Prognostic Factors for Mortality in Hospitalized Stroke Patients with COVID-19: A Retrospective Cohort Study
Received: 01-Apr-2023 / Manuscript No. JIDT-23-93878 / Editor assigned: 04-Apr-2023 / PreQC No. JIDT-23-93878 (PQ) / Reviewed: 18-Apr-2023 / QC No. JIDT-23-93878 / Revised: 25-Apr-2023 / Manuscript No. JIDT-23-93878 (R) / Published Date: 02-May-2023 DOI: 10.4172/2332-0877.1000540
Abstract
Patients with stroke are at increased risk of poor outcomes of COVID-19, yet data on prognostic factors of COVID-19 mortality in this population remained limited. In this multicenter retrospective cohort study, we investigated the clinical characteristics of hospitalized patients with COVID-19 and pre-existing stroke and assessed the risk factors for in-hospital mortality. Patients with COVID-19 and a history of stroke between January 1 and September 30, 2022, were included. Clinical data were extracted from electronic medical records. Univariate
and multivariable logistic regression analyses were conducted to identify the risk factors for in-hospital mortality. This study included 282 patients, comprising 57 (20.2%) who died (nonsurvivors) and 225 (79.8%) survivors. Multivariable analyses indicated that higher in-hospital mortality was associated with advanced age (>80 years; odds ratio [OR]: 3.46; 95% Confidence Interval [CI]: 1.70-7.03), residence in a long-term care facility (OR: 2.60; 95% CI: 1.70-5.70), a higher baseline modified Ranking scale score (OR: 1.53; 95% CI: 1.07-2.19), lymphopenia (OR: 3.12; 95% CI: 1.53-6.35), and elevated serum creatinine (OR: 3.86; 95% CI: 1.80-8.26) in patients with COVID-19 and pre-existing stroke.
Keywords: COVID-19; Mortality; Stroke; Risk factors
Introduction
Materials and Methods
Patient selection and definitions
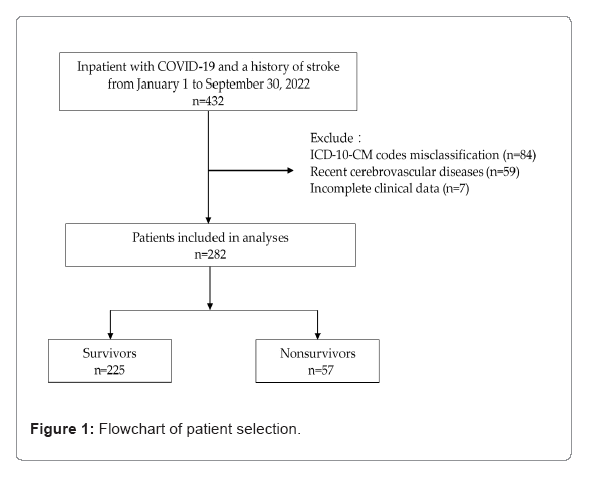
We conducted this retrospective multicenter study at a branch of the Chi Mei health-care system in Taiwan, which includes a tertiary referral hospital, regional hospital, and district hospital. Using the data based on International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10-CM) codes, we identified all patients hospitalized between January 1 and September 30, 2022 (Figure 1). Patients aged ≥ 20 years with a diagnosis of cerebrovascular disease (ICD-10-CM codes: I60–I69) before the index date were enrolled. The index date was defined as the date of diagnosis of SARS-CoV-2 infection based on a positive real-time fluorescence Reverse Transcription- Polymerase Chain Reaction (RT-PCR) test from a nasal or pharyngeal specimen obtained in an emergency department or ward. We manually reviewed the patients’ electronic medical records and excluded those with incorrect ICD-10-CM codes, cerebrovascular events within 7 days before the index date, or incomplete clinical data. This study was conducted in accordance with the principles of the Declaration of Helsinki and was approved by the Institutional Review Board of Chi Mei Medical Center (11111-007) (Figure 1).
Data collection and study outcomes
We extracted the following information from electronic medical records: baseline demographic data, COVID-19 vaccination information, admission and discharge dates, diagnoses and corresponding ICD-10-CM codes, modified Rankin Scale (mRS) scores, vital signs and laboratory measurements obtained within 48 h of arrival to the emergency department or the dates of confirmation of SARS-CoV-2 infection, and details of treatment during hospitalization, such as antiviral or antibiotic therapy or vasopressor use. Poststroke functional status was scored according to the mRS as follows: 0 indicated no symptoms, 1 indicated no significant disability despite symptoms (able to carry out all usual duties and activities), 2 indicated slight disability (unable to carry out all usual activities but able to look after own affairs without assistance), 3 indicated moderate disability (requiring some help but able to walk without assistance), 4 indicated moderately severe disability (unable to walk and attend to bodily needs without assistance), 5 indicated severe disability (bedridden, incontinent, and requiring constant nursing care and attention), and 6 indicated death. The study objective was to identify risk factors for in- hospital mortality in patients with pre-existing stroke and COVID-19.
Statistical analysis
Continuous and categorical variables are presented as medians with interquartile ranges and frequencies with percentages, respectively. Comparisons between survivors and nonsurvivors were performed using the Mann–Whitney U test for continuous variables and Pearson’s chi-square test or Fisher’s exact test for categorical variables. The associations between mortality and selected confounding factors were also estimated through logistic regression and reported as Odds Ratios (ORs) with 95% Confidence Intervals (CIs). Key predictors of mortality were identified through multivariable logistic regression analysis including only variables that were significant (p<0.05) in the univariate analyses. All analyses were performed using SAS software (version 9.4; SAS Institute, Cary, NC, USA). The statistical significance level was set at p<0.05.
Results
During the 9-month study period, 282 patients (182 men [65%]) met the inclusion criteria, of whom 57 (20.2%) died (nonsurvivors) and 225 (79.8%) survived. The median age of the study population was 79 (69-87) years, with 136 (48%) patients aged ≥ 80 years. In total, 82 people (29%) were transferred from long-term care facilities (LTCFs).
The baseline clinicodemographic characteristics differed between the survivors and nonsurvivors (Table 1). The nonsurvivors were older (p<0.001), more likely to reside in LTCFs (p<0.001), and had higher mRS scores (p<0.001) than were the survivors. However, the prevalence of comorbidities was similar between the two groups. Significantly higher percentages of the nonsurvivors had lymphopenia (absolute lymphocyte count <1 × 109/L; p=0.005) and creatinine levels >1.5 mg/ dL (p<0.001; Table 2). Moreover, antiviral drug use was comparable between the survivors and nonsurvivors, except for that of remdesivir (p=0.021; Table 3).
| Variables | Survivors (n=225) Median (IQR)/ n (%) | Nonsurvivors (n=57) Median (IQR)/ n (%) | p-value* |
| Characteristics | |||
| Age (years) | 77.00 (68.00, 86.00) | 84.00 (79.00, 89.00) | <0.001 |
| Age (years) | <0.001 | ||
| <80 | 131(58.22) | 15 (26.32) | |
| ≥80 | 94 (41.78) | 42 (73.68) | |
| Sex | 0.493 | ||
| Male | 143 (63.56) | 39 (68.42) | |
| Female | 82 (36.44) | 18 (31.58) | |
| Location before admission | <0.001 | ||
| Home | 171 (76.00) | 29 (50.88) | |
| Long-term care facility | 54 (24.00) | 28 (49.12) | |
| Pre-hospitalization mRS | 4.00 (3.00, 5.00) | 4.00 (4.00, 5.00) | <0.001 |
| Smoking | 0.475 | ||
| Never | 159 (70.67) | 43(75.44) | |
| Ever/current | 66 (29.33) | 14 (24.56) | |
| COVID-19 vaccination status (doses) | 0.103 | ||
| <2 | 107 (47.56) | 34 (59.65) | |
| ≥2 | 118 (52.44) | 23 (40.35) | |
| Comorbidities | |||
| Hypertension | 137 (60.89) | 37(64.91) | 0.577 |
| Diabetes | 89 (39.56) | 26 (45.61) | 0.406 |
| Atrial fibrillation | 43 (19.11) | 11 (19.30) | 0.974 |
| Heart failure | 19 (8.44) | 8 (14.04) | 0.2 |
| Chronic kidney disease | 23 (10.22) | 9 (15.79) | 0.237 |
| Chronic respiratory disease | 29 (12.89) | 12 (21.05) | 0.118 |
*Continuous variables were analyzed using the Mann–Whitney U test, and categorical variables were analyzed using the chi-square test.
Table 1: Baseline characteristics and outcomes of included patients.
| Variables | Survivors (n=225) n (%) | Non-survivors (n=57) n (%) | p-value* |
|---|---|---|---|
| Vital signs | |||
| Respiratory rate (breaths per minute) | 0.058 | ||
| ≤ 30 | 223 (99.11) | 54 (94.74) | - |
| >30 | 2 (0.89) | 3 (5.26) | |
| Systolic blood pressure (mmHg) | 1 | ||
| <90 | 19 (8.44) | 4 (7.02) | - |
| ≥ 90 | 206 (91.56) | 53 (92.98) | |
| SpO2 (%) | 0.011 | ||
| <94 | 23 (10.22) | 13 (22.81) | - |
| ≥ 94 | 202 (89.78) | 44 (77.19) | |
| Laboratory data | |||
| White blood cell count (× 109/L) | 0.252 | ||
| 4-10 | 141 (62.67) | 31 (54.39) | - |
| <4 or >10 | 84 (37.33) | 26 (45.61) | |
| Absolute lymphocyte count (× 109/L) | 0.005 | ||
| <1 | 111 (49.33) | 40 (70.18) | - |
| ≥ 1 | 114 (50.67) | 17 (29.82) | |
| Platelet count (× 103/cmm) | 0.633 | ||
| <150 | 60 (26.67) | 17 (29.82) | - |
| ≥ 150 | 165 (73.33) | 40 (70.18) | |
| Creatinine (mg/dL) # | <0.001 | ||
| ≤ 1.5 | 174 (77.33) | 32 (56.14) | - |
| >1.5 | 51 (22.67) | 25 (43.86) | |
| GPT (units/L) | 0.697 | ||
| ≤ 40 | 194 (86.22) | 48 (84.21) | - |
| >40 | 31 (13.78) | 9 (15.79) | |
Note: *Categorical variables were analysed using the chi-square test or Fisher’s exact test. #7 patients (1 non-survivor and 6 survivors) were excluded because their creatinine data were unavailable.
Table 2: Clinical data of survivors and non-survivors.
| Variables | Survivors (n=225) n (%) | Non-survivors (n=57) n (%) | p-value* |
|---|---|---|---|
| Remdesivir | 0.021 | ||
| No | 97 (43.11) | 15 (26.32) | - |
| Yes | 128 (56.89) | 42 (73.68) | |
| Nirmatrelvir-ritonavir | 0.538# | ||
| No | 210 (93.33) | 55 (96.49) | - |
| Yes | 15 (6.67) | 2 (3.51) | |
| Molnupiravir | 0.162 | ||
| No | 199 (88.44) | 54 (94.74) | - |
| Yes | 26 (11.56) | 3 (5.26) | |
| Tocilizumab | 0.121# | ||
| No | 219 (97.33) | 53 (92.98) | - |
| Yes | 6 (2.67) | 4 (7.02) | |
| Vasopressor | 0.043 | ||
| No | 206 (91.56) | 47 (82.46) | - |
| Yes | 19 (8.44) | 10 (17.54) | |
Note: *Categorical variables were analysed using the chi-square test or Fisher’s exact test. #Fisher’s exact test.
Table 3: Distribution of treatment with various drugs among survivors and non-survivors.
All available data were included in the univariate logistic regression analyses (Table 4). In the unadjusted analyses, the odds of mortality were higher for patients who were older (p<0.001), lived in LTCFs (p<0.001), had higher prehospitalization mRS scores (p<0.001), exhibited tachypnea (p = 0.049) or desaturation at baseline (p=0.013), had lymphopenia (p=0.006), or had elevated creatinine levels (p=0.002). In the multivariable analysis, an age ≥ 80 years (OR: 3.46, 95% CI: 1.70-7.03), transfer from an LTCF (OR: 2.60, 95% CI: 1.70- 5.70), higher baseline mRS score (OR: 1.53, 95% CI: 1.07-2.19), lower absolute lymphocyte count (OR: 3.12; 95% CI: 1.53-6.35) and higher creatinine levels (OR: 3.86; 95% CI: 1.80-8.26) remained independently associated with in-hospital mortality (Table 4).
| Variables | Univariate logistic regression | Multivariable logistic regression | ||
|---|---|---|---|---|
| Odds ratio (95% CI) | p-value | Odds ratio (95% CI) | p-value | |
| Characteristic | ||||
| Age ≥ 80 | 3.90 (2.04, 7.45) | <0.001 | 3.46 (1.70, 7.03) | <0.001 |
| Female | 0.81 (0.43, 1.50) | 0.493 | - | - |
| Long-term care facility before admission | 3.06 (1.67, 5.59) | <0.001 | 2.60 (1.70, 5.70) | 0.017 |
| Pre-hospitalization mRS | 1.71 (1.29, 2.27) | <0.001 | 1.53 (1.07, 2.19) | 0.021 |
| Ever or current smoking | 0.78 (0.40, 1.53) | 0.476 | - | - |
| COVID-19 vaccination with ≥ 2 doses | 0.61 (0.34, 1.11) | 0.105 | - | - |
| Comorbidity | ||||
| Hypertension | 1.19 (0.65, 2.18) | 0.577 | - | - |
| Diabetes | 1.28 (0.71, 2.30) | 0.406 | - | - |
| Atrial fibrillation | 1.01 (0.48, 2.12) | 0.974 | - | - |
| Heart failure | 1.77 (0.73, 4.28) | 0.205 | - | - |
| Chronic kidney disease | 1.65 (0.72, 3.79) | 0.24 | - | - |
| Chronic respiratory disease | 1.80 (0.85, 3.80) | 0.122 | - | - |
| Vital signs | ||||
| Respiratory rate >30 (breaths per minute) | 6.19 (1.01, 37.95) | 0.049 | 5.02 (0.47, 53.61) | 0.182 |
| SBP ≥ 90 (mmHg) | 1.22 (0.40, 3.74) | 0.726 | - | - |
| SpO2 <94 (%) | 2.60 (1.22, 5.51) | 0.013 | 1.90 (0.78, 4.61) | 0.159 |
| Laboratory data | ||||
| WBC 4-10 (×109/L) | 1.41 (0.78, 2.53) | 0.253 | - | - |
| ALC <1 (× 109/L) | 2.42 (1.29, 4.51) | 0.006 | 3.12 (1.53, 6.35) | 0.002 |
| Platelet ≥ 150 (× 103/cmm) | 0.86 (0.45, 1.62) | 0.633 | - | - |
| Creatinine >1.5 (mg/dL) | 2.67 (1.45, 4.90) | 0.002 | 3.86 (1.80, 8.26) | <0.001 |
| GPT >40 (units/L) | 1.17 (0.52, 2.63) | 0.698 | - | |
Table 4: Associations between mortality and selected risk factors.
Discussion
In this retrospective study, we explored the risk factors for the in- hospital mortality of patients with COVID-19 and pre-existing stroke. Of the 282 patients, 20.6% (57) died during hospitalization. This mortality rate is higher than the 7% reported by Portmann et al.; this difference may be due to their younger cohort (median age: 71 years) and exclusive analysis of patient with the Omicron variant [6]. By contrast, our cohort had a higher proportion of patients at an advanced age (≥ 80 years; 48.3%; 136/282), which was associated with a greater likelihood of in-hospital mortality (30.9%, 42/136). In another study, the mortality rate among COVID-19 people aged >80 years was high (30.6%), consistent with that in our study [7].
Our multivariable logistic regression analysis revealed the following risk factors to be independently associated with mortality: a higher poststroke mRS score, transfer from an LTCF, advanced age (>80 years), lymphopenia, and elevated creatinine level. The mRS is widely used and reliable for assessing functional status in patients with stroke, and higher mRS scores indicate more severe neurological disability, predisposing patients to infection, especially pneumonia, due to poor swallowing and cough function and higher risk of immunosuppression; and a higher risk of all-cause mortality [8-10]. Our data also indicated that poststroke mRS score is an independent risk factor for in-hospital mortality in patients with COVID-19, corroborating results suggesting worse COVID-19 outcomes in patients with stroke and higher mRS scores [11].
Consistent with previous studies, we discovered that patients who transferred from an LTCF exhibited a greater mortality risk from COVID-19 than did those who lived at home [12]. In addition, patients with stroke who reside in LTCFs are more severely functionally impaired or have a higher prevalence of comorbidities, and these have also been identified as independent risk factors for COVID-19 mortality [2,12].
As mentioned, advanced age is a risk factor for mortality [13]. Recent data indicate that patients with comorbidities such as hypertension and diabetes are at an increased risk of COVID-19 mortality [2]. Although over half of the COVID-19 nonsurvivors in our analysis had hypertension and 47% had diabetes, no comorbidities were discovered to be risk factors for COVID-19-related mortality. This lack of significant findings regarding comorbidities may be due to our limited sample size.
SARS-CoV-2 can directly or indirectly cause lymphocyte death, particularly that of T lymphocytes, inducing a more potent cytokine storm and inflammatory response and worsening the disease and prognosis [14,15]. Our data indicate an association between lymphopenia and increased mortality risk. In addition, creatinine levels were elevated among nonsurvivors in our study, and recent data suggest a correlation between impaired renal function and COVID-19 mortality [16].
Our study has several limitations. First, this study was conducted at three hospitals within a single health-care system and thus analyzed only a small number of cases. Second, we obtained data from electronic medical records; thus, missing data and misdiagnoses were possible. To minimize the potential for bias, we manually confirmed the accuracy and completeness of the data. Third, we defined COVID-19 on the basis of positive RT-PCR results for SARS-CoV-2; however, although the Omicron variant was the predominant strain during our study period, we did not differentiate and analyze individual viral strains, which may affect prognosis.
Finally, we analyzed all-cause mortality instead of COVID-19 mortality as the outcome, but this limited our ability to analyze the risk of COVID-19-specific deaths. Future studies should compare COVID-19 mortality with all-cause mortality in patients with stroke to assess the impact of COVID-19 on this population.
Conclusion
In conclusion, patients with a history of stroke who are hospitalized for COVID-19 are at a significantly higher risk of mortality if they have a high prehospitalization mRS score. Additionally, patients aged ≥ 80 years, residing in LTCFs before admission, having lymphopenia, or having creatinine levels >1.5 mg/dL may also have a greater risk of mortality. These patient groups require more rigorous monitoring and aggressive treatment.
Acknowledgement
This manuscript was edited by Wallace Academic Editing.
References
- World Health Organization. (2023) Coronavirus disease (COVID-19) pandemic.
- Ge E, Li Y, Wu S, Candido E, Wei X (2021) Association of pre-existing comorbidities with mortality and disease severity among 167,500 individuals with COVID-19 in Canada: A population-based cohort study. PLoS One 16: e0258154.
[Crossref] [Google Scholar] [PubMed]
- Patel U, Malik P, Shah D, Patel A, Dhamoon M, et al. (2021) Pre-existing cerebrovascular disease and poor outcomes of COVID-19 hospitalized patients: a meta-analysis. J Neurol 268: 240-247.
[Crossref] [Google Scholar] [PubMed]
- Kummer BR, Klang E, Stein LK, Dhamoon MS, Jette N (2020) History of stroke is independently associated with in-hospital death in patients with COVID-19. Stroke 51: 3112-3114.
[Crossref] [Google Scholar] [PubMed]
- Nia AM, Srinivasan VM, Hayworth MK, Lall RR, Kan P (2022) A history of cerebrovascular disease is independently associated with increased morbidity and mortality in patients with COVID-19: a cohort study of 369,563 COVID-19 cases in the USA. Cerebrovasc Dis 51: 20-28.
[Crossref] [Google Scholar] [PubMed]
- Portmann L, de Kraker MEA, Frohlich G, Thiabaud A, Roelens M, et al. (2023) Hospital outcomes of community-acquired SARS-CoV-2 omicron variant infection compared with influenza infection in switzerland. JAMA Netw Open 6: e2255599.
[Crossref] [Google Scholar] [PubMed]
- Diaz-Menendez M, de la Calle-Prieto F, Montejano R, Arsuaga M, Jimenez-Gonzalez M, et al. (2022) Clinical characteristics and outcome of hospitalized elderly patients with COVID- 19 after vaccine failure. Vaccine 40: 4307-4311.
[Crossref] [Google Scholar] [PubMed]
- Kasner SE (2006) Clinical interpretation and use of stroke scales. Lancet Neurol 5: 603-612.
[Crossref] [Google Scholar] [PubMed]
- Elkind M S V, Boehme A K, Smith C J, Meisel A, Buckwalter M S (2020) Infection as a stroke risk factor and determinant of outcome after stroke. Stroke 51: 3156-3168.
[Crossref] [Google Scholar] [PubMed]
- Huybrechts K F, Caro J J, Xenakis J J, Vemmos K N (2008) The prognostic value of the modified Rankin Scale score for long-term survival after first-ever stroke. Results from the athens stroke registry. Cerebrovasc Dis 26: 381-7.
[Crossref] [Google Scholar] [PubMed]
- Wang M, Zhang H, He Y, Qin C, Liu X, et al. (2021) association between ischemic stroke and covid-19 in china: a population-based retrospective study. Front Med (Lausanne) 8: 792487.
[Crossref] [Google Scholar] [PubMed]
- Andrade J A, Muzykovsky K, Truong J (2021) Risk factors for mortality in COVID-19 patients in a community teaching hospital. J Med Virol 93: 3184-3193.
[Crossref] [Google Scholar] [PubMed]
- Rosenthal N, Cao Z, Gundrum J, Sianis J, Safo S (2020) Risk factors associated with in-hospital mortality in a US national sample of patients with COVID-19. JAMA Netw Open 3: e2029058.
[Crossref] [Google Scholar] [PubMed]
- Qin C, Zhou L, Hu Z, Yang S, Zhang S, et al. (2020) Clinical characteristics and outcomes of COVID-19 patients with a history of stroke in Wuhan, China. Stroke 51: 2219-2223.
[Crossref] [Google Scholar] [PubMed]
- Niu J, Sareli C, Mayer D, Visbal A, Sareli A (2022) Lymphopenia as a predictor for adverse clinical outcomes in hospitalized patients with COVID-19: a single center retrospective study of 4485 cases. J Clin Med 11: 700.
[Crossref] [Google Scholar] [PubMed]
- Charif F, Mahdi Z, Bousgheiri F, Belafki H, Gourinda A, et al. (2022) Predictive factors of death and the clinical profile of hospitalized COVID-19 patients in Morocco: a one-year mixed cohort study. Cureus 14: e32462.
[Crossref] [Google Scholar] [PubMed]
Citation: Lu KH, Chang TC, Lai CC, Kan WC, Ho CH, et al. (2023) Prognostic Factors for Mortality in Hospitalized Stroke Patients with COVID-19: A Retrospective Cohort Study. J Infect Dis Ther 11: 540. DOI: 10.4172/2332-0877.1000540
Copyright: © 2023 Lu KH, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 2235
- [From(publication date): 0-2023 - Nov 08, 2025]
- Breakdown by view type
- HTML page views: 1894
- PDF downloads: 341