Prognostic and Predictive Value of Lymphoid Microenvironment in Locally Advanced Rectal Cancer
Received: 08-Oct-2021 / Accepted Date: 22-Oct-2021 / Published Date: 29-Oct-2021
Abstract
Objective: Tumor immunity infiltrate, density and location in the microenvironment could be predictive value for survival in patients with rectal cancer. The aims of this study is to evaluate tumor microenvironment using immune adaptive cells as CD3+ and CD8+ T lymphocytes and explore relationship between clinic pathological features, therapeutic response, survival outcomes and immunoscore.
Materials and Methods: Scoring system “immunoscore” in pretreated biopsies tissues from rectal cancer. Quantification of cytotoxic and memory T cells in the Core of Tumor (CT) and in the tumor’s Invasive Margin (IM)
Results: According to 5 immunoscore groups I0, I1, I2, I3 and I4, the frequency of each group was respectively 9.3%, 18.5%, 22.2%, 18.5% and 31.5%. When grouping immunoscore into 3 groups’ data allow to a proportion of 27.8% for low immunoscore, 22.2% for moderate immunoscore and 50% for high immunoscore. Furthermore, in two-tailed classification (I0, I1, I2 versus I3 and I4) 50% of tumors were ranged as low immunoscore versus 50% as high immunoscore.
Conclusion: High Lymphoid infiltration in CT and IM prove that tumor microenvironment could be a predictive factor, on pretreated biopsies, for tumor sensibility after neoadjuvant treatment in rectal cancer. This reflects the importance role of high immune infiltration in prediction of complete therapeutic effect. Immunoscore method should be considered as predictive marker for overall survival.
Keywords: Immunoscore, CD3, CD8, Therapeutic response, Rectal cancer, Neoadjuvant treatment, Morocco.
Introduction
Tumor microenvironment is an extrinsic factor that modifies the behavior of the tumor cell [1]. The understanding of the tumoral properties leads to appreciate the influence of the peripheral and intratumoral immune component on the prognosis and the evolution of the cancer [2]. The immune system alone is able to recognize and eliminate precancerous cells that are undergoing cancer transformation [3]. The concept of “immune surveillance of cancer” is currently demonstrated and validated, and has been defined as a new vision of “immunoediting of cancer” [4,5]. In Addition, the tumor immune infiltrate consists of adaptive immune cells such as B and T-lymphocytes presenting a specific antigen receptor, and which are responsible for the immune memory function. Furthermore innate immune system cells are part of the tumor infiltration [2]. The “immunoscore” method has been designed by several authors to focus on the concept of the tumor microenvironment, as well as to explore and quantifies the immune infiltrate characters in solid tumors [2,5]. The European Hospital of Georg Pompidou (EHGP) immunomonitoring platform demonstrate an excellent concordance rate of eye and machine count correlation for CD3 and CD8 (r>0.80) [2]. This immunologic method has been validated especially in colorectal cancer [6]. “Immunoscore” system is based on the recognition of CD expressed on immune cells. CD4 “helper” T-lymphocytes as well as cytotoxic effector CD8 T cells can be found at different densities in the tumor microenvironment of tumor [7,8]. Moreover, better disease free survival and overall survival has been associated with a strong intra-tumoral infiltration of CD3+, CD8+ T and CD45R0 lymphocytes in solid tumors [9,10]. Furthermore, current research has focused on establishment of a new classification based on immunoscore (TNM-Immune), to better characterize the prognosis and behavior of patients more accurately than classical pathologic TNM as reported by 7th AJCC staging system [11,12].
For this purpose, the aim of our study is to determine whether the immune infiltrate of the tumor, recently evaluated with the immunoscore methodology, could be a useful predictive and prognostic marker in patients with rectal cancer, also to encourage the use of this method as a routine test for the classification of rectal cancer.
Materials and Methods
Patients and pathological examination
In all, 54 patients diagnosed with adenocarcinoma at HASSAN II University Hospital Center of Fez were included in this study. Patients received neoadjuvant treatment followed by surgical procedure. We followed the methods of Otmani [13]. Samples collected and selected for Immunohistochemistry (IHC) were Formalin-Fixed-Paraffin- Embedded (FFPE) pre-therapeutic biopsies.
Histological slides based on a hematoxylin and eosin-stained slide were evaluated by a gastrointestinal pathologist. Histological parameters were investigated and performed according to the staging criteria of the American Joint Committee on Cancer, 7th edition (AJCC) (histological type, tumor differentiation, tumor regression grade with the Dworak Grading, post-neoadjuvant treatment TNM stage (ypTNM), lymph node status. And other clinic pathological characteristics). Data registered include demographic details, neoadjuvant treatment details, type and results of surgery, pathology reports, and cancer outcome.
The total density of cytotoxic CD3+ and CD8+ T lymphocytes were evaluated by immunohistochemistry and quantified by simple optical microscope analysis for 54 tumor biopsies taken before neoadjuvant treatment of patients with rectal cancer. Results were correlated with clinic pathological features, therapeutic response on surgical specimens after neoadjuvant therapy as well as
Evaluation of CD3 and CD8 expression by immunohistochemistry
Multiple fine sections (3ym) were cut and mounted on super frost glass slides from the biopsy tissues blocks for subsequent immunostaining. Manual immunohistochemistry was performed according to the manufacturer’s protocol. Slides incubated at 56°C during the night preceding the technique, were deparaffinized using toluene, rehydrated with serial alcohol and distilled water. EnVisionTM FLEX, High pH (Link), DAKO system was used in all Following steps. Unmasquage was performed by immersion slides in target retrieval solution 0.001 M (pH 9.0) at a preheated temperature of 65°C and then a target final temperature set at 95°C during 20 mn. Slides were immersed in a wash buffer for 5 mn and treated with peroxidase-blocking reagent for 3 mn to block endogenous peroxidase activity. Specific antibodies for each protein were used for membrane staining a CD3 DAKO monoclonal rabbit antibodies, clone 4B12, ready to use was performed. FLEX monoclonal mouse anti-human CD8, Clone C8/144B; Readyto- use (Link) was used to evaluate density of CD8. The tissue section was then incubated during 1 hour at room temperature. HRP-system detection was used during 30 mn followed by 5 mn incubation with 3,3’-diaminobenzidine for color reaction. A counterstaining using Hematoxylin for 2 mn was performed. Final serial ethanol washes were used followed by a mounting of the slats with specific glue. The slides were analyzed and the staining of each marker was evaluated according to a specific manner.
CD3 and CD8 density in the Core of Tumor (CT) and in the tumour’s Invasive Margin (IM)
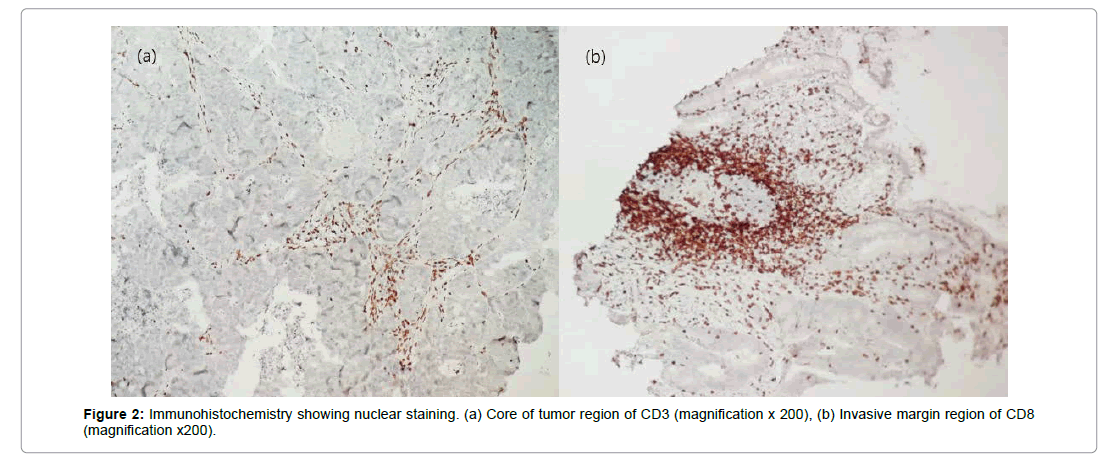
The density of total CD3 T cells was determined by simple counting using an optical microscope. The number of CD3 T lymphocytes was calculated with respect to an area equal to 1mm2. An observation with the Leica DM 2500 microscope using the x20 magnification, and an optic index equal to 1/16, allowed limiting a microscopic field of an area of 1.25 mm2. The number obtained by 1.25 mm2 was then calculated to obtain a density corresponding to a surface of 1 mm2. The number of T lymphocytes was counted in the Core of Tumor (CT), choosing the richest tumor zone in lymphocyte cells on a stained slide. Moreover the lymphocytes density in tumour’s Invasive Margin (IM) was calculated at the area of overlap between tumor and normal tissue [10]. This procedure was performed for both CD3 and CD8 T lymphocytes.
Immunoscore design
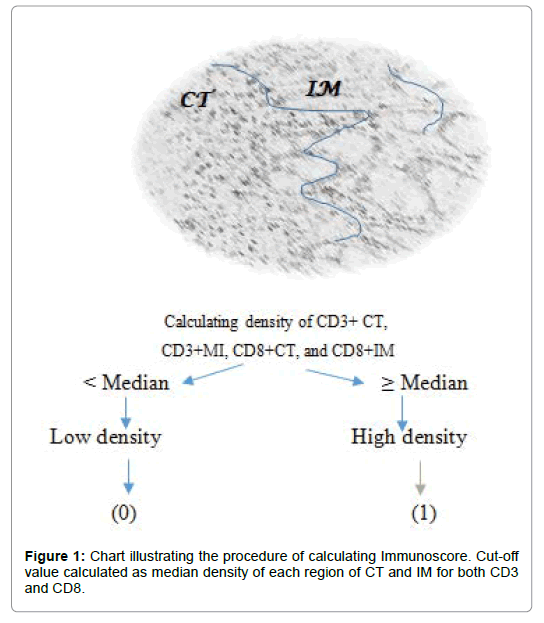
The median of each region was calculated; median cell densities of CD3 in the CT region (CD3+ CT) and in the IM region (CD3+ IM) as well as CD8 T cells in the CT region (CD8+ CT) and in IM region (CD8+ IM). The calculated median for each region was considered as threshold for classifying regions into two groups as high (1) or low (0) density as shown in Figure 1.
The next step in designing the immunological test “immunoscore” was to calculate the summation of scores of two markers of CD3 and CD8. Thus, immunoscore was classified in groups from I0 to I4 as described in Table 1. A low density of two markers CD3 and CD8 in two regions was defined as I0 and a high density of two markers CD3 and CD8 in two regions was defined as I4 (Table 1).
| CD3+ | CD8+ | Score | ||
|---|---|---|---|---|
| CD3+CT | CD3+IM | CD8+CT | CD8+IM | |
| 1 | 1 | 1 | 1 | I4 |
| 1 | 1 | 1 | 0 | I3 |
| 1 | 1 | 0 | 0 | I2 |
| 1 | 0 | 0 | 0 | I1 |
| 0 | 0 | 0 | 0 | I0 |
Table 1: Immunoscore of 5 groups calculated with density summation of 2 region in 2 markers CD3 and CD8.
Furthermore, immunoscore was classified, for large investigation, in 3 groups of low immunoscore corresponding to I0 and I1, moderate immunoscore corresponding to I2, and a high immunoscore witch correspond to I3 and I4. Another stratification was used, based on a split into two groups of immunoscore; A first group of low immunoscore (I0, I1, and I2) and a second group of high immunoscore (I3, I4).
The immune infiltration of each marker of CD3 and CD8 was also analyzed separately. Thus a high density of combined tumor region of CT/IM was classed as High Density (HD), a Moderate Density (MD) of CD marker was defined as a summation of high and low density of two region CT and IM, and a Low Density (LD) was defined as a low density in both CT and IM region [10].
Statistical analysis
Chi-Square test and t-student test were used when appropriate to analyses association between clinic pathological features and immunoscore. Kaplan-Meier curves were used to analyses Disease Free Survival (DFS) and Overall Survival (OS). Results were considered statistically significant when p<0.05. The SPSS version 21 was used for statistical analysis.
Results
Clinic pathologic data
Demographic and clinopathologic features of 54 patients were investigate and shown in Table 2. The patients study comprised 22 (40.7%) men and 32 (59.3%) women with a mean age of 55.26 years (range, 28-80 years). Histologic type of 51 (94.7%) of pre-treated biopsies were diagnosed with adenocarcinoma, and 3 (5.6%) were mucinous and signet ring carcinoma. 27 (55%) tumours out of 54 were well differentiated, 24 (44%) and 3 (5.6%) were moderate and poor and undifferentiated, respectively.
| Demographic characteristics | Patients N (%) |
|---|---|
| Age, mean (year) | 55.26 |
| Minimum | 25 |
| Maximum | 80 |
| Standard deviation | 14.66 |
| Age | |
| <50 | 22 (40.7) |
| ≥ 50 | 32 (59.3) |
| Sex | |
| Male | 22 (40.7) |
| Female | 32 (59.3) |
| Histopathology, clinical characteristics of pretreated biopsy | |
| Histologic type | |
| Adenocarcinoma | 51 (94.4) |
| Mucinous carcinoma/signet ring carcinoma | 3 (5.6) |
| Degrees of differentiation | |
| Well | 27 (55) |
| Moderate | 24 (44) |
| Poor/undifferentiated | 3 (5.6) |
| Tumor location | |
| High rectum | 4 (7.4) |
| Median rectum | 27 (50) |
| Low rectum | 23 (42) |
| Immunohistochemistry of pretreated biopsies | |
| Density of CD3 CT, mean (n cells/mm2) | 74.37 |
| Median (n cells/mm2) | 60 |
| Standard deviation | 39.45 |
| Minimum | 8 |
| Maximum | 180 |
| Density of CD3 IM, mean(n cells/mm2) | 54.28 |
| Median(n cells/mm2) | 50 |
| Standard deviation | 29.66 |
| Minimum | 10 |
| Maximum | 130 |
| Score of CD3 (CT/IM) (one marker/two regions) | |
| Ld | 10 (18.5) |
| Md | 22 (40.7) |
| Hd | 22 (40.7) |
| Density of CD8 CT, mean (n cells/mm2) | 41.94 |
| Median (n cells/mm2) | 40 |
| Standard deviation | 25.39 |
| Minimum | 10 |
| Maximum | 120 |
| Density of CD8 IM, mean (n cells/mm2) | 29.22 |
| Median (n cells/mm2) | 20 |
| Standard deviation | 20 |
| Minimum | 5 |
| Maximum | 80 |
| Score of CD 8 (CI/IM) (one marker/two regions) | |
| Ld | 14 (25.9) |
| Md | 14 (25.9) |
| Hd | 26 (48.1) |
| Immunoscore groups of 5 | |
| I0 | 5 (9.3) |
| I1 | 10 (18.5) |
| I2 | 12 (22.2) |
| I3 | 10 (18.5) |
| I4 | 17 (31.5) |
| Immunoscore groups of 3 | |
| L | 15 (27.8) |
| M | 12 (22.2) |
| H | 27 (50) |
| Immunoscore groups of 2 | |
| L | 27 (50) |
| H | 27 (50) |
| Characteristics of post therapeutic resection specimens | |
| Therapeutic response | |
| <50% | 19 (35.2) |
| ≥ 50% | 35 (64.8) |
| Therapeutic response | |
| Incomplete response | 45 (83.3) |
| Complete response | 9 (16.7) |
| Dvorak grading system | |
| 1 | 7 (13) |
| 2 | 19 (35.2) |
| 3 | 19 (35.2) |
| 4 | 9 (16.7) |
| Peri-neural invasion | |
| Presence | 6 (11.1) |
| Absence | 48 (88.9) |
| Vascular invasion | |
| Presence | 4 (7.4) |
| Absence | 50 (92.6) |
| ypN | |
| N0 | 36 (66.7) |
| N1 | 13 (24.1) |
| N2 | 5 (9.3) |
| ypT | |
| T0 | 9 (16.7) |
| T1 | 2 (3.7) |
| T2 | 20 (37) |
| T3 | 22 (40.7) |
| T4 | 1 (1.9) |
Abbrevations: CT: Core of tumor; IM: Invasive margin; Ld: Low density; Md: Moderate density; Hd: High density; L: Low; M: moderate; H: high
Table 2: Description analysis of clinicopathologica data.
In resected specimens after neoadjuvant therapy the Dvorak staging system resulting showed an incomplete therapeutic effect in 46 (85.2%) and a complete therapeutic effect in 8 (14.8%) surgical specimens. Perineural invasion and vascular invasion were observed in 6 (11.1%) and 4 (7.4%) tumours, respectively. The median follow-up duration for Overall Survival (OS) was 34 months (range, 4-80 months). Out of 54 patients, 5 (9.3%) died of tumor or other causes. The rate of relapse free survival (RFS) was 31.5% (n=17).
Immune density of CD3 and CD8
Tumor infiltration lymphocytes was analyzed using quantification of two markers of CD3 and CD8 in both CT and IM region, results were described in shows images of immunohistochemistry staining result in pre-treated rectal biopsies (Table 2 and Figure 2).
According to 5 immunoscore groups, I0, I1, I2, I3 and I4, frequency of each group was 9.3%, 18.5%, 22.2%. 18.5% and 31.5% respectively. When grouping immunoscore into 3 groups’ data allow to a proportion of 27.8% for low immunoscore, 22.2% for moderate immunoscore and 50% for high immunoscore. Furthermore, in two-tailed classification (I0, I1, I2 versus I3 and I4) 50% of tumours were ranged as low immunoscore vs 50% as high immunoscore.
Immunoscore and relationship with clinic pathological features
We analyzed immune infiltration using immunoscore grouped into 3 methods; 5 groups, 3 groups and 2 groups. The 3 methods were correlated with clinic pathological characteristics of pre-treated rectal cancer biopsies and post therapeutic resected specimens. High immunoscore (I4) was significantly associated with complete response (p=0.003) as shown in Table 3. In addition there was significant association between high immunoscore (I4) and lower ypT stage (p=0.008), as shown in Tables 3-5. Moreover, significant association was founded between 5 groups of immunoscore and lymph node status, low immune infiltration (I0, I1 I2) was associated presence of metastasis lymph node (N1)(p=0.039).
| Group of Immunoscore | p value | |||
|---|---|---|---|---|
| Low | Moderate | Height | ||
| Gender | 0.77 | |||
| Male | 5 (22.7) | 5 (22.7) | 12 (54.5) | |
| Female | 10 (31.3) | 7 (21.9) | 15 (46.9) | |
| Age | 0.341 | |||
| <50 | 6 (27.3) | 7 (31.8) | 9 (40.9) | |
| ≥ 50 | 9 (28.1) | 5 (15.6) | 18 (56.3) | |
| Histologic type | 0.422 | |||
| Adenocarcinoma | 13 (25.5) | 12 (23.5) | 26 (51) | |
| Mucinous carcinoma/signet ring carcinoma | 2 (66.7) | 0 (0) | 1 (0) | |
| Degrees of differentiation | 0.712 | |||
| Well | 9 (33.3) | 5 (18.5) | 13 (48.1) | |
| Moderate | 5 (20.8) | 7 (29.2) | 12 (50) | |
| poor/un differentiated | 1 (33.3) | 0 (0) | 2 (66.7) | |
| Therapeutic response | ||||
| <50% | 7 (36.8) | 3 (15.8) | 9 (47.4) | 0.526 |
| ≥ 50 % | 8 (22.9) | 9 (25.7) | 18 (51.4) | |
| Therapeutic response | 0.005 | |||
| incomplete response | 15 (33.3) | 12 (26.7) | 18 (40) | |
| complete response | 0 (0) | 0 (0) | 9 (100) | |
| Dvorak | ||||
| 1 | 2 (28.6) | 1 (14.3) | 4 (57.1) | 0.012 |
| 2 | 8 (42.1) | 3 (15.8) | 8 (42.1) | |
| 3 | 5 (26.3) | 8 (42.1) | 6 (31.6) | |
| 4 | 0 (0) | 0 (0) | 9 (100) | |
| Perineural invasion | 0.454 | |||
| Presence | 2 (33.3) | 0 (0) | 4 (66.7) | |
| Absence | 13 (27.1) | 12 (25) | 23 (47.9) | |
| Vascular invasion | 0.554 | |||
| Presence | 2(50) | 0 (0) | 2 (50) | |
| Absence | 13(26) | 12 (24) | 25 (50) | |
| ypT | 0.004 | |||
| T0 | 0 (0) | 0 (0) | 9 (100) | |
| T1 | 0 (0) | 1 (1) | 1 (50) | |
| T2 | 4 (20) | 6 (30) | 10 (50) | |
| T3 | 11 (50) | 4 (18.2) | 7 (31.8) | |
| T4 | 0 (0) | 1(100) | 0 (0) | |
| ypT | 0.012 | |||
| ypT0/T1/T2 | 4 (12.9) | 7 (22.6) | 20 (64.5) | |
| ypT3/T4 | 11 (47.8) | 5 (21.7) | 7 (30.4) | |
| YpN | 0.292 | |||
| N0 | 7 (19.4) | 10 (27.8) | 19 (52.8) | |
| N1 | 6 (46.2) | 2 (15.4) | 5 (38.5) | |
| N2 | 2 (40) | 0 (0) | 3 (60) | |
| ypN | 0.12 | |||
| N0 | 7 (19.4) | 10 (27.8) | 19 (52.8) | |
| N1/N2 | 8 (44.4) | 2 (11.1) | 8 (44.4) | |
Table 3 : Association between 5 groups of immunoscore and clinicopathological features.
In contrast when dividing patients into 3 and 2 groups of immunoscore, as shown in no other significant association was observed between clinicopathological parameters and Immune infiltration (Tables 4 and 5).
| Immunoscore of 2 group | |||
|---|---|---|---|
| Low | High | p value | |
| I0/I1/I2 | I3/I4 | ||
| Sex | 0.782 | ||
| Male | 10 (45.5) | 12 (54.5) | |
| Female | 17(53.1) | 15(46.9) | |
| Age | 0.406 | ||
| <50 | 13 (59.1) | 9 (40.9) | |
| ≥ 50 | 14 (43.8) | 18 (56.3) | |
| Histologic type | 1 | ||
| Adenocarcinoma | 25 (49) | 26 (51) | |
| Mucinous carcinoma/signet ring carcinoma | 2 (66.7) | 1 (33.3) | |
| Degrees of differentiation | 1 | ||
| Well | 14 (51.9) | 13 (48.1) | |
| Moderate | 12(50) | 12 (66.7) | |
| Poor/undifferentiated | |||
| Therapeutic response | 1 | ||
| <50% | 10 (52.6) | 9 (47.4) | |
| ≥ 50% | 17 (48.6) | 18 (51.4) | |
| Therapeutic response | 0.002 | ||
| Incomplete response | 27 (60) | 18 (40) | |
| Complete response | 0 (0) | 9 (100) | |
| Dvorak | |||
| 1 | 3 (42.9) | 4 (57.1) | 0.005 |
| 2 | 11 (57.9) | 8 (42.1) | |
| 3 | 13 (68.4) | 6 (31.6) | |
| 4 | 0 (0) | 9 (100) | |
| Perineural invasion | 0.669 | ||
| Presence | 2 (33.3) | 4 (66.7) | |
| Absence | 25 (52.1) | 23 (47.9) | |
| Vascular invasion | 1 | ||
| Presence | 2 (50) | 2 (50) | |
| Absence | 25 (50) | 2 (50) | |
| ypT | 0.004 | ||
| T0 | 0 (0) | 9 (100) | |
| T1 | 1 (50) | 1 (50) | |
| T2 | 10 (50) | 10 (50) | |
| T3 | 15 (68.2) | 7 (31.8) | |
| T4 | 1 (100) | 0 (0) | |
| ypN | 0.027 | ||
| ypT0/T1/T2 | 11 (35.5) | 20 (64.5) | |
| ypT3/T4 | 16 (69.6) | 7 (30.4) | |
| ypN | 0.688 | ||
| N0 | 17 (47.2) | 19 (52.8) | |
| N1 | 8 (61.5) | 5 (38.5) | |
| N2 | 2 (40) | 3 (60) | |
| ypN | 0.773 | ||
| N0 | 17 (47.2) | 19 (52.8) | |
| N1/N2 | 10 (55.6) | 8 (44.4) | |
Table 4: Association between 3 group of immunoscore and clinicopathological features.
| Immunoscore of 2 group | |||
|---|---|---|---|
| Low | High | p value | |
| I0/I1/I2 | I3/I4 | ||
| Sex | 0.782 | ||
| Male | 10 (45.5) | 12 (54.5) | |
| Female | 17(53.1) | 15(46.9) | |
| Age | 0.406 | ||
| <50 | 13 (59.1) | 9 (40.9) | |
| ≥ 50 | 14 (43.8) | 18 (56.3) | |
| Histologic type | 1 | ||
| Adenocarcinoma | 25 (49) | 26 (51) | |
| Mucinous carcinoma/signet ring carcinoma | 2 (66.7) | 1 (33.3) | |
| Degrees of differentiation | 1 | ||
| Well | 14 (51.9) | 13 (48.1) | |
| Moderate | 12(50) | 12 (66.7) | |
| Poor/undifferentiated | |||
| Therapeutic response | 1 | ||
| <50% | 10 (52.6) | 9 (47.4) | |
| ≥ 50% | 17 (48.6) | 18 (51.4) | |
| Therapeutic response | 0.002 | ||
| Incomplete response | 27 (60) | 18 (40) | |
| Complete response | 0 (0) | 9 (100) | |
| Dvorak | |||
| 1 | 3 (42.9) | 4 (57.1) | 0.005 |
| 2 | 11 (57.9) | 8 (42.1) | |
| 3 | 13 (68.4) | 6 (31.6) | |
| 4 | 0 (0) | 9 (100) | |
| Perineural invasion | 0.669 | ||
| Presence | 2 (33.3) | 4 (66.7) | |
| Absence | 25 (52.1) | 23 (47.9) | |
| Vascular invasion | 1 | ||
| Presence | 2 (50) | 2 (50) | |
| Absence | 25 (50) | 2 (50) | |
| ypT | 0.004 | ||
| T0 | 0 (0) | 9 (100) | |
| T1 | 1 (50) | 1 (50) | |
| T2 | 10 (50) | 10 (50) | |
| T3 | 15 (68.2) | 7 (31.8) | |
| T4 | 1 (100) | 0 (0) | |
| ypN | 0.027 | ||
| ypT0/T1/T2 | 11 (35.5) | 20 (64.5) | |
| ypT3/T4 | 16 (69.6) | 7 (30.4) | |
| ypN | 0.688 | ||
| N0 | 17 (47.2) | 19 (52.8) | |
| N1 | 8 (61.5) | 5 (38.5) | |
| N2 | 2 (40) | 3 (60) | |
| ypN | 0.773 | ||
| N0 | 17 (47.2) | 19 (52.8) | |
| N1/N2 | 10 (55.6) | 8 (44.4) | |
Table 5: Association between Two groups of Immunscore and clinicopathologic features.
Considering analysis according to one marker of CD3 and CD8. Density evaluated in combined CT and IM tumor region associated with clinicopathological features was illustrated in Table 6.
| CD3 CT/IM Immunoscore | p value | CD8 CT/IM Immunoscore | p value | |||||
|---|---|---|---|---|---|---|---|---|
| I0 | I1 | I2 | I0 | I1 | I2 | |||
| N(%) | N(%) | N(%) | N(%) | N(%) | N(%) | |||
| Gender | 0.031 | 0.077 | ||||||
| Male | 1 (4.5) | 13 (59.1) | 8 (36.4) | 5 (22.7) | 7 (31.8) | 10 (45.5) | ||
| Female | 9 (28.1) | 9 (28.1) | 14 (43.8) | 9 (28.1) | 7 (21.9) | 16 (50) | ||
| Age | 1 | 0.375 | ||||||
| <50 | 4 (18.2) | 9 (40.9) | 9 (40.9) | 8 (36.4) | 5 (22.7) | 9 (40.9) | ||
| ≥ 50 | 6 (18.8) | 13 (40.6) | 13 (40.6) | 6 (18.8) | 9 (28.1) | 17 (53.1) | ||
| Histologic type | 1 | 1 | ||||||
| Adenocarcinoma | 9 (17.6) | 21 (41.2) | 21 (41.2) | 13 (25.5) | 13 (25.5) | 25 (49) | ||
| Mucinous carcinoma/signet ring carcinoma | 1 (33.3) | 1 (33.3) | 1 (1) | 1 (33.3) | 1 (33.3) | 1 (33.3) | ||
| Degrees of differentiation | 0.898 | 0.147 | ||||||
| Well | 5 (18.5) | 12 (44.4) | 10 (37) | 6 (22.2) | 11 (40.7) | 10 (37) | ||
| Moderate | 5 (20.8) | 9 (37.5) | 10 (41.7) | 7 (29.2) | 3 (12.5) | 14 (58.3) | ||
| poor/undifferentiated | 0 (0) | 1 (33.3) | 2 (66.7) | 1 (33.3) | 0 (0) | 2 (66.7) | ||
| Therapeutic response | 0.429 | 0.175 | ||||||
| <50% | 3 (15.8) | 10 (52.6) | 6 (31.6) | 8 (42.1) | 4 (21.1) | 7 (36.8) | ||
| ≥ 50% | 7 (20) | 12 (34.3) | 16 (45.7) | 6 (17.1) | 10 (28.6) | 19 (54.3) | ||
| Therapeutic response | 0.008 | 0.002 | ||||||
| incomplete response | 10 (22.2) | 21 (46.7) | 14 (31.1) | 14 (31.1) | 14 (31.1) | 17 (37.8) | ||
| complete response | 0 (0) | 1 (11.1) | 8 (88.9) | 0 (0) | 0 (0) | 9 (100) | ||
| Dvorak | 0.078 | 0.007 | ||||||
| 1 | 1 (14.3) | 3 (42.9) | 3 (42.9) | 2 (28.6) | 2 (28.6) | 3 (42.9) | ||
| 2 | 5 (26.3) | 9 (47.4) | 5 (26.3) | 8 (42.1) | 3 (15.8) | 8 (42.1) | ||
| 3 | 4 (21.1) | 9 (47.4) | 6 (31.6) | 4 (21.1) | 9 (47.4) | 6 (31.6) | ||
| 4 | 0 (0) | 1 (11.1) | 8 (88.9) | 0 (0) | 0 (0) | 9 (100) | ||
| Perineural invasion | 0.54 | 0.398 | ||||||
| Yes | 2 (33.3) | 1 (16.7) | 3 (50) | 1 (33.3) | 0 (0) | 4 (66.7) | ||
| No | 8 (16.7) | 21 (43.8) | 19 (39.6) | 12 (25) | 14 (29.2) | 22 (45.8) | ||
| Vascular invasion | 1 | 0.359 | ||||||
| Yes | 1 (25) | 2 (50) | 1 (25) | 2 (50) | 0 (0) | 2 (50) | ||
| No | 9 (18) | 20 (40) | 21 (42) | 12 (24) | 14 (28) | 24 (48) | ||
| ypT stage | 0.09 | 0.011 | ||||||
| T0 | 0(0) | 1 (11.1) | 8 (88.9) | 0(0) | 0 (0) | 9 (100) | ||
| T1 | 0 (0) | 1 (50) | 1 (50) | 0 (0) | 1 (50) | 1(50) | ||
| T2 | 4 (20) | 10(50) | 6 (30) | 5 (25) | 5 (25) | 10 (50) | ||
| T3 | 6 (27.3) | 9 (40.9) | 7 (31.8) | 9 (40.9) | 7 (31.8) | 6 (27.3) | ||
| T4 | 0 (0) | 1 (100) | 0 (0) | 0(0) | 1(100) | 0(0) | ||
| ypT stage | 0.348 | 0.016 | ||||||
| T0/T1/T2 | 4 (12.9) | 12 (38.7) | 15 (48.4) | 5 (16.1) | 6 (19.4) | 20 (64.5) | ||
| T3/T3 | 6 (26.1) | 10 (43.5) | 7 (30.4) | 9 (39.1) | 8 (34.8) | 6 (26.1) | ||
| ypN status | 0.185 | 0.842 | ||||||
| N0 | 5 (13.9) | 14 (38.9) | 17 (47.2) | 8 (22.2) | 10 (27.8) | 18 (50) | ||
| N1 | 4 (30.8) | 4 (30.8) | 5 (38.5) | 5 (38.5) | 3 (23.1) | 5 (38.5) | ||
| N2 | 1 (20) | 4(80) | 0 (0) | 1 (20) | 1 (20) | 3 (60) | ||
| ypN status | 0.357 | 0.751 | ||||||
| N0 | 5 (13.9) | 14 (38.9) | 17 (47.2) | 8 (22.2) | 10 (27.8) | 18 (50) | ||
| N1/N2 | 5 (27.8) | 8 (44.4) | 5 (27.8) | 6 (33.3) | 4 (22.2) | 8 (44.4) | ||
Table 6: Association between CD 3 and CD 8 immunoscore and clinicopathological features.
Significant association was found between density of CD3 and sex, 43.8% of women had a high density infiltration compared to only 36.4 of men with a proportion of 36.4% in high score infiltration. Both CD3 and CD8 showed association with pathologic response to neoadjuvant treatment. We observed that 88.9% and 100% of CD3 and CD8, respectively, of patients who expressed high density had a complete response (p=0.008, p=0.002). Moreover, 64.5% of patients who had a T0, T1 and T3 stage was classed in High score of density infiltration of CD8 (p=0.016).
Survival analysis correlated with immunoscore
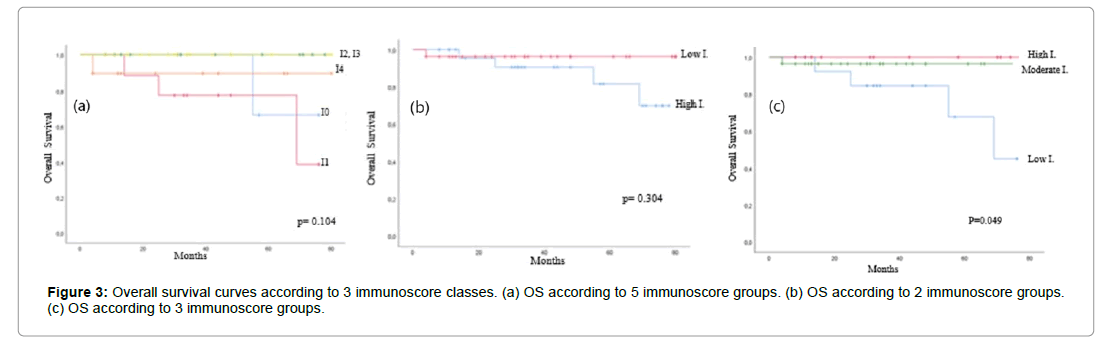
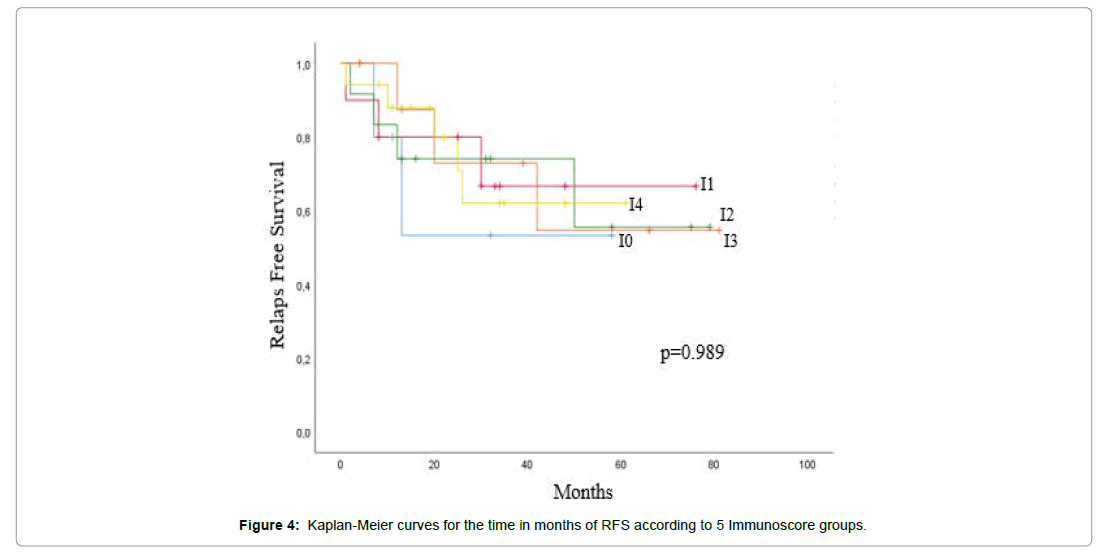
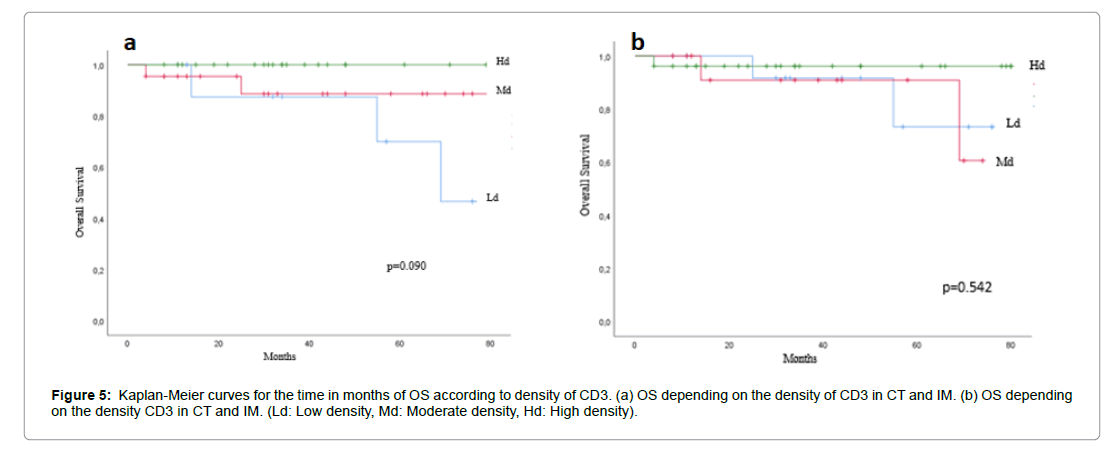
The correlation between survival analysis and immunoscore was investigated in rectal cancer. The effect of T-cell density was correlated to immune infiltration classified on 3 groups as showed in Figures 3a and 3b, a significantly association was found between OS and high expression of immunoscore (p=0.45), while RFS was not associated with immune density in different region of two markers. Patient’s survival curves according to density of each marker of CD3 and CD8 did not show significance as describe in Figures 3-5.
Discussion
The interaction between immune system cells and cancer cells is part of the mechanisms of “immunosurveillance” [14]. In a global way, a new type of biomarker can define the intratumoral component in immune cells. This biomarker may be complementary to other biomarkers linked directly to the tumor cell [3]. The technological revolution, which concerned nanotechnologies, the calculation and imaging system as well as digital pathology, made it possible to better characterize and identify the component of the tumor cell with great precision and robustness. This has created a favourable ground for research and development of new biomarkers [15]. Also allowed to understand the role that the immune system plays in the dynamics of cancer and to develop an original method called “Immunoscore” [5,6]. Studies have shown that in most solid tumours such as colorectal cancer, a strong immune infiltration in the tumor has been associated with prolonged survival [9].
Our study was conducted to evaluate relationships between immune infiltration using Immunoscore test, and therapeutic response, clinicopathological features and survival outcomes of patients. We here in demonstrate that high score of immune cells infiltration in pretreated tumours is associated with a complete pathologic response after neoadjuvant therapy. As a result, we demonstrate the importance role of densities of CD3 and CD8 in both CT and IM in therapy sensitivity.
The high level expression of immune cells of CD3 and CD8 proved necessary to ensure a complete response. This result is in accordance with publication of several studies [10,16] in which they report a significant correlation between high densities of CD3 cells and response to chemo radiation therapy evaluated using ypTNM and Dvorak classification. Similar finding was reported by Melanie J in a cohort involving 106 rectal tumours [17]. Therefore, these experiments suggested that the behavior of the tumor cell is the consequence of a balance between the tumor invasion process and the local immune response of the host [9,18]. Furthermore, this correlation can be explaining by an additional immune adjuvant acting involving innate and adaptive immune response [19]. Also previous studies on rectal cancer showed the impact of the association of cytotoxic T lymphocytes with an immune orientation of Th1 lymphocytes in tumor microenvironment [18,20,21]. Patients with a good response to neoadjuvant treatment present in the tumor area an increase in the transcript level of T lymphocyte activation genes and cytotoxic Th1 orientation [22].
We further demonstrate in our study that a high density of CD3 infiltration in both CT and IM within pre-treated rectal biopsies was correlated with a lower pathologic tumor infiltration (ypT) in rectal specimen resections after neoadjuvant treatment. The immune infiltration in tumor sites is not hindered to physical born, thus a broad expansion is confronted with an immunosuppressive environment [6,23,24]. Maria-Gabriele Anitei confirmed that the highest immunoscore I4 showed in 90% of patients was observed in localized tumor infiltration [10].
Similar research interesting 109 patients with rectal cancer was conducted by siyu Zhang between 2002 and 2005. He indicated that a highly expression of CD4+ and CD8+ Tumor Infiltration Lymphocytes (TIL) was correlated with more response to neoadjuvant therapy. Also he found a significant difference between TIL density between pretherapeutic and post-therapeutic tumours [24]. This explain the major role of neoadjuvant treatment as an enhancer of immune defences by stimulating the expression of receptors specific to cell death, as well as to increase immune cell density such as CD4+ and CD8+, and this lead to a positive activation of tumor immune microenvironment [25].
We also highlight the performance of immune infiltration measured in pre-treated biopsies to predict the survival outcome of patients after neoadjuvant and surgical treatment [10]. Tumor microenvironment will have a major role in the development of supplementary immunotherapy cancer therapy for altered and cold tumours with combination of radiotherapy or chemotherapy with immunotherapy [6,25].
The Immunoscore will have an interest in the immune evaluation of CD3 and CD8 in diagnostic biopsies in rectal cancer in order to help in the therapeutic management. Particularly in the decision making of a minimal conservative surgery or simple monitoring [26], taking into consideration the morbidity and mortality related to the surgical procedure, as well as the alteration of the quality of life of the patients [2]. To improve significant correlation with RFS, and to obtain a comparative surgical and outcomes with literatures the respect of 10-years follow-up is required for clinical studies in rectal cancer as recommended [27].
Conclusion
Our investigational analysis highlight the role of high Immunoscore of CD3 and CD8 in both CT and IM, to be a predictive factor for pathologic complete response after neoadjuvant therapy in rectal cancer. Immune infiltration analysis of the microenvironment was correlated to improved overall survival. DFS were not found to be significant depending on microenvironment immunity infiltration. Evaluation of the immune marker on a multicentre study should be taken into consideration.
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Conflict of Interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
Funding Statement
No funding has been granted for this research project.
Acknowledgments
Sincere thanks to Professor Amarti Afar from AL-AZHAR laboratory, and her team for expert advices in immunohistochemestry technique. Special gratitude to Mohamed Issaoui from Laboratory of Anatomic Pathology and Molecular Pathology at the University Hospital Hassan II at Fez, for technical assistance in immunohistochemistry techniques.
References
- Folkman J, Hahnfeldt P, Hlatky L (2000) Cancer: Looking outside the genome. Nat Rev Mol Cell Biol 1: 76-79.
- Sissy CE, Marliot F, Haicheur N, Kirilovsky A, Scripcariu D, et al. (2017) Mise au point sur l’immunoscore et ses potentielles implications cliniques focus on the immunoscore and its potential clinical implications. Ann Pathol 37: 29-38.
- Guillebon ED, Tartour E (2015) Immunite antitumorale (mécanismes, immunoediting, immunosurveillance). Oncologie 17: 337-344.
- Dunn GP, Old LJ, Schreiber RD (2004) The three Es of cancer immuno editing. Annu Rev Immunol 22: 329-360.
- Schreiber RD, Old LJ, Smyth MJ (2011) Cancer immunoediting: Integrating immunityls roles in cancer suppression and promotion. Science 331: 1565-1570.
- Galon J, Bruni D (2019) Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat Rev Drug Discov 18: 197-218.Â
- Albert ML, Sauter B, Bhardwaj N (1998) Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature 392: 86-89.
- Rahir G, Moser M (2012) Tumor microenvironment and lymphocyte infiltration. Cancer Immunol Immunother 61: 751-759.
- Fridman WH, Pages F, Fridman CS, Galon J (2012) The Immune contexture in human tumours: Impact on clinical outcome. Nat Rev Cancer 12: 298.
- Anitei MG, Zeitoun G, Mlecnik B, Marliot F, Haicheur N et al. (2014) Prognostic and predictive values of the immunoscore in patients with rectal cancer. Clin Cancer Res 20: 1891-1899.
- Mlecnik B, Tosolini, Kirilovsky A, Berger A, Bindea G, et al. (2011) Histopathologic-based prognostic factors of colorectal cancers is associated with the state of the local immune reaction. J Clin Oncol 29: 610-618.
- Pagès F, Mlecnik B, Marliot F, Bindea G, Ou FS, et al. (2018) International validation of the consensus immunoscore for the classification of colon cancer: A Prognostic and accuracy study. The Lancet 391: 2128-2139.
- Otmani IE, Agy FE, Baradai SE, Bouguenouch L, Lahmidani N, et al. (2020) Analysis of molecular pretreated tumor profiles as predictive biomarkers of therapeutic response and survival outcomes after neoadjuvant therapy for rectal cancer in Moroccan population. Dis Markers 2020: 2-3.
- Galon J, Pagès F, Marincola FM, Thurin M, Trinchieri G, et al. (2012) The Immune score as a new possible approach for the classification of cancer. J Transl Med 10: 1.
- Galon J, Costes A, Cabo FS, Kirilovsky A, Mlecnik B, et al. (2006) Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 313: 1960-1964.
- Yasuda K, Nirei T, Sunami E, Nagawa H, Kitayama J (2011) Density of CD4+ and CD8+ T lymphocytes in biopsy samples can be a predictor of pathological response to chemoradiotherapy (CRT) for rectal cancer. Radiat oncol 6: 49.
- McCoy MJ, Hemmings C, Anyaegbu CC, Austin SJ, Pullen TFL, et al. (2017) Tumour-infiltrating regulatory T cell density before neoadjuvant chemoradiotherapy for rectal cancer does not predict treatment response. Oncotarget 8: 19803-19813.
- Hanahan D, Weinberg RA (2011) Hallmarks of cancer: The Next generation. Cell 144: 646-674.
- Zitvogel L, Galluzzi L, Smyth MJ, Kroemer G (2013) Mechanism of action of conventional and targeted anticancer therapies: Reinstating immunosurveillance. Immunity 39: 74-88.
- Jass JR (1986) Lymphocytic infiltration and survival in rectal cancer. J Clin Pathol 39: 585-589.
- Nagtegaal ID, Marijnen CA, Kranenbarg EK, Stapel AM, Hermans J, et al. (2001) Local and distant recurrences in rectal cancer patients are predicted by the nonspecific immune response; specific immune response has only a systemic effect-a histopathological and immunohistochemical study. BMC Cancer 1: 7.
- Zeitoun G, Sissy CE, Kirilovsky A, Anitei G, Todosi AM, et al. (2019) The Immunoscore in the clinical practice of patients with colon and rectal cancers. Chirurgia 114: 152-161.
- Mlecnik B, Bindea G, Angell HK, Sasso MS, Obenauf AC, et al. (2014) Functional network pipeline reveals genetic determinants associated with in situ lymphocyte proliferation and survival of cancer patients. Sci Transl Med 6: 228-237.
- Zhang S, Bai W, Tong X, Bu P, Xu J, et al. (2019) Correlation between tumor microenvironment-associated factors and the efficacy and prognosis of neoadjuvant therapy for rectal cancer. Â Oncol Lett 17: 1062-1070.
- Kalbasi A, June CH, Haas N, Vapiwala N (2013) Radiation and immunotherapy: A Synergistic combination. J Clin Invest 123: 2756-2763.
- Gama AH (2006) Assessment and management of the complete clinical response of rectal cancer to chemoradiotherapy. Colorectal Dis 8: 21-24.
- Sadahiro S, Suzuki T, Ishikawa K, Nakamura T, Tanaka Y, et al. (2003) Recurrence patterns after curative resection of colorectal cancer in patients followed for a minimum of ten years. Hepatogastroent 50: 1362-1366.
Citation: Otmani IE, Agy FE, Lahmidani N, Abkari ME, Benajah DA, et al. (2021) Prognostic and Predictive Value of Lymphoid Microenvironment in Locally Advanced Rectal Cancer. J Oncol Res Treat S5: 003.
Copyright: © 2021 Otmani IE, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Open Access Journals
Article Usage
- Total views: 2109
- [From(publication date): 0-2021 - Feb 22, 2025]
- Breakdown by view type
- HTML page views: 1673
- PDF downloads: 436