Prognostic and Predictive Values of cell Cycle Proteins Centrosomal Protein 5 (CEPP 5)
Received: 19-Sep-2017 / Accepted Date: 09-Oct-2017 / Published Date: 19-Oct-2017 DOI: 10.4172/2476-2024.1000127
Abstract
Background: Disturbances in the expressions of centrosomal proteins (CEPs) and regulatory proteins that control G1-Sphase transition, like cyclins could participate in dysregulation of cell cycle control that has been incriminated in the pathogenesis of several malignancies. Centrosomal protein 55 (CEP55) has an important role in participation in the final stage of cell division, and cell cycle progression. CEP55 and Cyclin D1 expressions were detected in several tumors but their prognostic and predictive roles in epithelial ovarian carcinoma (EOC) are still studied.
Aim of the study: Explore tissue expressions of CEPP55 and Cyclin D1 in EOC correlating their expression with pathological, clinical and prognostic parameters.
Methods: CEP55 and Cyclin D1 expressions were evaluated in tissue biopsies that are retrieved from 60 cases of epithelial ovarian carcinoma using immunohistochemistry, patients that were followed up for 3 years. The relationship between their level of expressions and degree of differentiation, spread of the tumor, disease recurrence, response to therapy and survival were studied.
Results: CEP55 expression in EOC was positively correlated with loss of differentiation of the tumor, presence of L.N (p<0.001), and distant metastases (p=0.012) and advanced stage of the tumor (p=0.007), cyclin D1 expression in EOC was positively correlated with loss of differentiation and advanced stage of the tumor, presence of L.N (p<0.001), and distant metastases (p=0.009). CEPP 55 and Cyclin D1 were positively correlated with each other. Low CEPP 55 and Cyclin D1 expressions were strongly correlated with optimal surgical eradication of the tumor, increased 3-year overall survival (OS) and low incidence of tumor recurrence after therapy (P<0.001).
Conclusion: High levels of expression of CEPP 55 and Cyclin D1and are markers of poor prognosis in EOC patients.
Keywords: CEP55; Cyclin D1 epithelial ovarian carcinoma; Prognosfis
Introduction
Despite there is improvement in different therapeutic medical and surgical modalities for ovarian cancer patients managements, that have resulted in marked improvement in prognosis and survival rates of patients having such cancer type, it is still a serious type of female cancer that have the highest motility worldwide [1]. So several studies have been done regarding the prognostic roles of recent biomarkers in a trial to identify novel therapies that could improve the survival and relapse rates of ovarian carcinoma patients.
The centrosome that is an important cellular structure had many cellular functions e.g. a microtubule-organizer in human cells and it is involved in normal cell division [2]. Centrosomal proteins (CEPs) are the molecules that are found inside the centrosome and are participating in the regulation of its related functions [3]. The centrosomal-protein 55 (CEP55), is a centrosome- associated protein has assize of about ~55 kDa and had been mapped on the 10q23 chromosomal location [4]. CEP55 was detected to have many roles in centrosome associated cellular functions, like centrosomal duplication, progression of cell cycle and in cytokinesis regulation [5,6]. CEP55 expression have been detected in a plethora of malignant tumors that might be related to oncogenesis onset, malignant proliferation and invasion [7], so it is considered a promising biomarker for prognosis of cancer patients and targeted therapies [8]. But, its pathogenic and prognostic roles in EOC development remain unclear and need further investigations.
There are many disturbances were observed in cell cycle regulatory proteins expressions as cyclins which could control G1-Sphase transition. Such step is considered an important rate-limiting step in the progression of cell cycle. Cyclins and cyclin-dependent kinases (cdks) are essential structures of the cell cycle that are responsible for cell cycle regulation [9]. Cyclin D1 is a member of the D-cyclins family that has 3 members, cyclin D1, D2 and D3 and selectively could be able to control the progression of cell cycle [10]. Roles of Cyclin D1 expression in different tumors, especially in ovarian cancer, had been found to have contradictory results that need further clarifications.
Aim of the study was to explore tissue expressions of CEPP55 and Cyclin D1 in EOC correlating their expression with pathological, clinical and prognostic parameters.
Patients And Methods
This is a prospective cohort study where we included 60 patients, clinically and radiologically diagnosed to have ovarian cancer, that were admitted to general surgery hospital, oncology unit and department of gynecology and obstetrics, faculty of medicine, Zagazig university. Radical dissection of the tumor was done, and excised tumors sent to pathology department, faculty of medicine, Zagazig university where tissues were processed, prepared for routine hematoxylin and eosin staining, diagnosed as epithelial ovarian carcinoma (EOC) of different histopathological types, for staging of the EOC we have used TNM [tumor-node-metastasis and FIGO (International Federation of Gynecology and Obstetrics)] systems [11], while, we have used WHO grading system for pathological grading [12]. We stained sections from 60 paraffin blocks retrieved from all cases with both CEP55 and Cyclin D1 using immunohistochemistry, assessed expression of both markers in tumor tissue, analyzed correlations between the levels of markers expression with pathological parameters e.g. histopathological subtype, grade, stage, lymph node (LN) and distant metastases, clinical parameters as age of the patient, prognostic and follow up parameters like recurrence, survival and response to therapy. All slides are reviewed and revaluated by pathologists from pathology department, faculty of medicine, Benghazi university, Benghazi, Libya We followed our patients for 3 years in both medical and clinical oncology and nuclear medicine departments, faculty of medicine, Zagazig University.
Immunohistochemical staining
Immunohistochemistry was done by streptavidine-biotin method [13], we cut sections from the paraffin-embedded blocks of about 4μm thick put on positively charged slides then incubated them at 65°C for 30 min, we deparaffinized sections with xylene, then rehydrated them, submerged into EDTA buffer then microwaved for antigen retrieval, to antagonize endogenous peroxidase activity we added 3%hydrogen peroxide in methanol to sections, then we incubated them with 1% bovine serum albumin (BSA) to overcome any nonspecific binding. Sections were incubated with a rabbit monoclonal anti-CEP55 (Abcam, ab170414, 1:250) and anti-Cyclin D1 (Abcam, ab134175, 1:1-00) antibodies overnight at 4°C. we washed sections then incubated them with anti-rabbit biotinylated secondary antibody (Abcam), followed by a streptavidin-horseradish peroxidase complex (Abcam) and finally we counterstained sections with 10% Mayer’s hematoxylin, we dehydrated the slides and mounted them in crystal mount. The degree of immunoreactivity of CEP55 and Cyclin D1 was reviewed and evaluated by pathologist from pathology department, faculty of medicine, Zagazig university, Zagazig, Egypt and pathologists from pathology department, faculty of medicine, Benghazi university, Benghazi, Libya.
Scores of the intensity and extent of immune-reactivity that are given by all pathologists were averaged.
Evaluation of immunostaining of CEPP5 and Cyclin D1
We considered stained slides positive for CEPP5 and Cyclin D1 when we detected brown cytoplasmic expression and brown nuclear expression respectively, in more than or equal to ≥ 1% of the tumor cells. Then we scored the extent and intensity of stain, multiplied them in each other to result in the final staining index (SI).
We scored extent of stain as will follow: 1 (<10% positivity in cancer cells), 2 (10-50% positivity in cancer cells), 3 (50-75% positivity in cancer cells), and 4 (>75% positivity in cancer cells). We scored intensity of stain on a scale of 0 (no positivity in cancer cells), 1 (weak positivity in cancer cells=light yellow), 2 (moderate positivity in cancer cells=yellow brown), and 3 (strong positivity in cancer cells=brown). The final SI was ranged from 0 to 12, and optimal SI cutoff value 6 were chosen and we used SI of ≥ 6 to define tumors with CEP55 over expression, and a score of ≤ 6 to define tumors with low expression [14]. The final SI was ranged from 0 to 12, and optimal SI cutoff value 4 were chosen and we used SI of ≥ 4 to define tumors with Cyclin D1 over expression, and a score of ≤ 4 to define tumors with low expression [15].
Results
Patient clinicopathological results
Age of our 60 patients with EOC was ranged from (25-75) years and the Mean age is 55.53 ± 10.53 years. We diagnosed 35 (58.3%) cases as serous ovarian carcinoma (SOC), 15 (25%) as mucinous ovarian carcinoma (MOC) and 10 (16.7%) as endometroid ovarian carcinoma 37 (61.7%) cases have high grade and 23 (38.3%) cases with low grade EOC, distant metastases are present in 14 (23.3%) of our cases (Table 1).
| Characteristics | All patients (N=60) | Characteristics | All patients (N=60) | |
|---|---|---|---|---|
| Age (years) | Operation | |||
| Mean ± SD | 55.53 ± 10.53 | Radical surgery | 15 (25%) | |
| Median (Range) | 57 (25 – 75) | Suboptimal | 18 (30%) | |
| 4 (6.7%) Optimal27 (45%) | ||||
| 41-59 years | 34 (56.7%) | |||
| ≥ 60 years | 22 (36.7%) | |||
| Histopathology | ECOG PS | |||
| Serous | 35 (58.3%) | ECOG 1 | 42 (70%) | |
| Mucinous | 15 (25%) | ECOG 2 | 18 (30%) | |
| Endometroid | 10 (16.7%) | |||
| Positive cytology | Number of cycles | (N=57) | ||
| Absent | 39 (65%) | 4 cycles | 10 (17.5%) | |
| Present | 21 (35%) | 6 cycles | 8 (14%) | |
| 8 cycles | 39 (68.4%) | |||
| CA125 | Response | (N=45) | ||
| ≤ 35U/ml | 21 (35%) | NR | 10 (22.2%) | |
| >35U/ml | 39 (65%) | OAR | 35 (77.8%) | |
| Bilaterality | Response after 4-6 cycles | (N=45) | ||
| Unilateral | 44 (73.3%) | PD | 3 (6.7%) | |
| Bilateral | 16 (26.7%) | SD | 10 (22.2%) | |
| Implants | PR | 29 (64.4%) | ||
| Absent | 38 (63.3%) | CR | 3 (6.7%) | |
| Present | 22 (36.7%) | |||
| Ascites | Response after 8 cycles | (N=45) | ||
| Absent | 38 (63.3%) | PD | 3 (6.7%) | |
| Present | 22 (36.7%) | SD | 7 (15.6%) | |
| Grade | PR | 7 (15.6%) | ||
| Low | 23 (38.3%) | CR | 28 (62.2%) | |
| High | 37 (61.7%) | |||
| LN | Follow-up duration (months) | |||
| Node negative | 21 (35%) | Mean ± SD | 17.01 ±9.15 | |
| Node positive | 39 (65%) | Median (Range) | 11 (10 – 36) | |
| M | Recurrence | (N=43) | ||
| M0 (non-metastatic) | 46 (76.7%) | Absent | 12 (27.9%) | |
| M1 (metastatic) | 14 (23.3%) | Present | 31 (72.1%) | |
| FIGO Stage | Chemosensitivity | (N=31) | ||
| Stage IA | 2 (3.3%) | Chemosensitive | 11 (35.5%) | |
| Stage IB | 1 (1.7%) | Chemorefractory | 20 (64.5%) | |
| Stage IC | 2 (3.3%) | Death | ||
| Stage IIA | 3 (5%) | Alive | 28 (46.7%) | |
| Stage IIB | 7 (11.7%) | Died | 32 (53.3%) | |
| Stage IIC | 6 (10%) | |||
| Stage IIIA | 9 (15%) | |||
| Stage IIIB | 12 (20%) | |||
| Stage IIIC | 4 (6.7%) | |||
| Stage IV | 14 (23.3%) |
Categorical variables were expressed as number (percentage), Continuous variables were expressed as mean ± SD & median (range).
Table 1: Clinicopathological and follow up criteria of our patients
Immunohistochemical results
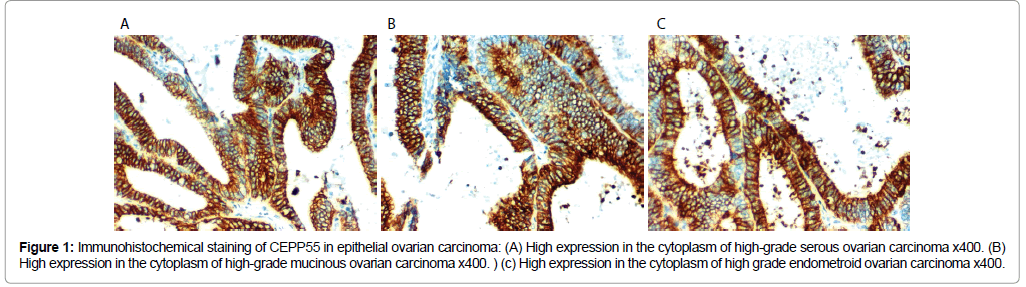
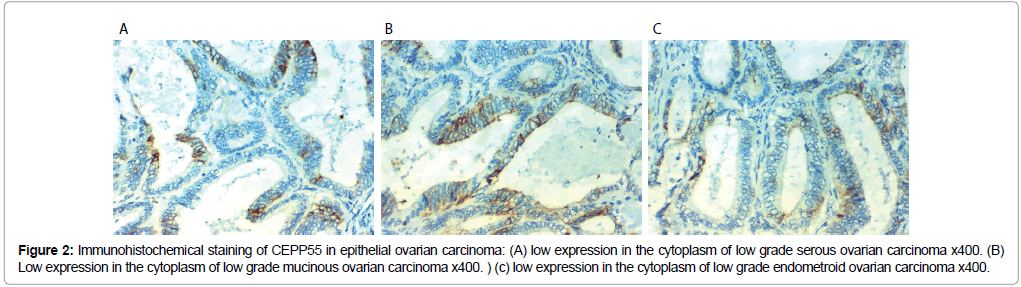
CEPP55 immunoreactivity results: CEP55 over expression in EOC was associated with SOC more than mucinous or endometroid, positively correlated with advanced age of the patient, higher grade of the tumor, higher CA125 level, positive peritoneal cytology, presence of peritoneal implants, L.N (p<0.001), and distant metastases (p=0.012) and advanced stage of the tumor (p=0.007). No statistically significant correlations between CEP55 expression and presence of ascites or bilateral tumors (Table 2 and Figures 1 and 2).
| Characteristics | All (N=60) | Cyclin D1 | p-value | CEP55 | p-value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Low (N=22) |
High (N=38) |
Low (N=26) |
High (N=34) |
||||||||
| No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | |||||||
| Age (years) | |||||||||||
| Mean ± SD | 55.53 ±10.53 | 47.54 ± 10.10 | 60.15 ± 7.68 | <0.001* | 48.88 ±10.06 | 60.61 ±7.75 | <0.001Ÿ | ||||
| Median (Range) | 57 (25-75) | 45 (25-65) | 59 (46-75) | 47 (25-65) | 60 (46-75) | ||||||
| 4 (6.7%)4 (100%)0 (0%)0.002‡4 (100%)0 (0%)0.002‡ | |||||||||||
| 41-59 years | 34 (56.7%) | 15 (44.1%) | 19 (55.9%) | 18 (52.9%) | 16 (47.1%) | ||||||
| ≥ 60 years | 22 (36.7%) | 3 (13.6%) | 19 (86.4%) | 4 (18.2%) | 18 (81.8%) | ||||||
| Serous Histopathology | 35 (58.3%) | 5 (14.3%) | 30 (85.7%) | <0.001‡ | 8 (22.9%) | 27 (77.1%) | 0.001‡ | ||||
| Mucinous | 15 (25%) | 9 (60%) | 6 (40%) | 10 (66.7%) | 5 (33.3%) | ||||||
| Endometroid | 10 (16.7%) | 8 (80%) | 2 (20%) | 8 (80%) | 2 (20%) | ||||||
| Positive cytology | |||||||||||
| Absent | 39 (65%) | 21 (53.8%) | 18 (46.2%) | <0.001‡ | 24 (61.5%) | 15 (38.5%) | <0.001‡ | ||||
| Present | 21 (35%) | 1 (4.8%) | 20 (95.2%) | 2 (9.5%) | 19 (90.5%) | ||||||
| CA125 | |||||||||||
| ≤ 35U/ml | 21 (35%) | 17 (81%) | 4 (19%) | <0.001‡ | 17 (81%) | 4(19%) | <0.001‡ | ||||
| >35U/ml | 39 (65%) | 5 (12.8%) | 34 (87.2%) | 9 (23.1%) | 30 (76.9%) | ||||||
| Bilaterality | |||||||||||
| Unilateral | 44 (73.3%) | 18 (40.9%) | 26 (59.1%) | 0.258‡ | 20 (45.5%) | 24 (54.5%) | 0.582‡ | ||||
| Bilateral | 16 (26.7%) | 4 (25%) | 12 (75%) | 6 (37.5%) | 10 (62.5%) | ||||||
| Implants | |||||||||||
| Absent | 38 (63.3%) | 20 (52.6%) | 18 (47.4%) | 0.001‡ | 23 (60.5%) | 15 (39.5%) | <0.001‡ | ||||
| Present | 22 (36.7%) | 2 (9.1%) | 20 (90.9%) | 3 (13.6%) | 19 (86.4%) | ||||||
| Ascites | |||||||||||
| Absent | 38 (63.3%) | 18 (47.4%) | 20 (52.6%) | 0.024‡ | 20 (52.6%) | 18 (47.4%) | 0.056‡ | ||||
| Present | 22 (36.7%) | 4 (18.2%) | 18 (81.8%) | 6 (27.3%) | 16 (72.7%) | ||||||
| Grade | |||||||||||
| Low | 23 (38.3%) | 18 (78.3%) | 5 (21.7%) | <0.001‡ | 19 (82.6%) | 4 (17.4%) | <0.001‡ | ||||
| High | 37 (61.7%) | 4 (10.8%) | 33 (89.2%) | 7 (18.9%) | 30 (81.1%) | ||||||
| LN | |||||||||||
| Node negative | 21 (35%) | 17 (81%) | 4 (19%) | <0.001‡ | 17 (81%) | 4 (19%) | <0.001‡ | ||||
| Node positive | 39 (65%) | 5 (12.8%) | 34 (87.2%) | 9 (23.1%) | 30 (76.9%) | ||||||
| M | |||||||||||
| M0 (non-metastatic) | 46 (76.7%) | 21 (45.7%) | 25 (54.3%) | 0.009‡ | 24 (52.2%) | 22 (47.8%) | 0.012‡ | ||||
| M1 (metastatic) | 14 (23.3%) | 1 (7.1%) | 13 (92.9%) | 2 (14.3%) | 12 (85.7%) | ||||||
| FIGO Stage | |||||||||||
| Stage IA | 2 (3.3%) | 2 (100%) | 0 (0%) | <0.001§ | 2 (100%) | 0 (0%) | 0.007§ | ||||
| Stage IB | 1 (1.7%) | 1 (100%) | 0 (0%) | 1 (100%) | 0 (0%) | ||||||
| Stage IC | 2 (3.3%) | 2 (100%) | 0 (0%) | 2 (100%) | 0 (0%) | ||||||
| Stage IIA | 3 (5%) | 3 (100%) | 0 (0%) | 3 (100%) | 0 (0%) | ||||||
| Stage IIB | 7 (11.7%) | 5 (71.4%) | 2 (28.6%) | 5 (71.4%) | 2 (28.6%) | ||||||
| Stage IIC | 6 (10%) | 4 (66.7%) | 2 (33.3%) | 4 (66.7%) | 2 (33.3%) | ||||||
| Stage IIIA | 9 (15%) | 1 (11.1%) | 8 (88.9%) | 3 (33.3%) | 6 (66.7%) | ||||||
| Stage IIIB | 12 (20%) | 2 (16.7%) | 10 (83.3%) | 2 (16.7%) | 10 (83.3%) | ||||||
| Stage IIIC | 4 (6.7%) | 1 (25%) | 3 (75%) | 2 (50%) | 2 (50%) | ||||||
| Stage IV | 14 (23.3%) | 1 (7.1%) | 13 (92.9%) | 2 (14.3%) | 12 (85.7%) | ||||||
| Cyclin D1 | |||||||||||
| Low | 22 (36.7%) | 22 (100%) | 0 (0%) | <0.001‡ | |||||||
| High | 38 (63.3%) | 4 (10.5%) | 34 (89.5%) | ||||||||
| CEP55 | |||||||||||
| Low | 26 (43.3%) | 22 (84.6%) | 4 (15.4%) | <0.001‡ | |||||||
| High | 34(56.7%) | 0 (0%) | 34 (100%) | ||||||||
Categorical variables were expressed as number (percentage), continuous variables were expressed as mean ± SD & median (range); *Independent samples Student's test; Mann Whitney U test; ‡ Chi-square test; § Chi-square test for trend; p
Table 2: correlations between clinicopathological criteria, Cyclin D1 and CEP55 expression in our patients.
Figure 1: Immunohistochemical staining of CEPP55 in epithelial ovarian carcinoma: (A) High expression in the cytoplasm of high-grade serous ovarian carcinoma x400. (B) High expression in the cytoplasm of high-grade mucinous ovarian carcinoma x400. ) (c) High expression in the cytoplasm of high grade endometroid ovarian carcinoma x400.
Figure 2: Immunohistochemical staining of CEPP55 in epithelial ovarian carcinoma: (A) low expression in the cytoplasm of low grade serous ovarian carcinoma x400. (B) Low expression in the cytoplasm of low grade mucinous ovarian carcinoma x400. ) (c) low expression in the cytoplasm of low grade endometroid ovarian carcinoma x400.
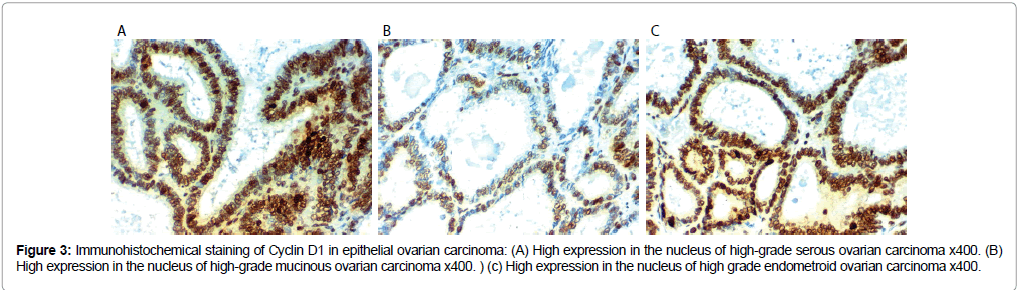
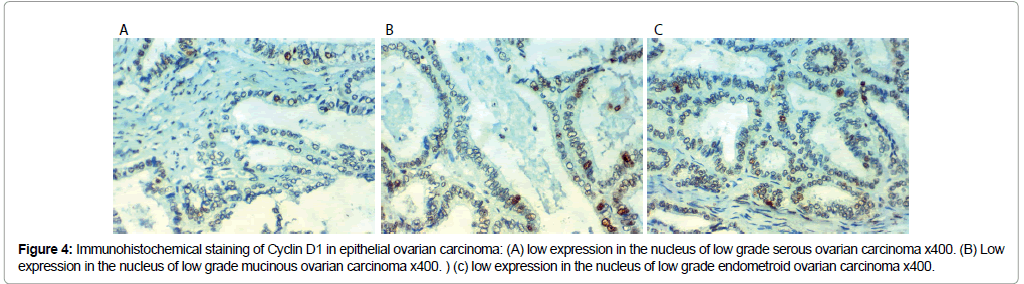
Cyclin D 1 immunoreactivity results: Cyclin D 1 over expression in EOC was associated with SOC more than mucinous or endometroid, related to older age of the patient, advanced grade and stage of the tumor, presence of peritoneal implants, higher CA125 level, positive peritoneal cytology, L.N (p<0.001), distant metastases (p=0.009) and presence of ascites (p=0.009).
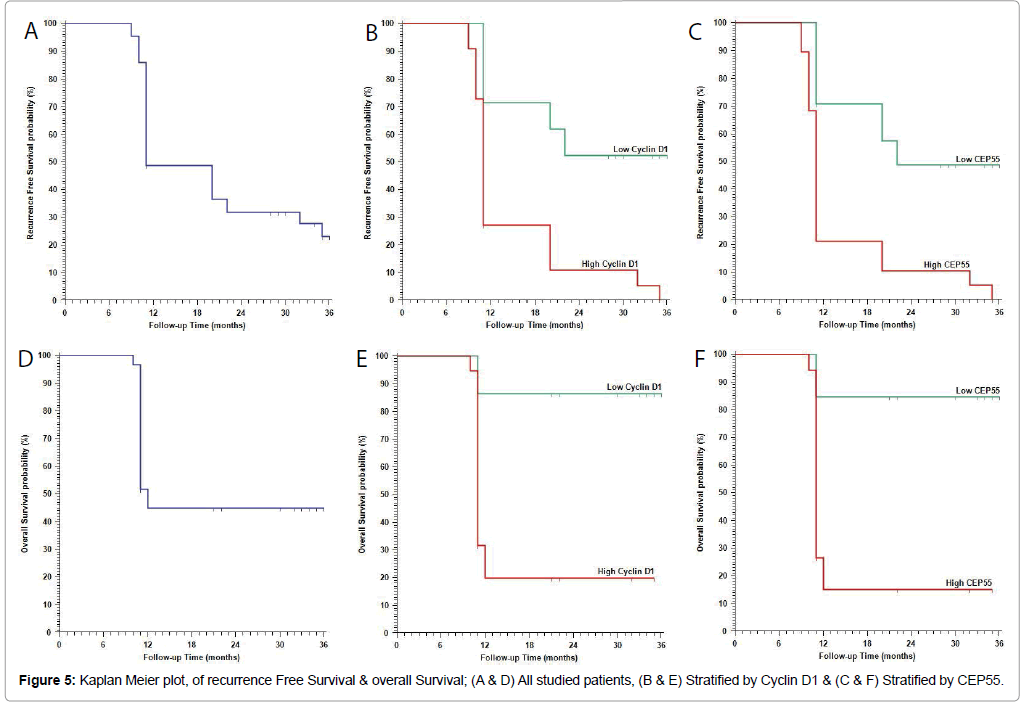
No statistically significant correlations between Cyclin D 1 expression and the presence of bilateral disease. Low CEPP 55 and Cyclin D1 expression were strongly related to optimal surgical eradication of the tumor, increased 3 year overall survival (OS) and low incidence of recurrence after therapy (P<0.001). No statistically significant correlations were found between markers expression, chemosensitivity or response to therapy (Table 3 and Figures 3 and 4).
| Characteristics | All No. (%) |
Cyclin D1 | p-value | CEP55 | p-value | |||
|---|---|---|---|---|---|---|---|---|
| Low No. (%) |
High No. (%) |
Low No. (%) |
Low No. (%) |
High No. (%) |
||||
| Operation | (N=60) | (N=22) | (N=38) | (N=35) | (N=26) | (N=34) | ||
| Radical surgery | 15 (25%) | 13 (59.1%) | 2 (5.3%) | <0.001‡ | 0 (0%) | 13 (50%) | 2 (5.9%) | <0.001‡ |
| Suboptimal | 18 (30%) | 2 (9.1%) | 16 (42.1%) | 17 (48.6%) | 4 (15.4%) | 14 (41.2%) | ||
| Optimal | 27 (45%) | 7 (31.8%) | 20 (52.6%) | 18 (51.4%) | 9 (34.6%) | 18 (52.9%) | ||
| ECOG PS | (N=60) | (N=22) | (N=38) | (N=35) | (N=26) | (N=34) | ||
| ECOG 1 | 42 (70%) | 17 (77.3%) | 25 (65.8%) | 0.350‡ | 22 (62.9%) | 20 (76.9%) | 22 (64.7%) | 0.306‡ |
| ECOG 2 | 18 (30%) | 5 (22.7%) | 13 (34.2%) | 13 (37.1%) | 6 (23.1%) | 12 (35.3%) | ||
| Number of cycles | (N=57) | (N=19) | (N=38) | (N=35) | (N=23) | (N=34) | ||
| 4 cycles | 10 (17.5%) | 8 (42.1%) | 2 (5.3%) | 0.001‡ | 0 (0%) | 8 (34.8%) | 2 (5.9%) | 0.010‡ |
| 6 cycles | 8 (14%) | 4 (21.1%) | 4 (10.5%) | 5 (14.3%) | 4 (17.4%) | 4 (11.8%) | ||
| 8 cycles | 39 (68.4%) | 7 (36.8%) | 32 (84.2%) | 30 (85.7%) | 11 (47.8%) | 28 (82.4%) | ||
| Response | (N=45) | (N=9) | (N=36) | (N=35) | (N=13) | (N=32) | ||
| NR | 10 (22.2%) | 0 (0%) | 10 (27.8%) | 0.173‡ | 10 (28.6%) | 0 (0%) | 10 (31.3%) | 0.042‡ |
| OAR | 35 (77.8%) | 9 (100%) | 26 (77.8%) | 25 (71.4%) | 13 (100%) | 22 (68.8%) | ||
| Response after 4-6 | (N=45) | (N=9) | (N=36) | (N=35) | (N=13) | (N=32) | ||
| PD | 3 (6.7%) | 0 (0%) | 3 (8.3%) | 0.366‡ | 3 (8.6%) | 0 (0%) | 3 (9.4%) | 0.557‡ |
| SD | 10 (22.2%) | 1 (11.1%) | 9 (25%) | 10 (28.6%) | 2 (15.4%) | 8 (25%) | ||
| PR | 29 (64.4%) | 8 (88.9%) | 21 (58.3%) | 19 (54.3%) | 10 (76.9%) | 19 (59.4%) | ||
| CR | 3 (6.7%) | 0 (0%) | 3 (8.3%) | 3 (8.6%) | 1 (7.7%) | 2 (6.3%) | ||
| Response after 8 | (N=45) | (N=9) | (N=36) | (N=35) | (N=13) | (N=32) | ||
| PD | 3 (6.7%) | 0 (0%) | 3 (8.3%) | 0.269‡ | 3 (8.6%) | 0 (0%) | 3 (9.4%) | 0.136‡ |
| SD | 7 (15.6%) | 0 (0%) | 7 (19.4%) | 7 (20%) | 0 (0%) | 7 (21.9%) | ||
| PR | 7 (15.6%) | 1 (11.1%) | 6 (16.7%) | 7 (20%) | 2 (15.4%) | 5 (15.6%) | ||
| CR | 28 (62.2%) | 8 (88.9%) | 20 (55.6%) | 18 (51.4%) | 11 (84.6%) | 17 (53.1%) | ||
| Recurrence | (N=43) | (N=21) | (N=22) | (N=18) | (N=24) | (N=19) | ||
| Absent | 12 (27.9%) | 11 (52.4%) | 1 (4.5%) | <0.001‡ | 1 (5.6%) | 12 (50%) | 0 (0%) | <0.001‡ |
| Present | 31 (72.1%) | 10 (47.6%) | 21 (95.5%) | 17 (94.4%) | 12 (50%) | 19 (100%) | ||
| Chemosensitivity | (N=31) | (N=10) | (N=21) | (N=17) | (N=12) | (N=19) | ||
| Chemosensitive | 11 (35.5%) | 6 (60%) | 5 (23.8%) | 0.106‡ | 4 (23.5%) | 7 (58.3%) | 4 (21.1%) | 0.056‡ |
| Chemorefractory | 20 (64.5%) | 4 (40%) | 16 (76.2%) | 13 (76.5%) | 5 (41.7%) | 15 (78.9%) | ||
| RFS | (N=43) | (N=21) | (N=22) | (N=17) | (N=24) | (N=19) | ||
| Mean (months) (95%CI) |
20.2 months (16.9 – 23.5) |
26 months (21.3 – 30.7) |
14.6 months (11.3 – 17.9) |
<0.001† | 14 months (10.9 – 17.2) |
25.3 months (20.9 – 29.8) |
13.9 months (10.5 – 17.3) |
<0.001† |
| 1 year RFS | 48.8% | 71.4% | 27.3% | 27.8% | 70.8% | 21.1% | ||
| 2 year RFS | 31.4% | 52.4% | 10.9% | 6.9% | 48.7% | 10.5% | ||
| 3 year RFS | 23.2% | 52.4% | --- | --- | 48.7% | --- | ||
| Death | (N=60) | (N=22) | (N=38) | (N=35) | (N=26) | (N=34) | ||
| Alive | 28 (46.7%) | 19 (86.4%) | 9 (23.7%) | <0.001‡ | 6 (17.1%) | 22 (84.6%) | 6 (17.6%) | <0.001‡ |
| Died | 32 (53.3%) | 3 (13.6%) | 29 (76.3%) | 29 (82.9%) | 4 (15.4%) | 28 (82.4%) | ||
| OS | (N=60) | (N=22) | (N=38) | (N=35) | (N=26) | (N=34) | ||
| Mean (months) (95%CI) |
22.3 months (19 – 25.5) |
32.6 months (29 – 36.2) |
15.8 months (12.5 – 19.1) |
<0.001† | 14.1 months (11.5 – 16.8) |
32.2 months (28.7 – 35.6) |
14.7 months (11.6 – 17.8) |
<0.001† |
| 1 year OS | 44.9% | 86.4% | 19.7% | 14.7% | 84.6% | 15.1% | ||
| 2 year OS | 44.9% | 86.4% | 19.7% | 14.7% | 84.6% | 15.1% | ||
| 3 year OS | 44.9% | 86.4% | 19.7% | 14.7% | 84.6% | 15.1% | ||
Continuous variables were expressed as mean (95%CI); categorical variables were expressed as number (percentage); 95%CI: 95%Confidence Interval; ‡ Chi-square test; † Log rank test; p
Table 3: correlations between Cyclin D1 and CEP55 expression and outcome of our patients.
Figure 3: Immunohistochemical staining of Cyclin D1 in epithelial ovarian carcinoma: (A) High expression in the nucleus of high-grade serous ovarian carcinoma x400. (B) High expression in the nucleus of high-grade mucinous ovarian carcinoma x400. ) (c) High expression in the nucleus of high grade endometroid ovarian carcinoma x400.
Figure 4: Immunohistochemical staining of Cyclin D1 in epithelial ovarian carcinoma: (A) low expression in the nucleus of low grade serous ovarian carcinoma x400. (B) Low expression in the nucleus of low grade mucinous ovarian carcinoma x400. ) (c) low expression in the nucleus of low grade endometroid ovarian carcinoma x400.
Statistical analysis
Continuous variables were expressed as the mean ± SD and median (range), and the categorical variables were expressed as a number (percentage). Continuous variables were checked for normality by using Shapiro-Wilk test. Independent samples Student’s t-test was used to compare between two groups of normally distributed variables while Mann Whitney U test was used for non-normally distributed variables. Percent of categorical variables were compared using Pearson’s Chisquare test or Fisher’s exact test when was appropriate. Trend of change in distribution of relative frequencies between ordinal data were compared using Chi-square test for trend. Overall Survival (OS) was calculated as the time from diagnosis to death or the most recent follow-up contact (censored). Recurrence Free Survival (RFS) was calculated as the time from start of treatment to date of recurrence or the most recent follow-up contact that patient was known as recurrence free. Stratification of OS and RFS was done according markers. These time-to-event distributions were estimated using the method of Kaplan-Meier plot, and compared using two-sided exact log-rank test. All tests were two sided. A p-value Figure 5).
Discussion
In our results CEP55 overexpression in EOC was related to worse clinical and pathological criteria and aggressive phenotype of EOC that strongly supports the hypothesis that this protein expression has an essential role in ovarian cancer progression and poor patient prognosis.
Zhang et al., found the same results, moreover they stated that CEP55 suppression could decrease cancer cells invasion, which clarified its role in increasing ovarian cancer cells migratory and invasive capabilities [14].
Furthermore, we found that over expression of the protein CEP55 is considered an independent prognostic factors for worse 3 year OS, RFS rates in patients with EOC.
Under normal non neoplastic conditions, CEP55 had many roles during cytokinesis which is needed during cell division in two daughter cells by guiding the process of segregation of chromosomes that are replicated, properly into the two daughter cells. But in case of CEP55 overexpression which may lead to defects in cytokinesis that resulted in many chromosome instabilities. Such abnormalities are common criteria in malignant EOC cells [14]. Also, CEP55 overexpression promoted growth signaling pathways that could result in cancer cell metastasis and poor patient prognosis [16]. All these findings have suggested that CEP55 played a major role in cancer initiation and progression, which explain our results about association between CEPP55 overexpression, tumor aggressiveness, worse clinicopathological and prognostic parameters
Similar to our findings CEP55 protein overexpression had been found to be related to tumor aggressiveness in a plethora of cancers [17,18].
Our patients with CEP55 over-expression showed a shorter OS rate than patients with lower levels of expression that protein that provides essential evidence about the significance of CEP55 expression in EOC as a reliable prognostic marker for ovarian cancer patients.
We found that patients with CEP55 protein overexpression showed no detected statistically significant difference with chemosensitivity that was different from results of Zhang et al. that proved that patients with increased CEPP55 expression are better to take neo-adjuvant chemotherapy. Such differences might be due to different patients’ number between their study and ours which gave a statistical difference [14].
The value of exploring the prognostic role of CEP55 biomarker in EOC could help to detect which patients had a worse outcome, which subsequently might help to choose better treatment for patients to reduce mortality and improve their prognosis. LN metastasis is a poor prognostic parameter for patients with ovarian cancer [19], moreover early detection of intraperitoneal implants can help to improve prognosis and survival of patients with EOC [20].
So if we had the ability to predict LN and intra-peritoneal metastasis by tissue protein marker expression it will be essential to predict patient prognosis. In our study, we tried to give solution to such problem and found that CEP55 protein over expression was related to LN and intra-peritoneal metastasis that was also similar to Zhang et al., results which explained the roles of CEP55 in ovarian carcinoma patients [14]. Epithelial mesenchymal transition (EMT) is the recently discovered process that is responsible for cancer cells invasion, dissemination, and therapy resistance criteria of cancer cells [21]. CEP55 found to have a critical role in EMT regulation e.g. in nasopharyngeal carcinoma it promotes EMT by activation of osteopontin/ CD44 pathway [22]. Moreover, Chen et al. showed that CEP55 regulate EMT by activation of CEP55/FOXM1/MMP-2 pathway in squamous cell carcinoma of the oral cavity, and the VEGF-A/PI3K/AKT pathway in bronchogenic carcinoma [23]. Zhang et al., have proved that CEP55 down regulation could inhibit malignant cells invasion of ovarian cancer and inhibit EMT [14]. But, more studies are needed to explain the signaling pathways that are related to CEP55 in regulating EMT in ovarian carcinoma.
A promising management of advanced EOC included neoadjuvant chemotherapy, then surgical cyto-reduction [23]. CEP55 protein over expression was associated with accepting neoadjuvant chemotherapy. This adds to our results that CEP55 could be considered a critical prognostic bio-marker for patients with ovarian cancer [24]. Similar findings to us was detected by Chen et al., that CEP55 overexpression in cancer lung promotes cell invasion and migration [25], also Jiang et al. have reported that overexpression of CEP55 is related to advanced cancer stage, poor tumor differentiation, increased incidence of visceral pleural invasion, shorter five-year OS rate and poor outcome of patients with stage I adenocarcinoma of the lung [26].
Cyclin D1 is a cell cycle protein that regulates the progression of G1 phase to S phase of DNA synthesis in the cell cycle [27]. Any disturbances of cyclin expression were found to result in enhanced cellular proliferation and progression to cancer [28]. The association between cyclin D1 overexpression and malignant transformation of cells was explored and studied in many types of malignant tumors [29]. In the present study, we found significant associations between over expression of cyclin D1, advanced stage and higher grade of EOC which was in line with results of Mehmet Kanter et al., that have detected a higher cyclin D1 expression in malignant ovarian tumors [30], and results of Turan et al. who found that cyclin D1 over expression was associated with advanced stage and higher grade in EOC [31]. Lin et al. have demonstrated that cyclin D1 was over expressed during progression from benign to borderline to malignant ovarian tumors [29].
Sui et al., have proved that cyclin D1 was overexpressed in borderline and well differentiated malignant ovarian tumors, and decreased the expression in high grade tumors [32], such results were contradictory with our results.
Also many conflicting results were found by previous studies that explored prognostic role of cyclin D 1 in carcinoma of the ovary and other organs [33,34].
The cause of such conflict may be due to different clones of the antibody used, different number of patients or different method of assessment of the immune-reactivity, moreover EOC had marked heterogeneity and variable alterations in regulatory genes of the cell cycle and there are multiple cellular pathways that occur during EOC development.
We detected a significant association between cyclin D1 overexpression and poor patient OS rate that was similar to results of Barbieri et al. and . Bali et al., who found that cyclin D1 overexpression, had been related to worse progression-free and overall survival rates in ovarian cancer patients [35,36].
On the contrary other studies have found a strong correlations between cyclin D1 expression and better survival rates in male having colorectal carcinoma (CRC) [37], in addition Ioachim et al., [38], and Al-Maghrabia, et al., [39], found no statistically significant correlation between cyclin D1 expression and OS rate or pathological parameters in patients with CRC, except with lympho-vascular invasion. Causes of such discrepancy is that Al-Maghrabia, et al., [39], study have included tissue microarray (TMA) that uses only a small part of the tumor tissue specimen which might be not representative of the actual cyclin D1 protein expression or distribution within the cancer, and also had heterogeneous patterns of staining in different regions of the tumor [40]. Many former researchers proved results similar to ours, that overexpression of cyclin D1 had been found as a bad prognostic parameter in several malignancies such as bronchogenic, pancreatic and tongue carcinoma [41].
Our results could be clarified by that in addition to Cyclin D1 role in the control of cell cycle, it increased cell proliferation rate that contributed to malignancy [28], moreover, Li et al. [42], investigated relation between cyclin D1 and Cyclooxgenase-2 (COX-2) that is an inflammatory mediator and stimulus for and tumor initiation and is found to be upregulated in a many of cancers including EOC, and they found that celecoxib, a COX-2 inhibitor found to significantly found to reduce tumor growth and also decreased the cyclin D1 expression that indicated a dependent mechanism of cyclin D1 and COX-2. So, cyclin D1 targeting by inhibitors COX-2 might be used in management of EOC in addition to the current therapies [42,43]. The main causes of variability of such previous study results regarding the prognostic role of CEP55 and Cyclin D expression in EOC and other cancers might be due to that the great majority of these studies used only immunohistochemistry to detect the expression of cyclin D1, as although that method has an accepted degree of sensitivity but its results are not quantitative and lacking a fixed and standardized scoring system or a uniform accepted positivity threshold for all studies that are serious limitations to immunohistochemistry results interpretations.
We found a significant positive association between the expression of CEP55 and Cyclin D in EOC and found that increased expression of both markers together is related to aggressive clinicopathological parameters and could predict poor prognosis in patients with EOC.
In our study, we demonstrated the upregulated expression of CEP55 and Cyclin D in EOC and correlated their expression with its clinical stage, LN, intra-peritoneal and distant metastasis, in addition to correlation with patient survival, disease recurrence and response to therapy and we finally proved that both markers induced LN and distant metastasis via regulating EMT. Taken together, our results suggest that CEP55 and Cyclin D 1 are markers predicting unfavorable outcomes in EOC and play significant roles in the invasion and spread of EOC.
Future studies are needed to prove the roles of both markers in EOC using large number of patients and different methods of assessment to explore the possible discovery of therapeutic targets against such markers to decrease aggressively of EOC and improve patients’ prognosis.
References
- Liu T, Gao H, Chen X, Lou G, Gu L, et al. (2013) TNFAIP8 as a predictor of metastasis and a novel prognostic biomarker in patients with epithelial ovarian cancer. Br J Cancer 109: 1685-1692.
- Zyss D, Gergely F (2009) Centrosome function in cancer: guilty or innocent? Trends Cell Biol 19: 334–346.
- Andersen JS, Wilkinson CJ, Mayor T, Mortensen P, Nigg EA, et al. (2003) Proteomic characterization of the human centrosome by protein correlation profiling. Nature 426: 570-574.
- Jeffery J, Sinha D, Srihari S, Kalimutho M, Khanna KK (2015) Beyond cytokinesis: the emerging roles of CEP55 in tumorigenesis. Oncogene 35: 683-690.
- Carlton JG, Martin-Serrano J (2007) Parallels between cytokinesis and retroviral budding: a role for the ESCRT machinery. Science 316: 1908-1912.
- Suzuki H, Kawasaki M, Inuzuka T, Okumura M, Kakiuchi T, et al. (2008) Structural basis for Ca2+-dependent formation of ALG-2/Alix peptide complex: Ca2+/EF3-driven arginine switch mechanism. Structure 16: 1562-1573.
- Martin KJ, Patrick DR, Bissell MJ, Fournier MV (2008) Prognostic breast cancer signature identified from 3D culture model accurately predicts clinical outcome across independent datasets. PLoS One 3: e2994.
- Gemenetzidis E, Bose A, Riaz AM, Chaplin T, Young BD, et al. (2009) FOXM1 upregulation is an early event in human squamous cell carcinoma and it is enhanced by nicotine during malignant transformation. PLoS One 4: e4849.
- Motokura T, Arnold A (1993) Cyclins and oncogenesis. Biochim Biophys Acta 1155: 63-78.
- Sherr CJ, Roberts JM (1999) CDK inhibitors: positive and negative regulators of G1-phase progression. Gene Dev 13: 1501-1512.
- Prat J (2014) FIGO Committee on Gynecologic Oncology. Staging classification for cancer of the ovary, fallopian tube, and peritoneum. Int J Gynaecol Obstet 124: 1-5.
- Kurman RJ, Carcangiu ML, Herrington CS, Young RH (2014) WHO Classification of Tumours of Female Reproductive Organs. 4th edn. International Agency for Research on Cancer; Lyon, France.
- Hsu SM, Raine L, Fanger H (1981) Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem 29: 577-580.
- Zhang W, Niu C, He W, Hou T, Sun X, et al. (2016) Upregulation of centrosomal protein 55 is associated with unfavorable prognosis and tumor invasion in epithelial ovarian carcinoma. Tumor Biol 37: 6239-6254.
- Saawarn S, Astekar M, Saawarn N, Dhakar N, Kumar S, et al. (2012) Cyclin D1 Expression and Its Correlation with Histopathological Differentiation in Oral Squamous Cell Carcinoma. Scientific World Journal 2012: 978327.
- Chen CH, Shiu LY, Su LJ, Huang CY, Huang SC, et al. (2012) FLJ10540 is associated with tumor progression in nasopharyngeal carcinomas and contributes to nasopharyngeal cell proliferation, and metastasis via osteopontin/CD44 pathway. J Transl Med 10: 93.
- Tao Ji, Zhi X, Tian Y, Li Z, Zhu Y, et al. (2014) CEP55 contributes to human gastric carcinoma by regulating cell proliferation. Tumour Biol 35: 4389-4399.
- Singh PK, Srivastava AK, Rath SK, Dalela D, Goel MM, et al. (2015) Expression and clinical significance of centrosomal protein 55(CEP55) in human urinary bladder transitional cell carcinoma. Immunobiol 220: 103-108.
- Ataseven B, Grimm C, Harter P, Prader S, Traut A, et al. (2014) Prognostic value of lymph node ratio in patients with advanced epithelial ovarian cancer. Gynecol Oncol 135: 435–440.
- Ye Y, Yin M, Huang B, Wang Y, Li X, et al. (2015) CLIC1 a novel biomarker of intraperitoneal metastasis in serous epithelial ovarian cancer. Tumour Biol 36: 4175–4179.
- Fujiwara T, Bandi M, Nitta M, Ivanova EV, Bronson RT, et al. (2005) Cytokinesis failure generating tetraploids promotes tumorigenesis in p53-null cells. Nature 437: 1043–1047.
- Hwang CF, Shiu LY, Su LJ, Yin YF, Wang WS, et al. (2013) Oncogenic fibulin-5 promotes nasopharyngeal carcinoma cell metastasis through the FLJ10540/AKT pathway and correlates with poor prognosis. PLoS One 8: e84218.
- Chen CH, Chien CY, Huang CC, Hwang CF, Chuang HC, et al. (2009) Expression of FLJ10540 is correlated with aggressiveness of oral cavity squamous cell carcinoma by stimulating cell migration and invasion through increased FOXM1 and MMP-2 activity. Oncogene 28: 2723-2737.
- Baruah U, Barmon D, Kataki AC, Deka P, Hazarika M, et al. (2015) Neoadjuvant chemotherapy in advanced epithelial ovarian cancer: a survival study. Indian J Med Paediatr Oncol 36: 38-42.
- Chang SJ, Bristow RE, Chi DS, Cliby WA (2015) Role of aggressive surgical cytoreduction in advanced ovarian cancer. J Gynecol Oncol 26: 336–342.
- Chen CH, Lai JM, Chou TY, Chen CY, Su LJ, et al. (2009) VEGFA upregulates FLJ10540 and modulates migration and invasion of lung cancer via PI3K/AKT pathway. PLoS One 4: e5052.
- Jiang W, Wang Z, Chen G, Jia Y (2016) Prognostic significance of centrosomal protein 55 in stage I pulmonary adenocarcinoma after radical resection. Thorac Cancer 7: 316–322.
- Biliran H Jr, Wang Y, Banerjee S, Xu H, Heng H, et al. (2005) Overexpression of cyclin D1 promotes tumor cell growth and confers resistance to cisplatin-mediated apoptosis in an elastasemyc transgene-expressing pancreatic tumor cell line. Clin Cancer Res 11: 6075–6086
- Yasui M, Yamamoto H, Ngan CY, Damdinsuren B, Sugita Y, et al. (2006) Antisense to cyclin D1 inhibits vascular endothelial growthfactor-stimulated growth of vascular endothelial cells: implicationof tumor vascularization. Clin Cancer Res 12: 4720–4729.
- Lin S, Yu HS (2011) Clinical significance of nucleostemin expression and its correlation with cyclin D1 expression in malignant ovarian tumors. Int J Gynecol Cancer 21: 1166-1171
- Kanter M, Turan G, Usta C, Usta A, Esen H, et al. (2016) Survivin and cycline D1 expressions are associated with malignant potential in mucinous ovarian neoplasms J Mol Hist 47: 145-152.
- Turan G, Usta CS, Usta A, Kanter M, Tavli L, et al. (2014) The expression of HER-2/neu (c-erbB2), survivin and cycline D1 in serous ovarian neoplasms: their correlation with clinicopathological variables. J Mol Histol 45: 679–687.
- Sui L, Tokuda M, Ohno M, Hatase O, Hando T (1999) The concurrent expression of p27(kip1) and cyclin D1 in epithelial ovarian tumors. Gynecol Oncol 73: 202–209.
- Myklebust M, Li Z, Tran TH, Rui H, Knudsen H, et al. (2012) Expression of cyclin D1a and D1b as predictive factors for treatmentresponse in colorectal cancer. Br J Cancer 107: 1684–1691.
- Ogino S, Nosho K, Irahara N, Kure S, Shima K, et al. (2009) A cohortstudy of cyclin D1 expression and prognosis in 602 colon cancercases. Clin Cancer Res 15: 4431-4438.
- Barbieri F, Lorenzi P, Ragni N, Schettini G, Bruzzo, et al. (2004) Overexpression of cyclin D1 is associated with poor survival in epithelial ovarian cancer. Oncology 66: 310-315.
- Bali A, O’Brien PM, Edwards LS, Sutherland RL, Hacker NF, et al. (2004) Cyclin D1, p53, and p21Waf1/Cip1 expression is predictive ofpoor clinical outcome in serous epithelial ovarian cancer. Clin Cancer Res 10: 5168-5177.
- Wangefjord S, Manjer J, Gaber A, Nodin B, Eberhard J, et al. (2011) Cyclin D1 expression in colorectal cancer is a favorable prognosticfactor in men but not in women in a prospective, population-basedcohort study. Biol Sex Differ 2: 10.
- Ioachim E (2008) Expression patterns of cyclins D1, E and cyclin dependentkinase inhibitors p21waf1/cip1, p27kip1 in colorectal carcinoma:correlation with other cell cycle regulators (pRb, p53 and Ki-67 and PCNA) and clinicopathological features. Int J Clin Pract 62: 1736-1743.
- Al-Maghrabia J, Muftib S, Gomaab W, Buhmeidad A, Al-Qahtanid M, et al. (2015) Immunoexpression of cyclin D1 in colorectal carcinomas is not correlated with survival outcome. J Microsc Ultrastruct 3: 62-67.
- Chen WC, Lin MS, Zhang BF, Fang J, Zhou Q, et al. (2007) Survey of molecular profiling during human colon cancer development and progression by immunohistochemical staining on tissue microarray. World J Gastroenterol 13: 699-708.
- Bova RJ, Quinn DI, Nankeruis JS, Cole IE, Sheridan BF, et al. (1999) Cyclin D1 and p16INK4A expression predict reduced survival in carcinoma of the anterior tongue. Clin Cancer Res 5: 2810-2819.
- Li W, Jiang HR, Xu XL, Wang J, Zhang J, et al. (2010) Cyclin D1 expression and the inhibitory effect of celecoxib on ovarian tumor growth in vivo. Int J Mol Sci 11: 3999-4013.
Citation: Harb O, Elhasadi I, El.Ammari SAM, Inbaig A, Saad RHF, et al. (2017) Prognostic and Predictive Values of cell Cycle Proteins Centrosomal Protein 5 (CEPP 5) and Cyclin D1 Expression in Epithelial Ovarian Carcinoma (EOC). Diagn Pathol Open 2: 127. DOI: 10.4172/2476-2024.1000127
Copyright: ©2017 Harb O, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 4511
- [From(publication date): 0-2017 - Nov 22, 2024]
- Breakdown by view type
- HTML page views: 3817
- PDF downloads: 694