Special Issue Article Open Access
Production of the Human Anti-Metastatic Melonoma Interleukin-2 in Maize Vegetative Biomass for Clinical Use
| Thang Xuan Nguyen, Hussien Alameldin, Patrick Thomas and Mariam Sticklen* | ||
| Department of Plant, Soil and Microbial Sciences, Michigan State University, 361 Plant Soil Sciences Building, East Lansing, MI 48824, USA | ||
| Corresponding Author : | Mariam Sticklen Department of Crop Soil and Microbial Sciences Michigan State University East Lansing, MI 48824, USA Tel: 517-230- 2929 E-mail: Stickle1@msu.edu |
|
| Received November 29, 2014; Accepted May 20, 2015; Published May 22, 2015 | ||
| Citation: Nguyen TX, Alameldin H, Thomas P, Sticklen M (2015) Production of the Human Anti-Metastatic Melonoma Interleukin-2 in Maize Vegetative Biomass for Clinical Use. Adv Crop Sci Tech S1:002. doi: 10.4172/2329-8863.1000S1-002 | ||
| Copyright: ©2015 Nguyen TX, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | ||
Related article at Pubmed Pubmed  Scholar Google Scholar Google |
||
Visit for more related articles at Advances in Crop Science and Technology
Abstract
The E.coli produced recombinant human interleukin-2 (rhIL-2) has been approved by the Food and Drug Administration for the immunotherapeutic treatment of the end-stage metastatic melanoma and renal cell cancer. However, the E.coli produced rhIL-2 is very expensive (~$11,400 USD per treatment). In the present study, we explored the feasibility of producing rhIL-2 in transgenic Zea mays (maize) vegetative biomass instead of its production in E. coli because (1) maize vegetative biomass is abundant, (2) maize can cheaply produce recombinant proteins while obtaining its energy from freely available sun via photosynthesis, has an easy scale-up via reproduction system, can be easily grown by farmers in the field with minimum level of training, and has conserved protein folding machinery including glycosylation similar to that of human. The human hIL-2 gene was codon optimized to maximize its expression in plants. A plasmid construct containing the rhIL-2 regulated by a rubisco green-specific promoter, an endoplasmic reticulum-specific signal peptide, 6-histodin tag was developed and nos terminator The construct was transferred into the maize genome via the gene gun bombardment, and fertile plants developed. Molecular analysis confirmed that the human IL-2 had integrated, transcribed and translated in up to the 4th (T3) generation maize plants. When the same gene construct was transferred into the tobacco genome and the rhIL-2 was purified and its biological activity compared with the FDA approved commercially available E coli- produced version against the marine splenic CD4+ the mice cells, the plant-produced version was as effective as the commercially available E. coli-produced version. Research is needed to test the rhIL-2 producing maize in the field, and to perform preclinical and clinical trial for its potential commercial release.
| Keywords | |
| Maize; Recombinant DNA; Biopharmaceutical; Interleukin-2; Plant | |
| Introduction | |
| Historically, biopharmaceuticals including the recombinant human interleukin-2 (rhIL-2) have been produced in E. coli expression system. The plant expression system is believed to be superior over the E. coli expression system for production of recombinant human biopharmaceutical proteins because plants directly use solar energy rather than chemical energy inputs; plants have an easy scale-ups, plants can be easily grown by farmers in the field with minimum level of training, most plants have abundant vegetative biomass, and plants have conserved protein folding machinery including glycosylation similar to that of human [1-3]. | |
| Developed in late 1970’s, genetic modification of crops has benefitted humans for the production of food, feed, fuel and industrial matters [4]. Multiple numbers of companies have been pursuing the use of agricultural crops for the production of biopharmaceuticals [5]. Due to its high vegetative biomass, maize can be genetically modified in a manner that it can produce biopharmaceuticals and other products such as biodegradable plastic [6] and microbial cellulase enzymes in its leaves, stalk and husk i.e. not in its seeds, flowers or roots [7,8]. With the growing needs for biopharmaceuticals and the needs to reduce their production costs, and the fact that maize produces abundant vegetative biomass wastes, maize is an ideal crop for production of biopharmaceuticals [9] including the recombinant human interleukin-2 (hrIL-2). | |
| ¨The E. coli-produced IL-2 is a cytokine that has been approved [10] by the Food and Drug Administration (FDA) and commercialized by Novartis Pharmaceuticals under the generic name “Aldesleukin” or “Proleukin” and released commercially under the name Proleukin or Aldesleukin for its immunotherapeutic clinical treatment of the endstage monastic melanoma cancer (skin cancer) and renal kidney cancer [1]. | |
| Currently, the commercially available rhIL-2 is produced in E. coli. While E. coli is a safe and a common expression host for production of biopharmaceuticals, it costs patients ~$11,400 USD per clinical treatment [1]. | |
| In 2013, the authors reported the production of rhIL-2 in the endoplasmic reticulum (ER) of tobacco vegetative biomass, its purification and testing of the purified tobacco-produced rhIL-2 against the Splenic CD4+T-Cells [1]. ER is a sub-cellular compartment in plant cells that is naturally in charge of correct protein folding [11]. | |
| Accumulation of recombinant proteins in the lumen of the ER in the plant cells does not affect the plant host. Other befits of targeting of heterologous proteins into the plant ER is because ER contains many molecular chaperones, carries protein glycosylation, and it is lacking proteases , and therefore can help with correct folding and assembly of recombinant proteins. ER also alleviates downstream protein processing in plants, and therefore is considered an ideal compartment for production of most recombinant proteins including biopharmaceuticals in plants [1,12,13]. | |
| Here, we report the production of hrIL-2 in the ER of maize vegetative biomass. We also report the development of 4th (T3) generation rhIL-2 producing maize that is also tolerant to drought as it expresses the barley drought tolerance HVA1 transgene to better adapt to drier environment. HVA1 has been proven to be a single gene of importance used for drought tolerance in oat [14-16], rice [17,18] and wheat [19]. The authors recently reported the production of drought tolerance maize via the transfer of the barley HVA1 gene into its genome [20]. | |
| Materials and Methods | |
| The plant expression vector (Constructs) | |
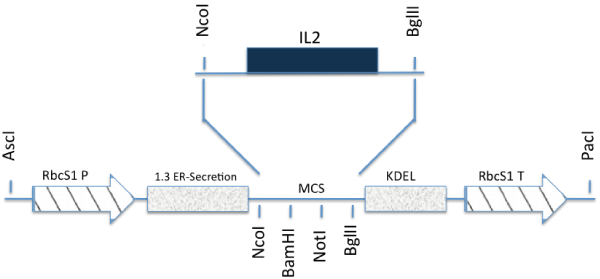
| A plant Expression vector (Figure 1) containing the rhIL-2, a rubisco green-specific promoter, endoplasmic reticulum transit peptide, nos terminator and 6-histodin tag was developed [1]. | |
| A second plasmid, pBY520 containing the barley HVA1 gene regulated by the rice actin promoter and nos terminator [20] was also used for genetic co-transformation. This construct also contains a second cassette containing the bar herbicide resistance gene regulated by the 35S promoter and nos terminator. | |
| Plant tissue culture and gene bombardment | |
| Maize plants were grown in a greenhouse to maturity. Immature seeds were sterilized and immature embryos dissected and cultured on a maize culture growth media containing MS salts and vitamins [21], sucrose, and myo-innsitol [20]. Small (2-3 mm) long undifferentiated tissue (callus) clumps were bombarded with a 1:1 ration of the two plasmids using a solution containing 150 μL of M10 Tungsten, along with 150 μL calcium chloride and 60 μL spermidine. The solution was placed on yellow disks designed for bombardment and shot in immature embryo-derived Petri dishes at a pressure of 25-26 PSI. | |
| The bombarded calli were left to grow in the dark for approximately two weeks, or until very small shootlets were developed. Then, the shootlets were transferred to a shoot growing media containing MS Salt and vitamins, supplemented with sucrose, myo-inositol and biolaphous. Once the shootets emerged to develop and roots could be seen, the plantlets were transferred to a rooting media containing the MS salt and vitamines supplemented with indole butyric acid (IBA) as described before and kept under light condition [20]. | |
| Genetic transformation and regeneration of the 4th generation transgenic maize plants | |
| Biolistic particle delivery (gene gun) system was used for stable transformation of maize following the author’s standard procedures [20]. | |
| Confirmation of transgenes integration and expression | |
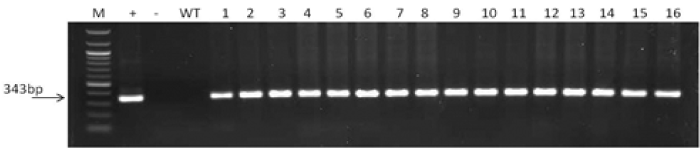
| Polymerase chain reaction: DNA extraction was performed using the CTAB method [20]. The primers used to screen for the IL-2 transgene lines were designated IL-2OPT_F1 and IL-2OPT_R1. The sequences of these primers are CATGGCGGCACCCACTTCAA (forward primer) and GGCGGTCTCGTCAGCGTATT (reverse primer). They amplify a 343bp segment of the IL-2 transgene. The PCR thermo cycler was used through the following cycles: 940C for 3 min (1 cycle); and then 940C for 30s, 620C for 30s, 720C for 45s (35 cycles); 720C for 5 min (1 cycle), and 40C indefinitely. The plasmid DNA containing the IL-2 analog was used as a positive control for these reactions. | |
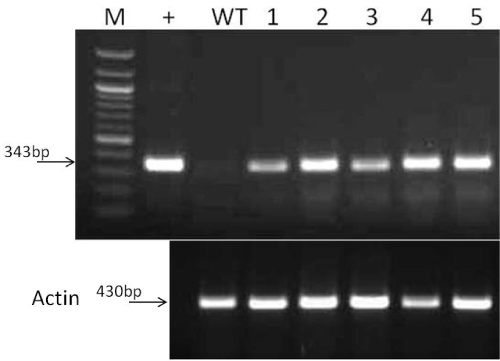
| Semi-quantitative PCR (reverse transcriptase-PCR): RNeasy Plant Mini Kit was used to perform the RNA Extraction from plant leaves. First-strand cDNA was synthesized using the SuperScript® III Reverse Transcriptase (RT). Actin mRNA was used for normalization in quantifying cDNA. The specific primers was designed based on the mRNA of IL2 gene (CATGGCGGCACCCACTTCAA) as forward primer and (GGCGGTCTCGTCAGCGTATT) as reverse primer; (ctggattctggagacggtgt) as forward and (gccatacaggtccttcctga) as reverse based on actin mRNA. The reactions consisted of 940C for 3 min (1 cycle); and then 940C for 30s, 620C for 30s, 720C for 45s (35 cycles); 720C for 5 min (1 cycle), and 40C indefinitely. Amplified PCR products were visualized on a 1.5% agarose gel. The PCR products were quantified using the Bio- Rad Quantity One software, Actin cDNA was used as a standard for normalization. | |
| Protein extraction and western blotting: Protein extraction: Fresh leaf samples of maize lines that transcribed the rhIL-2 was homogenized in phosphate grinding buffer containing 100mM sodium phosphate pH 5.8, 1mM EDTA, 10mM diethyldithio carbamic acid, and 0.5% Tween 20. A protease inhibitor cocktail (2X) was purchased from the Sigma-Aldrich; St. Louis, MO, and was added in order to the buffer to prevent proteases to degrade the rhIL-2 during protein extraction. Protein quantification took place using the commercially available Coomassie Plus (Bradford) Protein Assay reagent (Thermo Scientific Co.). All steps associated with protein extraction and purification was conducted on ice. Protein samples were then incubated at 80°C for 10 minutes before Western blotting. In more details, three leaf disks were placed in SDS buffer (2.4 μL 10X reducing agent, six μL 4X loading dye and 15.6 μL Crude protein extract) and kept chilled. The samples were then boiled at 80 °C for 10 minutes. | |
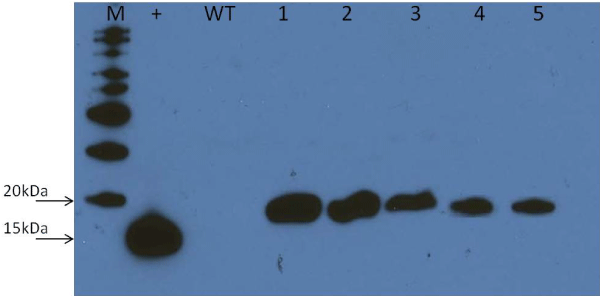
| Western blotting was performed as per standard method [1] using a monoclonal antibody to the human IL-2 as described before [20] The gel electrophoresis apparatus was filled with water and ran at 30V for one hr. The transferred membrane was then rocked in 20mL of blocking buffer composed of 1X PBS, 0.1% TWEEN 20 and 5% (w/w) non-fat dry milk for 45 min. After removing the blocking buffer, membrane was rocked in the primary antibody solution (20mL of 1X PBS with 0.2 ug/mL of polyclonal goat IgG specific for IL-2 (R&D systems AF-202-NA) for 1.5 hrs. The membrane was rocked thrice washing buffer (1X PBS with 0.1% TWEEN 20) for every ten mins. The secondary antibody solution [HRP conjugated donkey anti-goat IgG (R&D System) HAF109] was applied to the membrane and rocked for 1.5 hrs. The procedure was then followed by three times of wash steps. The SuperSignal West Pico Kit (CN # 34079, Thermo Scientific) and autoradiography film (Premier Autoradiography Film, E3018, Denville Scientific Inc, Metuchen, NJ) was used for visualization as per manufacturer’s instructions. | |
| Production of transgenic maize progeny: Plants that produced the rhIL-2 protein were self-bred (Figure 2) for three generations following the authors previous maize research [20], and seeds were collected. Seeds were germinated and grown in a maize greenhouse, where they were maintained at 25 degree Celsius with a photoperiod of 16L/8D. | |
| Results and Discussions | |
| The PCR, RT-PCR and Western blot analysis were respectively performed to test for the integration, transcription and correct translation of rhIL-2 in maize vegetative tissues (Figures 3 and 4). The expected DNA size for IL-2 is 35bp, and the expected protein size is 15 kDa. The PCR analysis confirmed that the human IL-2 has been correctly incorporated into the maize plant genome. However, the Western blotting showed the maize produced rhIL-2 to be~20 KDa. The extra base pairs are mostly due to the inclusions of tags and other seqences in the plasmid construct (Figure 1). Molecular studies also confirmed the integration, transcription and translation of the barley HVA1 and the bar herbicide resistance gene in the maize vegetative biomass [20]. Furthermore, comprehensive drought tolerance tests of up to the 4th-generation plants confirmed the drought tolerance of HVA1 transgenic maize [20]. | |
| Expected band size is 343 bp. M: a molecular marker; +: commercially available E. coli-produced rhIL-2; (Water as a negative control); WT: Wild-type control non-transgenic plant. Expected band size is 343bp for IL2. The expression levels are the same for all five plants. The lower panel of this Figure 5 represents the cDNA loading control, showing the expression of maize actin. The expected band size is 430 bp. | |
| Economic analysis needs to be performed to estimate the the amount of rhIL-2 produced per ton of maize vegetative biomass, and/ or maize crop produced per hectare for a comprehensive comparison of cost effectiveness of maize expression system over that of the E. coli expression platform. | |
| It is expected that production of rhIL-2 produced in maize vegetative wastes to have the potential to benefit the western nations, as well as the developingt nations where maize can be easily grown in a large-scale in the fields, and the protein can be purified for clinical use. | |
| References | |
References
- id="Reference_Titile_Link" value="1">Matakas Jason D, Balan V, Carson WF IV, Gao D, Sticklen M, et al. (2013)Plant-produced recombinant human interleukin-2 and its activity against splenic CD4+T-cells. Intl J Life SciBiotechnol&Pharma Res 2: 192-203.
- id="Reference_Titile_Link" value="2">Gomord V, Sourrouille C, Fitchette AC, Bardor M, Pagny S, et al. (2004) Production and glycosylation of plant-made pharmaceuticals: the antibodies as a challenge.Plant Biotechnol J 2: 83-100.
- id="Reference_Titile_Link" value="3">Gomord V, Fitchette AC, Menu-Bouaouiche L, Saint-Jore-Dupas C, Ps lasson C, et al. (2010) Plant-specific glycosylation patterns in the context of therapeutic protein production.Plant Biotechnol J 8: 564-587.
- id="Reference_Titile_Link" value="4">Theresa P (2008) Genetically Modified Organisms (GMOs): Transgenic Crops and Recombinant DNA Technology. Nature Education 1:213.
- id="Reference_Titile_Link" value="5">Giddings G, Allison G, Brooks D, Carter A (2000) Transgenic plants as factories for biopharmaceuticals.Nat Biotechnol 18: 1151-1155.
- id="Reference_Titile_Link" value="6">Heng Z, Teymouri F, Chapman B, Maqbool S, Sticklen MB, et al. (2003)The dicot pea (Pisumsativum L.) rbcS transit peptide directs the Alcaligeneseutrophuspolyhydroxybutyrate enzymes into the monocot maize (Zea mays L.) chloroplasts. Plant Science 165: 455-462.
- id="Reference_Titile_Link" value="7">Park SH, Ransom C, Mei C, Sabzikar R, Sticklen M,et al. (2011) In the quest of alternatives to microbial cellulase mix production: Corn stover-produced heterologous multi-cellulases readily deconstruct lignocellulosic biomass to fermentable sugars. J ChemTechnolBiotechnol. Online.
- id="Reference_Titile_Link" value="8">Bakhsh Allah, AbdulQayyumRao, SeeshamShamim, TayyabHusin (2011) A Mini-Review: Rubisco Small Subunit as a strong green tissue-specific promoter. Arch BiolSci Belgrade 63: 299-307.
- id="Reference_Titile_Link" value="9">Sticklen M (2014) Plantipharma Technology: Production of Antibodies, Anti-HIV, Anti-Ebola Virus, Anti-End-Stage Metastatic Melanoma and Other Recombinant Biotech Drugs in Crops. Adv Crop Sci Tech 2:e120.
- id="Reference_Titile_Link" value="10">Diehl B (2011) “Approval of New Melanoma Therapy Changes Landscape for Patients" Melanoma Research Foundation, Washington DC.
- id="Reference_Titile_Link" value="11">Vitale A, Denecke J (1999) The endoplasmic reticulum-gateway of the secretory pathway Plant Cell 11: 615-628.
- id="Reference_Titile_Link" value="12">Chuansheng M, Park SH, Sabzikar R, Qi, Mariam Sticklen B (2008) Green Tissue-Specific Production of a Microbial Endo-Cellulase in Maize (Zea mays L.)Endoplasmic-Reticulum and Mitochondria Converts Cellulose into Fermentable Sugars. JCTB 84:689-695.
- id="Reference_Titile_Link" value="13">Kleizen B, Braakman I (2004) Protein folding and quality control in the endoplasmic reticulum.CurrOpin Cell Biol 16: 343-349.
- id="Reference_Titile_Link" value="14">Maqbool SB, Zhong H, El-Maghraby Y, Ahmad A, Sticklen MB, et al (2002) Competence of oat (Avena sativa L.) shoot apical meristems for integrative transformation, inherited expression, and osmotic tolerance of transgenic lines containing HVA1. TheorAppl Genet 105:201-208.
- id="Reference_Titile_Link" value="15">Oraby HF, Ransom CB, Kravchenko AN, Sticklen MB (2005) Barley HVA1 gene confers salt tolerance in R3 transgenic oat. Crop Sci 45: 2218-2227.
- id="Reference_Titile_Link" value="16">Bahieldin A, Mahfouz HT, Eissa HF, Saleh OM,Madkour MA (2005) Field evaluation of transgenic wheat plants stably expressing the HVA1 gene for drought tolerance. PhysiologiaPlantarum 123: 421-427.
- id="Reference_Titile_Link" value="17">Rohila JS, Wu R (2002) Genetic improvement of Basmati rice for salt and drought tolerance by regulated expression of a barley HVA1 cDNA. Plant Sci 163: 525-532.
- id="Reference_Titile_Link" value="18">Babu RC, Zhang J, Blum A, David Ho T, Wu R, et al. (2o12) "HVA1, a LEA Gene from Barley Confers Dehydration Tolerance in Transgenic Rice (Oryza Sativa L.) via Cell Membrane Protection. Plant Science Web. 08 Sept. 2012.
- id="Reference_Titile_Link" value="19">Sivamani E, Bahieldin A, Wraith JM, Al-Niemi TS,Qu RD, et al. (2000) Improved biomass productivity and water use efficiency under water deficit conditions in transgenic wheat constitutively expressing the barley HVA1 gene. Plant Sci155:1-9.
- id="Reference_Titile_Link" value="20">Nguyen Thang,Mariam Sticklen (2013) Barley HVA1 Gene Confers Drought and Salt Tolerance in Transgenic Maize (Zea Mays L.) Adv Crop SciandTechnol 1: 1-8.
- id="Reference_Titile_Link" value="21">Murashige T,SkoogF (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. PhysiologiaPlantarum 15: 473-497.
Figures at a glance
 |
 |
 |
 |
 |
||||
| Figure 1 | Figure 2 | Figure 3 | Figure 4 | Figure 5 |
Relevant Topics
- Agricultural science
- Agronomy
- Climate impact on crops
- Crop Productivity
- Crop Sciences
- Crop Technology
- Field Crops Research
- Hybrid Seed Technology
- Irrigation Technology
- Organic Cover Crops
- Organic Crops
- Pest Management
- Plant Genetics
- Plant Breeding
- Plant Nutrition
- Seed Production
- Seed Science and Technology
- Soil Fertility
- Weed Control
Recommended Journals
Article Tools
Article Usage
- Total views: 13785
- [From(publication date):
specialissue-2015 - Nov 21, 2024] - Breakdown by view type
- HTML page views : 9377
- PDF downloads : 4408
Peer Reviewed Journals
Make the best use of Scientific Research and information from our 700 + peer reviewed, Open Access Journals
