Stransky N, Venkatesan K, Margolin AA, et al. (2012) The Cancer Cell Line Encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature 483: 603-607.
- Feng YH, Wu CL, Tsao CJ, Chang JG, Lu PJ, et al. (2011) Deregulated expression of sprouty2 and microRNA-21 in human colon cancer: correlation with the clinical stage of the disease. Cancer Biology and Therapy 11: 111-121.
Citation: Haggag R, Khairy DA, Mahmoud AS, Harb OA, Salem RA, et al. (2017) Prognostic Role of H3K9 Methyltransferase G9a and Sprouty 1 in Colorectal Carcinoma. J Oncol Res Treat 2: 111.
Copyright: © 2017 Haggag R, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Open Access Journals
Article Usage
- Total views: 5429
- [From(publication date): 0-2017 - Apr 05, 2025]
- Breakdown by view type
- HTML page views: 4481
- PDF downloads: 948