Research Article Open Access
Production of Proteases by Genetically Improved Bacillus subtilis for Enhanced Skin Penetration of Antibacterial Topical Formulation
Faisal Shahzad1, Sohail Ahmad2, Habib Ahmad1, Qazi Abdur Rashid2, Nadia Parveen3, Fatima Javed3, Rida Khan4, Muhammad Zeeshan Ashraf3 and Faiza Naseer3*1Department of Genetics, Hazara University, Mansehra, Pakistan
2Department of Biochemistry, Hazara University, Mansehra, Pakistan
3Department of Pharmacy, Government College, University Faisalabad, Pakistan
4Department of Physiology and Pharmacology, Agriculture University, Faisalabad, Pakistan
- Corresponding Author:
- Faiza Naseer
Department of Pharmacy, Government College
University Faisalabad, Pakistan
E-mail: faiza.naseer@ymail.com
Received date:: June 06, 2015; Accepted date:: July 07, 2015; Published date:: July 14, 2015
Citation: Shahzad F, Ahmad S, Ahmad H, Rashid QA, Parveen N, et al. (2015) Production of Proteases by Genetically Improved Bacillus subtilis for Enhanced Skin Penetration of Antibacterial Topical Formulation. J Biotechnol Biomater 5:186. doi:10.4172/2155-952X.1000186
Copyright: © 2015 Shahzad F, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Biotechnology & Biomaterials
Abstract
The present study was designed for the production, isolation and extraction of protease enzyme from Bacillus subtilis and to investigate the role of protease enzyme mixed with standard antibacterial formulation, i.e. Silver Sulphadiazine 1% w/w cream in burnt abscessed wounds to improve and speed up the healing process. 18 male rabbits weighing between 1.8-2 kg were divided into 3 groups. A burnt abscessed wound model was created on the back of all the rabbits and pathogenic Streptococci introduced into the wound. Then the burnt abscessed areas in 1st, 2nd and 3rd groups were applied daily with cold cream (control), Silver Sulphadiazine 1% w/w cream and Silver Sulphadiazine 1% + Protease w/w cream respectively. The wounds were examined on a daily basis for the healing process; sizes were measured and recorded in the form of photographs on 2nd, 5th, 10th and 14th day. The rabbits were anesthetized and skin tissue samples of each group were collected for histo-pathological examinations. Significant improvement in wound healing with Silver Sulfadiazine 1% w/w and Proteases cream was noticed as compared to Silver Sulphadiazine 1% w/w cream and cold cream (control). It was concluded that application of Silver Sulfadiazine 1% w/w and Proteases was appreciably useful in healing of burnt and abscessed skin wounds in animal model (rabbit) and antibacterial formulation Silver Sulphadiazine mixed protease enzyme can be exploited in pharmaceutical field as an effective promoter of wound healing. The studies can be extended further for formulation stability and clinical trials on human.
Keywords
Bacillus subtilis; Silver sulphadiazine 1% w/w; Protease cream; Topical formulation
Introduction
One of the major problems with all the topical antibacterial formulations is their weak penetration at the wound site and low bioavailability due to which the wound takes a long healing time period or even no response to the antibacterial agent. Since the rate of absorption of topical anti-bacterial preparations is normally towards the slower side as compared to other routes, so in order to overcome the low bioavailability/ poor penetration, improved drug delivery system has been the subject of worldwide pharmaceutical research for many years [1].
Proteases acquire fundamental character of biotechnological interest due to which these have become the most imperative enzymes [2]. Almost all proteases are thermal resistant. They diverge broadly in their particular actions, optimum pH, pH stability range 02-13 and heat sensitivity. Proteases are exclusive kind of enzymes since they occupy a special position with reference to their industrial applications such as laundry, leather preparation, and protein recovery and meat tenderization [3]. The main function of proteolytic enzymes is to catalyze the pierce of peptide bonds proteins. Protease enzymes are therefore recognized as degradative agents that catalyze the total hydrolysis of proteins through cleavage of peptide bonds [4].
Bacillus species usually produce two groups of proteases, alkaline and neutral. These proteases have relatively low stability due to heat sensitivity. However, this property has a certain advantage [5]. For example, during the process of food hydrolysis, low degree of hydrolysis can be achieved through temperature control for the activity of enzymes. To produce low cost protease enzymes for industrial use, to find, isolate and characterize new strains which can grow on low cost media using not expensive Carbon and Nitrogen source is the main focus of recent research [6].
Bacilus subtilis is one of the most widely used bacteria and a major source of amylase and protease enzymes. It is also used in baking, brewing, meant tenderization, peptide synthesis, medical diagnosis, cheese making, used in certain inflammatory diseases, virulent lesions and in un-hiring of sheepskins. It also has wide application in Bioremediation process [3]. Through the course of study, Bacilus subtilis was genetically modified through radiation and then grown in suitable media for optimal production of protease.
The present studies were therefore designed to investigate any possible role of protease enzymes in the topically applied antibacterial formulations in order to enhance the wound healing by increasing the bioavailability of therapeutic agents. These studies were aimed to examine the influence of proteases ofBacillus subtilis ATCC 6633 in burnt, abscessed chronic and surgical induced skin wounds along with antibacterial skin cream.
Materials and Methods
Production of protease enzyme from genetically modified Bacillus subtilis (ATCC 6633) through UV radiation mutagenesis
Bacillus subtilis among protease producing strains was used to observe the changes in the protease gene expression after exposing to UV radiation. UV radiation is one of the well-known mutagen sources. Rasool Bibi Research Foundation, Islamabad, providedBacillus subtilis ATCC 6633 strain. Cultures of Bacillus strains were grown at 37ºC on Luria casein (1%) agar [7]. The pH of the medium was kept at 7.2 by using the sterile 20 percent (w/v) sodium carbonate.
Mutagenesis of Bacillus subtilis was done by using different exposure times and distances from UV source. Induction of mutation was carried out at 0, 15, 20, 25, and 30 minute exposure time to UV radiation and at the different distances between the treated bacterial cultures and UV radiation source. Samples from culture were taken and centrifuged, resuspended in 0.9% saline solution. 5 ml sample was again taken and placed in small glass petri dishes at a distance of 15 cm in dark and irradiated at the above-mentioned time. Germicidal lamp of 15 watts (254 nm) used for the UV radiation. The portion of 0.5 ml of sample was taken and was spread on Luria casein (1%) agar plates. These plates were put in incubator at 37ºC for 24 hrs. Numbers of colonies formed were transferred to the Luria casein milk agar [8].
Standardization of culture requirements of B. subtilis
Culture requirement was optimized for optimum culture time, pH, temperature and substrate concentration were established to get maximum yield. The purified protease activity was tested for the incubation results by observing clear zone in various incubation periods, i.e., 6, 12, 24, 36, 48, 60, 72 and 84 hrs. The diameter of clear zones were measured and calculated for each incubation periods. The purified protease activity was tested for the incubation results by using the same technique at various incubation temperatures, i.e., 25, 30, 35, 40, 45, 50, 55 and 60ºC. The diameter of clear zones were measured and calculated for each incubation period.
The effect of pH on alkaline protease production fromBacillus subtilis under study was carried out using different pH like 4.5-8.5. The optimization, media with the above pH was inoculated with the test sample and the protease assay was done after 24 hrs. The best pH was concluded at 7.2 to 8.0. The purified protease activity was measured by arranging gelatin agar plates with various gelatin concentrations, i.e.. 0.05, 0.1, 0.5, 2.0, 3.0, 4.0 and 5.0% (w/v). The purified enzyme was applied; incubated and clear zones were calculated. The mean diameter of each concentration was measured.
Protease activity of Bacillus subtilis
Enzyme detection was done using Luria casein agar added with 1% skim milk [9]. Using simple plate techniques performed screening test. With this technique proteolytic activity was detected on the basis of appearance of clear zones around the bacterial colonies. Luria casein agar (1%) plates were used for this purpose. Composition of the medium:
Peptone…………… 10.0 g
Yeast extract……… 5.0 g
NaCl………………. 10.0 g
Casein……………..10.0 g
Agar………………. 18.0 g
Distilled water……. 1000 ml
pH…………………. 7.2
Casein was autoclaved separately and after cooling was mixed aseptically in the medium before pouring of medium in plates. These plates were inoculated with bacterial cultures using sterile needles. Individual colonies were spotted visibly at equidistant a parting form each other to check the development of milk-casein digestion zone around each colony. These zones indicate the capability if any of Bacillus subtilis to produce proteases which digest the milk-casein and produce a clear zone of digestion of substrate. Diameter of each zone was measured to evaluate the qualitative capacity of each colony. After incubation for 24 hours at 37°C, clear zones were formed around bacterial colonies. Plates were then flooded with 10% glacial acetic acid that made clear zones around colonies more prominent. Diameter of each zone was measured and recorded.
Quantitative test for production of proteases
Bacillus subtilis ATCC 6633 strain was grown on nutrient broth medium and incubated for 48 hours at 40º C having pH 7.2. The cell free supernatant was obtained by centrifugation at 9000 RPM, for 20 minutes, and then the filtration of the obtained supernatant which is already a crude enzyme was performed. This crude enzyme was subjected to different purification methods. Composition of Medium;
Gelatin……………..…15.0 g
Casein Hydrolysate…..0.5 g
Glycerol………………. 3.0 ml
Distilled water………… 250 ml
pH……………………..8.0
In two 1000 ml Erlenmeyer flasks 250 ml medium was poured and the pH was adjusted using 0.1 N NaOH and 0.1 N CH3COOH. The medium was autoclaved at 121ºC, 15-pound pressure for 20 minutes. Three ml of glycerol solution sterilized in a dry oven at 100ºC for half an hour was poured into the medium aseptically. Then 1x106 of Bacillus subtilis were inoculated in these flasks. Flasks were kept on shaking incubator at 37ºC with 150 RPM. Samples were collected after 24, 48, 72 and 96 hours for quantitative analysis.
Extraction of protease enzyme produced
a) Separation of enzyme: All culture broth of 24, 48, 72 and 96 hours were centrifuged at 10,000 RPM for 30 min at 4ºC temperature. Bacterial cells of Bacillus subtilis and debris were removed by filtration and supernatant was used as the crude enzyme source for further purification enzyme.
b) Ammonium sulfate precipitation: The crude enzyme was precipitated by steady adding of solid ammonium sulfate with nonstop stirring and allowed to settle. The precipitate was put in centrifugation for 20 min at 9000 RPM. Then protein-ammonium sulfate pellet was dissolved in 10 ml of 0.2 M phosphate buffer (pH-7). Enzyme activity was determined for each separate fraction. A partially purified enzyme preparation was obtained by this method and column chromatography was used for purification.
c) Chromatography: The partially purified enzyme preparations were applied into a column packed with sephadex G-200 (mesh 200) and equilibrated with 0.2 M phosphate buffer pH 7, then eluted with the same buffer, preparation of the gel column and the fractionation procedure was carried out.
7 grams of sephadex G-200 were suspended in 200 ml of 0.2 M phosphate buffer pH-7 and allowed to swell for 24 hours at room temperature. A Pharmacia column (2.5 X 50 cm) was packed by adding cautiously, a previously prepared degaussed thin slurry gel in the buffer solution into a vertical column fractional filled with the same buffer. The gel was continuously added until a bed height of 40 cm in the column was reached. The buffer flow rate was preserved at a rate of approximately 5ml/hour. 1 ml of the crude extract was applied carefully to the top of the gel and passed into it by running the column. Then buffer was put cautiously without causing disturbance in the gel surface. 10 fractions, each of 5 ml, were prepared. Both enzyme activity and protein content were concluded for each separated fraction and curve was plotted.
Assay for proteases
Reagents: 0.02 M Tris (Hydroxymethyl) amino methane, pH 8.5, 5% TCA solution, 1% casein solution in Tris buffer (it was stored in 4ºC).
1 ml of casein solution was added into 1 ml crude enzyme. The mixture was put in the incubator at 45ºC for 1 hour. 3 ml of TCA was added after incubation and the tubes were placed on ice for 15 min. The precipitates were removed by centrifugation at 5000 RPM for 15 min at 4ºC. TCA was added in the blanks before incubation. The optical density of the supernatant was measured at 280 nm. Graph of standard curve was prepared using tyrosine as standard. Solutions of tyrosine 2, 4, 6, 8, 10, 12, 14, 16, 18, 20, 22 mg/ml were prepared to draw a standard curve at 280 nm through a UV spectrophotometer.
Unit of enzyme: Single unit of enzyme’s caseinolytic activity is defined as that quantity of enzyme, which releases 1 μ mole tyrosine under standard conditions of assay method.
Augmentation of proteases enzyme in topical antibacterial formulation
a) Topical applicant formulation: In Rabbit model, the effect of proteases enzyme augmentation in the skin topical application was studied beside control and plain preparations. The formulations for trial were developed by the addition of specified enzyme concentrations into the plain skin cream mentioned below to equalize both preparations.
b) Animal treatment model: Premature rabbits of 1.8-2.0 kg (local) were obtained from private farm and acclimatized for a week in stainless steel separate cages before the experiment started. They were fed with commercial diets, vegetables, crushed wheat and corn during the experimental process.
c) Experimental plan: The rabbits were divided into 3 groups (6 animals each), one control and 2 experimental groups.
• Group (T1): Cold cream (control) (12.5% Botatarma + 12% white wax + 56% liquid paraffin + 0.5% borate of soda + distilled water) used as topical applicant.
• Group (T2): Rabbits with septic burnt wounds treated with Silver Sulphadiazine 1% w/w (10 mg/g).
• Group (T3): Rabbits with burnt septic wound treated with Silver Sulphadiazine skin cream augmented with proteases enzymes having Silver Sulphadiazine 1 % and 3 Kunitz units/ gram of proteases (=3 μ mole of Tyrosine).
d) Wound creation and sepsis: Marked areas on the shaved skin were treated with 1 ml per spot of concentrated HCl to induce chemically burnt wound. After 24 hrs burnt wounds of each group of rabbits were impregnated with 1 ml of fresh culture broth of Streptococcus aureus having 1x106 CFU per locus
e) Treatment Trials: The skins of rabbits were sheared and left for 24 hours. Then 3 drops of concentrated HCl (80%) were applied topically on the shaved skin with cautions. The burned skin rabbits were kept separately under sterile conditions in an isolated room cleaned with a sterile solution. The burnt areas in the 1st, 2nd and 3rd groups were covered with (protease + Silver Sulphadizine 1% w/w), Silver Sulphadizine 1% w/w skin cream and cold cream (control) respectively. These applications were repeated every day. 2nd, 5th, 10th and 14th days later the animals were anesthetized and the burnt skin tissue samples were collected from rabbits for histopathological studies (Table 1).
| T1 (Treatment group ) |
T2 (Treatment group 2) |
T3 (Treatment group 3) |
|---|---|---|
| Control | Silver Sulphadiazine 1% w/w | Silver Sulphadiazine 1% w/w + Proteases 3 iu/ gram |
| Burnt wound + sepsis | Burnt wound + sepsis | Burnt wound + sepsis |
| Sampling Day | Sampling Day | Sampling Day |
| 0 day | 0 day | 0 day |
| 5th day | 5th day | 5th day |
| 10th day | 10th day | 10th day |
| 14th day | 14th day | 14th day |
| 28th day | 28th day | 28th day |
Table 1: Treatment model.
f) Histological processing and microscopic analysis: The lesion biopsies were fixed at 10% neutral formalin for not less than a week. The sections were building up on the precoated slides before passing through the hematoxylin, including alcohol dehydration and xylene clearing. The processed sections were protected by cover sips and studied with a light microscope for histopathological analysis. The images were photographed at using an Olympus camera mounted microscope. Magnification of each photograph was calculated by the standard procedure, i.e., = Power of eyepiece x objective = Magnification X
g) Data analysis: The width of granulation tissue was observed and calculated at the center of each lesion. Statistically, all data was articulated in millimeter as mean ± standard error of the mean. The differences between 2nd and 14th days were compared.
Results
Morphology and bacterial characters of Bacillus subtilis: Bacillus subtilis, one of many bacteria found naturally inside the human body, rod-shaped, a flagellum, that are grown in the mesophilic temperature range, i.e. 25-35ºC (Figure 1). It has evolved a set of strategies that allow survival under harsh conditions and from stress-resistant endospores. An agar colony of B. subtilis is traditionally circular, with ragged edges, cream or white colored. The bacteria spread out from the center and keeping the ragged circular shape of the colony. On gram staining the bacteria are noticed as gram-positive rod shaped.
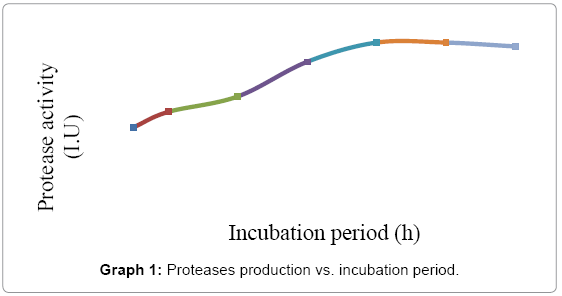
Standardization of culture requirements of B. subtilis: Culture requirement was optimized for optimum culture time, pH, temperature and substrate concentration were established to get maximum yield. Effect of incubation period in the purified protease activity was tested on Luria casein medium with 1% skim milk technique under different incubation periods, i.e., 6, 12, 24, 36, 48, 60, 72 and 84 hrs. The maximum yield of proteases was obtained at 48 hrs, which remains static up to 54 hrs, and starts declining on 60 hrs. However, the proteases production was above 45 IU at 48 hrs. which declined to 40 IU at 72 hrs. as shown in Graph 1. The data indicated harvesting during 36 hrs. to 96 hrs. was the best time for harvesting of protease enzyme to get maximum yield. The clear zones were measured and the mean diameter for each incubation period was calculated. The diameter of different proteolytic areas was from 8 mm to 20 mm.
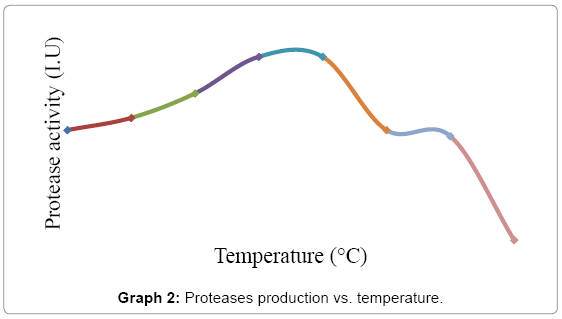
Effect of temperature on the purified protease activity was tested by using Luria casein agar with 1% skim milk technique at different incubation temperatures, i.e., 25, 30, 35, 40, 45, 50, 55 and 60ºC. The clear zones were measured and the mean diameter for each zone was calculated. The best results of max enzyme yield were obtained at 37ºC to 40ºC as shown in Graph 2.
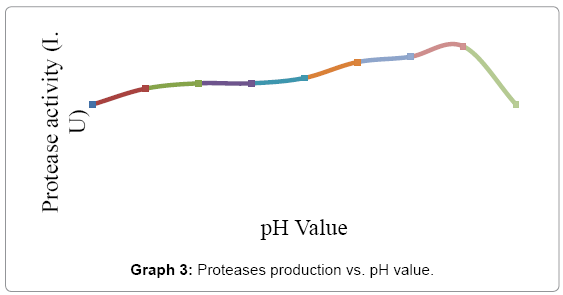
The effect of pH on alkaline protease production from Bacillus subtilis under study was carried out using different pH like 4.5 - 8.5. The optimization, media with the above pH was inoculated with the test sample and the protease assay was done after 24hrs. The best pH concluded was 7.2 to 8.0 as shown in Graph 3.
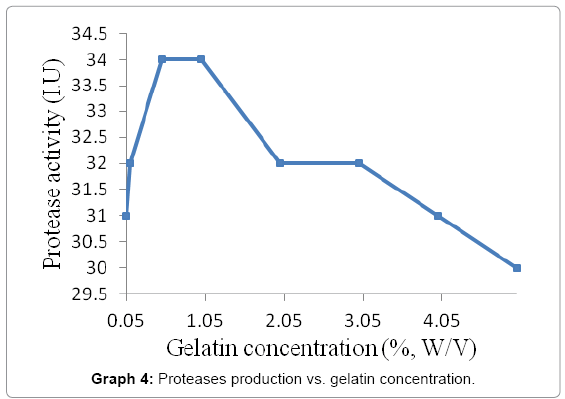
The purified protease activity was tested by preparing gelatin agar plates with different gelatin concentrations i.e. 0.05, 0.1, 0.5, 2.0, 3.0, 4.0 and 5.0 percent (w/v). The purified enzyme was applied; incubated and clear zones were measured. The mean diameter of each concentration was calculated and best results were found using 1.0 to 1.5 concentrations of gelatin (Graph 4).
Purified protease activity of Bacillus subtilis: Enzyme detection was done using Luria casein agar added with 1% skim milk. Using simple plate techniques performed screening test. With this technique proteolytic activity was detected on the basis of appearance of clear zones around the bacterial colonies. Luria casein agar (1%) plates were used for this purpose.
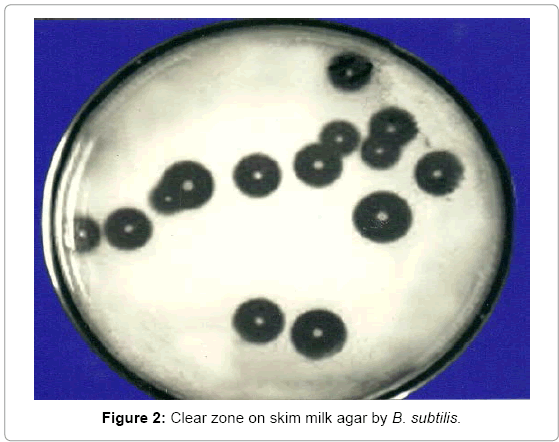
Casein was autoclaved separately and after cooling was mixed aseptically in the medium before pouring of medium in plates. These plates were inoculated with bacterial cultures using sterile needles. Individual colonies were spotted visibly at equidistant a parting form each other to check the development of milk-casein digestion zone around each colony shown in Figure 2. These zones indicated the capability if any of Bacillus subtilis to produce proteases which digest the milk-casein and produce a clear zone of digestion of substrate. Diameter of each zone was measured to evaluate the qualitative capacity of each colony.
After incubation for 24 hours at 37ºC, clear zones were formed around bacterial colonies. Plates were then flooded with 10% glacial acetic acid that made clear zones around colonies more prominent. The diameter of each zone was measured and recorded.
To study the comparative therapeutic wound healing effects of new formulation having protease enzyme with the non-protease conventional formulation in the burnt and the abscessed wound on rabbit model:
Experimentation for wound healing: During the experimental period, no animal death was observed. Wounds treated with Silver Sulphadizine 1% w/w displayed a higher degree of inflammation by three clinical signs of the inflammatory process include heat, redness and swelling. These symptoms were displayed to be lessened in wounds treated with (Silver Sulphadizine 1% w/w + protease) skin cream.
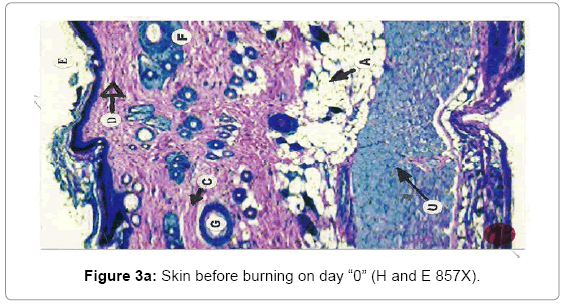
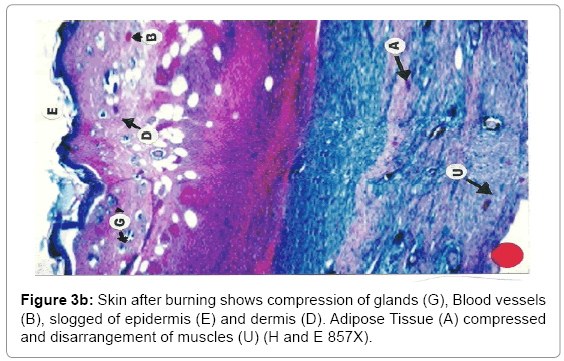
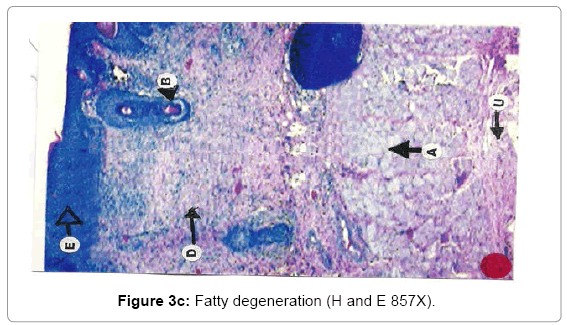
Histological analysis: A true burn depth was observed by histopathological studies; the state of viable adnexal structures (hair follicles) provided helpful information on the level of burn depth. Apart from the necrosis observed in the surrounding tissues, it could be inferred that thermal injury had reached at least depth if such structure appeared damaged. On 5th day after the introduction of burn injury, histological samples taken from both the Silver Sulphadiazine 1% w/w and protease and Silver Sulphadiazine 1% w/w treated groups showed that the burn depth had reached the deep dermis (D) layer that was equivalent to deep second to third degree burn injury (Figure 3a, b and c). The skin epidermis of both groups was completely smashed, leaving behind a few hair follicles (F) with the hair shafts gone. The deep dermal structures and the fibro adipose tissues (M) were workable, demonstrating at least deep second-degree burns.
Due to burning, the epithelial layer sloughed off in almost all cases, whereas the deeper layer of skin was affected seriously. The epidermis and dermis layer of tissue facing degenerative changes with the progression of time. The degenerative changes exposed encompassing of hydropic degeneration and fatty degeneration. These changes extended to the dermis layer and muscularis mucosa. The inflammatory changes were in abundance as the dermis and muscular layers of skin shown leukocyte infiltration, edema and proliferation of inflammatory cells. Due to heat the adipose layer exposed to liquefactive and degenerative changes. Fatty degeneration was maximum in adipose tissues. Collapsing of the architectural details of the adipose tissue layer was a constant feature of burning.
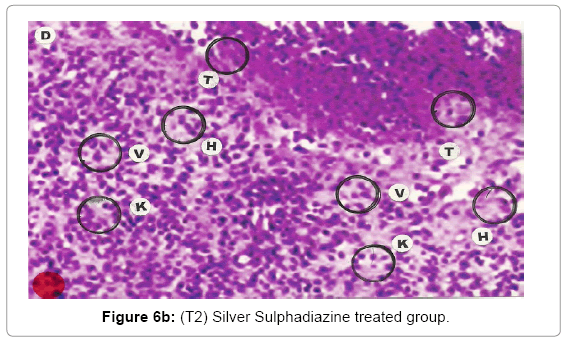
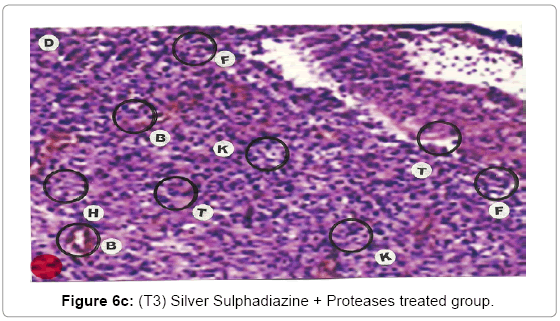
With the passage of time wound showed necrosis areas which later on replaced with the connective tissue, debris and necrosis nuclei of the cells. In early stages, degeneration showed vacuolation of the cytoplasm of the cells and presence of hydropic and fatty degeneration. Consequently, the wound showed degenerative changes followed by inflammation and necrosis of the tissues. The necrosed tissues infiltrated with PMN’s and later the gap is filled with the fibroblast and connective tissue. The mature wound gets contracted and depression was found in form of dent. The healing indicates the replacement of epidermis cells with irregular proliferation of the connective tissues. Presence of fibroblasts indicated the initiation of the healing process. Presence of fibroblasts in abundance was more in the treatment group (T3) where wounds were treated with SSD+ Proteases, earlier in comparison with SSD treated group (T2) and control group (T1).
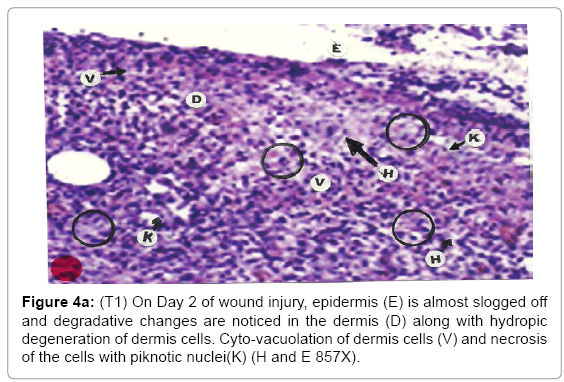
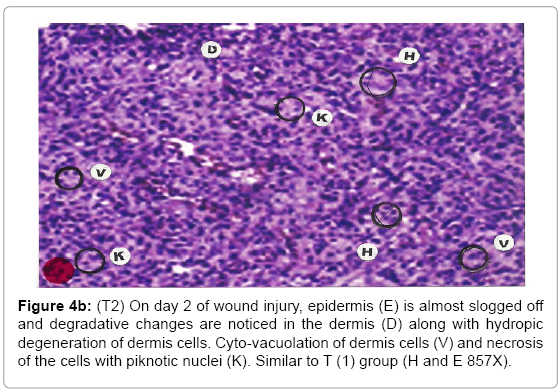
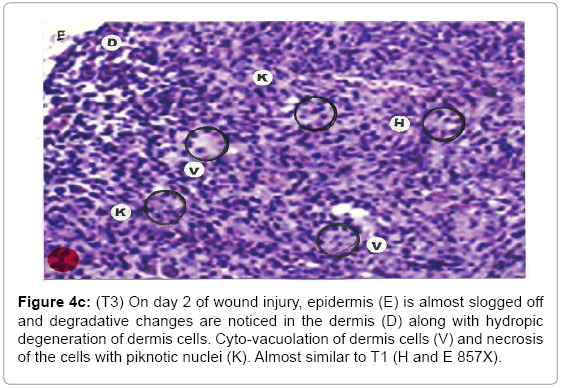
Comparative histopathological changes on day- 2: The skin histogram on day-2 of wound injuries showed histological findings of control group (T1) showed in histogram, almost identical picture on day 2nd. The epidermis was almost sloughed off and dermis showing degenerative changes. Hydropic degeneration of dermis cells along with cyto-vacuolation of cellular cytoplasm of dermis cells was common in control group (T1), SSD treated group (T2) and SSD+ proteases treated group (T3). Cells exposed to necrosis with piknotic nuclei. The histological representation is at Figure 4a (T1), b (T2) and c (T3).
Figure 4b: (T2) On day 2 of wound injury, epidermis (E) is almost slogged off and degradative changes are noticed in the dermis (D) along with hydropic degeneration of dermis cells. Cyto-vacuolation of dermis cells (V) and necrosis of the cells with piknotic nuclei (K). Similar to T (1) group (H and E 857X).
Figure 4c: (T3) On day 2 of wound injury, epidermis (E) is almost slogged off and degradative changes are noticed in the dermis (D) along with hydropic degeneration of dermis cells. Cyto-vacuolation of dermis cells (V) and necrosis of the cells with piknotic nuclei (K). Almost similar to T1 (H and E 857X).
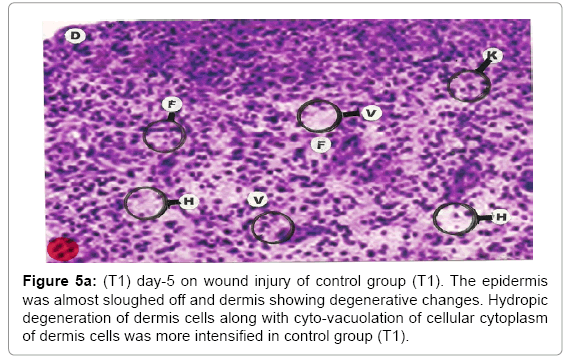
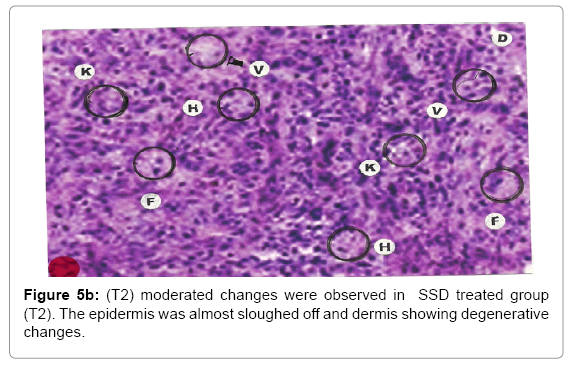
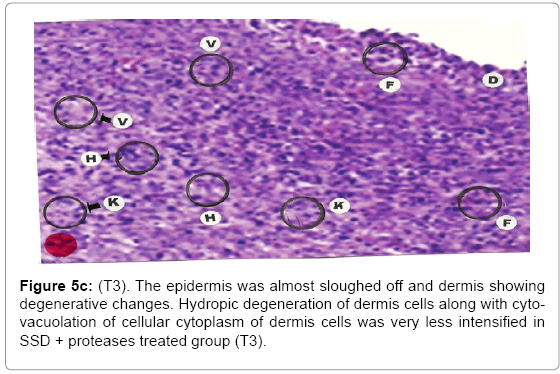
Comparative histopathological changes on day- 5: The skin histogram on day-5 on wound injury showed histological findings of control group (T1) showed in histogram, almost identical picture on day 2nd. The epidermis was almost sloughed off and dermis showing degenerative changes. Hydropic degeneration of dermis cells along with cyto-vacuolation of cellular cytoplasm of dermis cells was more intensified in control group (T1) whereas moderated changes were observed in SSD treated group (T2) and it was lessen in SSD+ proteases treated group (T3). Granulation of the dermis was slightly initiated in control group (T1). Whereas granulation of dermis on day 5th was extensive in treatment group (T2) and it was on higher side in treatment group (T3). Histological photographs are at Figure 5a (T1), b (T2) and c (T3).
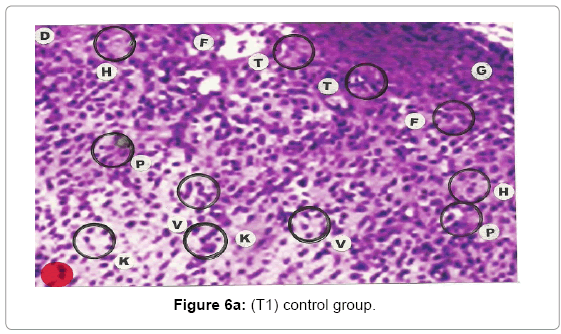
Comparative histopathological changes on day- 10: Granulation of the dermis was slightly initiated in the control group (T1). Whereas granulation of dermis on day 10th was extensive in treatment group (T2) and it was on higher side in treatment group (T3). On day 10 in control group (T1) dermis tissue showed extensive necrotic changes and fatty degeneration. However, fibroblast and connective tissue proliferation slightly initiated. In SSD treated group (T2) medium frequency of fibroblast proliferation was observed and moderate granulation of the dermis was observed. Looking into the treatment effect of SSD+ Proteases a marvelous improvement has been seen in the dermis tissue where blood vessels were starting to appear. High frequency of fibroblasts and collective tissue proliferation was observed. These changes indicated that the treatment effect is more pronounced in SSD+ Proteases treated group in comparison with control group and non-enzyme topical formulation treated group (T2). On day 10 the control group wound reached a stage compared with the day 7 position of SSD treated group and day 5 of SSD+ Proteases treated group. In treated groups on day 10 skin tissues showed heavy granulation and proliferation of connective tissue and fibroblasts. Necrotic and degenerative changes were still present in control group (T1) and to some extent in SSD treated group (T2) while it was marginal in SSD+ Proteases treated group. A remarkable sign of healing appeared in SSD+ Proteases treated group where tissue started showing organization of dermal layer. Connective tissue and fibroblasts were many times higher in (T3) treated group. The histological representations are given at Figure 6a (T1), b (T2) and c (T3).
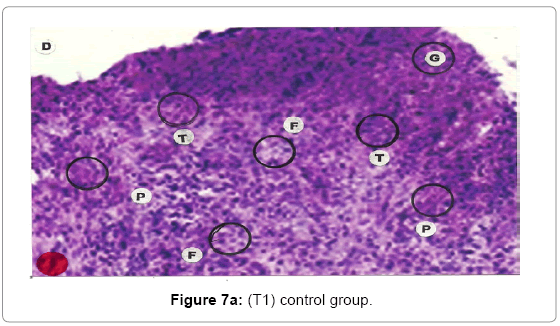
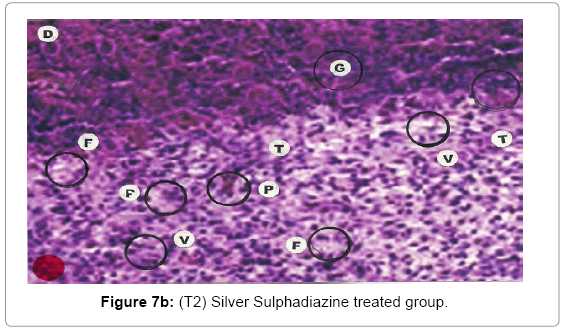
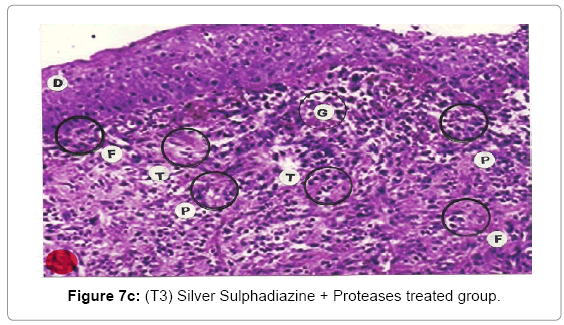
Comparative histopathological changes on day- 14 to 28: The skin histogram on day - 14 to 28th on wound injury showed histological findings of control group (T1) showed in histogram, almost identical picture on day 14th to 28th. The healing process was completed on day 14 in SSD+ Proteases treated group where complete replacement of dead and necrosis cells with fibroblasts and connective tissue followed by SSD treated group on day 21 and in control group on day 28. The healing process of the wound was perfect in group (T3) followed by group (T2) and on day 28 in control group (T1) wound was still in immature. These changes indicated a high response of treatment with the enzyme augmentation. Histological figures are at 7a (T1), b (T2) and c (T3) for the day 14.
Histopathology of Silver Sulphadiazine 1% w/w treated wound, severe hyperemia and granulation tissues, close to the dermis were present because blood vessel dilatation, infiltration of white blood cells and proliferation of fibroblast cells were seen. Since the microcirculation was still in a dilated and congested state, the infiltrated inflammatory cells were dispersed around the blood vessels. Hyalinization, a glass-like appearance could be observed, when the skin was thermally smashed, the fibers lost their linearity and fused with their neighbors forming dense aggregate degeneration of collagens. The appearance of hyalinization also suggested that thermal damage took place in that particular depth. Histopathological evaluations, on the 14th day showed that burn healing was better in (Silver Sulphadizine 1% w/w+ protease group than the other groups. An injury (formed from necrotic tissue remnants and mononuclear cell infiltration) was observed in (Silver Sulphadizine 1% w/w+ protease) group. Silver sulphadiazine 1% w/w+ protease treated wounds illustrated the complete healing Microscopic examination revealed complete regeneration of the epidermis (E) and dermis (D) with few injuries present in Silver Sulphadiazine 1% w/w+ protease groups. The skin healing occupied the epithelization with epithelium proliferation at the ends of scars and formed the granulation tissue. Furthermore, fibrous scar was seen evidently with some condensed nuclei of inactive fibroblasts and capillaries persisted, accounting for the red appearance of recent scars. In addition, there were no skin appendages in the lesions. A region with lots of vacuoles (V) remaining was noticed in the Silver Sulphadiazine 1% w/w+ protease wound that was left behind from previous inflammatory response. Concerning the current experiment, it was important to point out that there was a histological difference between each group. In conclusion, application of Silver Sulphadiazine 1% w/w + Proteases cream is significantly effective in healing of burned skin wounds in rabbit model. The mean values of thickness of granulation tissue in the center of the wounds for (Silver Sulphadizine 1% w/w+ protease) cream, Silver Sulphadiazine 1% w/w and control are shown in Table 2.
| Treatment Group | Percent of wound contraction (mean ± SE) | Period of epithelization in days | |||
| 2nd day | 5th day | 10th day | 14th day | ||
| Control (T1) | 15.1±3.1 | 19.1±3.3 | 27.6±4.3 | 34.9±2.4 | 28 ±1.5 |
| Silver Sulphadiazine 1% w/w (T2) | 10.2±4.5 | 29.8±3.2 | 63.2±5.9 | 75.5±4.4 | 20.0±1.5 |
| Silver Sulphadiazine 1% w/w+ protease (T3) | 10.1±4.2 | 43.1±12.6 | 82.1±7.2 | 92.1±4.1 | 14 ± 2.0 |
Table 2: The mean values of thickness of granulation tissue in the center of the wounds for Silver Sulphadizine 1% w/w+ protease cream, Silver Sulphadiazine 1% w/w and control group.
Discussion
Production of protease enzyme from Bacillus subtilis
The number of enzymes secreted by Bacillus subtilis includes amylase, several proteases, levansucrase, RNAse and alkaline phosphatase. In the present investigation, protease was produced under the optimum conditions and the factors affecting the activity of the protease were studied. Total protein content in the supernatant at different fractions represented in Figure 3, Data presented here has shown that Bacillus subtilis ATCC 6633 produces an extracellular protease. Results indicated that the optimum incubation period for protease production was 48 h.
The optimum temperature for protease productivity by Bacillus subtilis ATCC 6633 was 40ºC and various studies were in the range of 02-70ºC or more. Because they were designed for different type of organisms, the type and conditions of the medium and the type of enzyme. Secades with his co-authors observed the same results that the most favorable temperature for an extracellular protease produced by Flavobacterium psychrophilum was at temperatures ranging from 25 and 40ºC [10]. Further, the most advantageous temperature for the production of protease was between 30 and 45ºC [11] Jobin and Grenier in 2003 investigated the protease production by Streptococcus suis serotype 2 and recorded that the best possible temperature for production of protease ranged from 25 to 42ºC [12].
Optimum culture conditions were 37ºC pH 8 and 48 hrs incubation period [13]. This medium was introduced to produce protease for protease purification where the protein pellet obtained after 60% saturation when ammonium sulphate was dissolved in 2 M phosphate buffer, pH 7 and loaded onto column of Sephadex G-200 (2.5 50 cm). It was concluded that fraction 3 and 4 exhibited the maximum protease activity. However, it doesn't characterize the maximum protein content.
The factors affecting enzyme productivity including incubation period, temperature, pH, and substrate were executed. The maximum protease activity of the protease produced by Bacillus subtilis was observed after 48 hours of incubation time. These results were in correlation with the information saved for protease activity produced by Bacillus licheniformis LBBL-11 and Bacillus firmus 7728 and exhibited their best activity after 48 hours of incubation [14]. The results displayed in Fig.3 have shown the effect of various incubation periods of protease activity. The optimum incubation period for the maximum protease activity was 31 I.U at 48-72 hrs.
The paramount pH for production of protease was pH-8, concluded by the results. Usharani and Muthuraj in 2010 reported that the optimum pH of protease activity produced by Bacillus laterosporus was 7.0 [15]. The optimal pH of proteases from Bacillus subtilis strain 38 was found to be at 6.5 [16].
The results displayed in fig. 6 have shown that the effect of various pH on protease activity. Maximum protease activity was observed in a pH range between 7.5-8.0. B. subtilis displayed its maximum protease productivity and protein content at a pH range 7.5-8.0. This was matched with the results of previous researchers. Massucco et al., 1980 had been reported that maximum protease activity by Bacillus lincheniformis was in pH 7.5, Do et al, 2004 said at 6.9 by Bacillus sp.SMIA-2 and Wang, 2006 said at pH 7 by Bacillus sp.TKU004 [17].
The optimum gelatin concentration that shown maximum protease activity was 0.5-01% (w/v). This result was approximately matched with the similar results who depicted that maximum protease activity and productivity by Bacillus sp. were shown with gelatin concentration between 1.5-2% (w/v) for Bacillus anthracis s-44 and Bacillus sp. K30.
So, the present finding has shown that Bacillus subtilis ATCC 6633 under study is a good producer of extra cellular protease. This enzyme can be used to various industrial and medical fields and it can be produced in large scale from the microorganism, Bacillus subtilis by applying the fermentation technology.
Application of protease enzyme from Bacillus subtilis
Experimentation for wound healing: In recent times, pharmaceutical research has been particularly focused on application of enzyme technologies. The scope of this review is limited and the prime focus of using enzymes for the increase in the efficacy of topical formulations to promote healing. In the past, a great number of proteolytic enzymes of bacterial and plant origin have been investigated for a replacement for the mechanical debridement (removal of dead skin) of burns. Regrettably, the results have been erratic, perhaps due to the questionable quality of the enzymes which were used. Until recently, the overall mortality rate for burns has been 40%, even in specialized treatment centers and in the more extensive cases, it has been prohibitively high.
In burns of 50% or more of the body surface, infection can be overwhelming and most deaths in the past have been caused by septicemia. Infection also increases the morbidity in burns by augmenting burn anemia and malnutrition and accelerating the catabolic loss of body weight. It causes the conversion of second degree burns to full thickness lesions, thus increasing the severity of the injury and delaying epithelial healing.
Since the beginning of recorded medical history, various compounds and applications have been applied to the burn wound. In recent years, there has become available an even increasing list of topical antibacterial agents for the treatment of burns, as well as a host of differences of opinion regarding the advantage and disadvantages of each. Silver Sulphadiazine 1% w/w, as a topical agent, was first introduced by Fox in 1967 and has been in use by us for the treatment of burns since 1971.
Dead tissue covering the burn wound also serves or a medium for bacterial growth reduces the host’s resistance to infection and delays the formation of granulation tissue and the re-epithelialization. Therefore, wound debridement is an imperative to the healing of burns. Surgical tangential excision of dead tissue, the repeated application of moistened dressings, hydrocolloid or semi occlusive dressings, dextranomers, intracavity gets, hydro surgery, or various enzyme preparations can be used burn debridement [18]. While effective, surgical tangential excision has several major disadvantages. This method is non-selective and it is technically difficult to control the amount of tissue to be removed, often converting a partial thickness burn into a full thickness defect.
The syndrome of 'burn wound sepsis' was first established by Brentano in 1966. He showed that it was due to the rapid proliferation of bacteria in the burn wound with an active invasion of the adjacent unburned tissues by these microorganisms in numbers exceeding 100,000/gram of tissue. He demonstrated that it was the gram negative bacilli, predominantly Pseudomonas aeruginosa, which were the primary organisms responsible. These spreads initially by way of the lymphatics, but later invade the vascular network as septicemia occurred.
The initial infecting organisms were predominantly staphylococcus and streptococcus gram negative septicemia and occurred between the 2nd and 5th week after injury, with a mean post burn day of death of 19 days. This coincides with the highest incidence of Pseudomonas aeruginosa cultured from the burn wounds. This change in the bacteriological flora of the burn wound, from an initial infection by gram positive cocci to gram negative infection during the course of therapy, has been observed by other authors. Pseudomonas is present for more than a brief time in a wound; it often becomes the dominant organism, either by directly suppressing other species or by surviving their elimination due to a greater resistance to antimicrobial agents.
While topical antibacterial agents provide only one aspect of treatment for burn wound sepsis and should be considered in relation to all other adjunctive therapeutic procedures, they still from the mainstay of the treatment program in most burn centers. All the topical agents who have been in use in recent years have been effective to some degree in controlling burn wound sepsis. They do not completely eliminate bacteria from the burn wound, but when properly used, prevents invasive infection. Their main difference revolves around 3 basic factors, namely, the relative efficacy in controlling or suppressing infections; the presence or absence of side-effects during their use and the effect of the agent on the regeneration of the epidermis.
The efficiency of Silver Sulphadiazine 1% w/w has been confirmed by the combined experiences of many burns therapists in other countries. In addition to the advantages mentioned, the other factors contributing to the acceptability of this agent include-
1. Its potent bactericidal activity against a wide range of organisms such as B. proteus, Klebsiella, Staphylococci, Candida and Pseudomonas. The minimum inhibitory concentration against these organisms is 35-100 times less than that of sulfamylon acetate or the sulfonamides. Unlike the sulfonamides, it is not inhibited by para amino benzoic acid. The levels of the drug in the wound exudate 24 hour after application ranged from 40 to 95 mg % and represented a satisfactory reservoir of antibacterial activity even at that dilution.
2. Only 10% or less of the sulfonamide moiety is absorbed and absorption is less during the immediate post burn period. The levels of sulfonamide in the blood and urine in 357 samples investigated by Fox were consistently low. No instance of renal toxicity has been described, nor would any be expected at these low concentrations.
3. The silver ions from the material is not absorbed from the burn wound, in contrast to the detection of 5-30 ug % in the plasma of patients after silver nitrate soaks. This may account for the potent inhibition of bacterial growth achieved by Silver Sulphadiazine 1% w/w.
4. The drug does not inhibit the action of proteolytic enzymes or collagenase. These are normally responsible for autolyzing necrotic tissues. Hence, separation of the eschar ins not delayed.
5. Unlike silver nitrate and sulfamylon acetate, which resulted in inhibition of epithelial regeneration, no such effect was observed with Silver Sulphadiazine 1% w/w.
Silver Sulphadiazine 1% w/w, introduced by Fox about 7 years ago, has been responsible for an increase in the efficiency of topical burn therapy. The low incidence of positive wound cultures, reduced mortality rate from sepsis, in addition to its convenience of use as well as an almost total absence of side effects, has made it widely accepted as a topical agent for the control of burn wound sepsis.
The present study showed that early nonsurgical removal of injured tissue is an effective treatment for thermal burns. Histopathological comparison of the 3 groups indicated that healing of the burned skin wound was best in the Silver Sulphadiazine 1% w/w + protease group.
Pickens, (2000) reported topical ointment therapy may enhance epidermal barrier function by protecting the stratum corneum, leading to improved skin integrity and less risk of nosocomial infection [1]. Application of an emollient ointment may serve as a protective barrier that enhances skin integrity and epidermal maturation.
Enzymatic Debridement of wounds enjoyed a brief popularity when introduced a decade ago. These early agents – fibrinolysin and desoxyribonuclease (Elase) – reportedly affected necrotic tissue by fibrinolytic action, but the clinical results left much to be desired.
Debridement of wound is preparation for healing. Surgical or enzymatic debridement can be elected, depending on the patient's individual circumstance. Ease of bedside or outpatient application makes the enzymes an attractive alternative to the more complicated and expensive formal surgical operation. Further, both approaches may be used on the same patient, singly or simultaneously [19].
Enzymatic debridement with sultilains ointment is widely used in the treatment for pressure sores and leg ulcers. Best results are obtained when the agent is in a moist environment. A thin layer of ointment is applied directly to the necrotic wound surface, and then covered with moistened gauze. An antibacterial solution may be used for moistening, excluding compounds that deactivate the enzymes (such as the iodophors). The entire dressing system is replaced every six to eight hours, removing the loose debris with each change. Proteolytic activity should be noted within 24 to 48 hours. If no appreciable beneficial changes occur in this period, the enzymes may be considered ineffective and an alternative plan instituted [20].
Liu and Hansen noted that, in the course of isolating subtilin from Bacillus subtilis ATCC 6633, two peptides with potent antibacterial activity were observed. The previous authors proposed feasibility of an alternative mode of delivery and application of proteolytic enzymes for wound debridement. Continuous controlled streaming of protease solutions onto a targeted treated area, providing optimal and controlled environmental conditions for full exploitation of the protease’s potential debridement activity. The efficacy of the streaming approach was successfully demonstrated in removal of coagulated blood and debridement of experimental burn wounds in small lab animals by a series of proteases [21].
In the present study, comparison of the three groups indicated that healing of burned skin wounds was best in (Silver Sulphadiazine 1% w/w + protease enzyme) group. Therefore, protease enzyme produced by Bacillus subtilis plays a role in healing of burned skin wounds possible via its enzymatic debridement effect [22]. The present studies are therefore designed to investigate any possible role of protease enzymes in the topically applied antibacterial formulations in order to enhance the wound healing by increasing the bioavailability of therapeutic agents. The studies are aimed to examine the influence of proteases of Bacillus subtilis ATCC 6633 in burnt, abscessed chronic and surgical induced skin wounds.
Conclusion and Recommendations
It is concluded that protease enzyme produced by Bacillus subtilis can be provided as an agent that acts an effective promoter of wound healing through debridement. The studies can be extended for further research for compatibility and stability of topical skin formulation with proteases. The formulation can also be investigated for different strengths for its efficacy at the optimum enzyme composition. Further studies involving clinical trials on humans at a large scale in suitable hospital fulfilling criteria for clinical trials may prove the better efficacy of innovative topical pharmaceutical preparations with blend of protease enzymes.
References
- Pickens WL, Warner RR, Boissy YL, Boissy RE, Hoath SB (2000) Characterization of vernixcaseosa: water content, morphology, and elemental analysis. J Invest Dermatol 115: 875-881.
- Barindra S,DebashishG, Malay S,Joydeep M (2006) Purification and characterization of a salt, solvent, detergent and bleach tolerant protease from a new gamma Proteobacterium isolated from the marine environment of the Sundarbans. Process Biochem 41: 208-215.
- Gupta R, Beg QK, Khan S, Chauhan B (2002) An overview on fermentation, downstream processing and properties of microbial alkaline proteases. ApplMicrobiolBiotechnol 60: 381-395.
- Joo HS, Kumar CG, Park GC, Kim TK, Paik SR, et al. (2002) Optimization of the production of an extracellular alkaline protease from Bacillus horikoshii. Process Biochem 38: 155-159.
- Lee H, Suh DB, Hwang JH, Suh HJ (2002) Characterization of a keratinolyticmetalloprotease from Bacillus sp. SCB-3. ApplBiochemBiotechnol 97: 123-133.
- Tang XM, Shen W, Lakay FM, Shao WL, Wang ZX, et al. (2004) Cloning and over-expression of an alkaline protease from Bacillus licheniformis. BiotechnolLett 26: 975-979.
- Davis RW, Botstein D, Roth JR(1980) A Manual for Genetic Engineering Advanced Bacterial Genetics.Cold Spring Harber, NewYork.
- Courcelle J, Khodursky A, Peter B, Brown PO, Hanawalt PC (2001) Comparative gene expression profiles following UV exposure in wild-type and SOS-deficient Escherichia coli. Genetics 158: 41-64.
- Adinarayana K, Ellaiah P, Prasad DS (2003) Purification and partial characterization of thermostable serine alkaline protease from a newly isolated Bacillus subtilis PE-11. AAPS PharmSciTech 4: E56.
- Secades P, Alvarez B, Guijarro JA(2001) Purification and characterization of a psychrophilic, calcium-induced, growth-phase-dependent metalloprotease from the fish pathogen Flavobacteriumpsychrophilum. Appl Environ Microbiol 67: 2436-2444.
- Wery N, Gerike U, Sharman A, Chaudhuri JB, Hough DW, et al. (2003) Use of a packed-column bioreactor for isolation of diverse protease-producing bacteria from Antarctic soil. Appl Environ Microbiol 69: 1457-1464.
- Jobin MC, Grenier D (2003) Identification and characterization of four proteases produced by Streptococcus suis. FEMS MicrobiolLett 220: 113-119.
- Younis MAM, Hezayen FF, Moustafa A,Eldein, Shabeb MSA(2009) Optimization of Purified Protease produced in Low-cost medium by Bacillus subtilisKost rain.
- Naidu KSB, Devi KL(2005) Optimization of thermostable alkaline protease production from species of Bacillus using rice bran. African Journal of Biotechnol 7: 724-726.
- Usharani B, Muthuraj M(2010) Production and characterization of protease enzyme from Bacilluslaterosporus. African Journal of Microbiology Research 4: 1057-1063.
- Chantawannakul P, Oncharoen A, Klanbut K(2002) Characterization of proteases of Bacillus subtilis strain 38 isolated from traditionally fermented soybean in Northern Thailand. Science Asia 28: 241-245.
- Wang HY, Liu DM, Liu Y, Cheng CF, Ma QY, et al. (2007) Screening and mutagenesis of a novel Bacilluspumilus strain producing alkaline protease for dehairing. LettApplMicrobiol 44: 1-6.
- Mekkes JR, Le Poole IC, Das PK, Bos JD, Westerhof W(1998) Efficient debridement of necrotic wounds using proteolytic enzymes derived from Antarctic krill: A double-blind, placebo-controlled study in a standardized animal wound model.
- Ramakrishna SV, Jamuna R, Emery AN(1992) Production of ethanol by immobilized yeast. ApplBiochemBiotechnol 37: 275-282.
- Sen S, Satyanarayana T(1993) Optimization of alkaline protease production by thermophilicBacilluslicheniformis S-40. Indian J Microbiol 33: 43-47.
- Yaakobi T, Cohen-Hadar N, Yaron H, Hirszowicz E, Simantov Y, et al. (2007) Wound Debridement by Continuous Streaming of Proteolytic Enzyme Solutions: Effects on Experimental Chronic Wound Model in Porcin. Wounds 19: 192-200.
- Gupta R, Beg QK, Lorenz P (2002) Bacterial alkaline proteases: Molecular approaches and industrial applications. ApplMicrobiolBiotechnol 59: 15-32.
Relevant Topics
- Agricultural biotechnology
- Animal biotechnology
- Applied Biotechnology
- Biocatalysis
- Biofabrication
- Biomaterial implants
- Biomaterial-Based Drug Delivery Systems
- Bioprinting of Tissue Constructs
- Biotechnology applications
- Cardiovascular biomaterials
- CRISPR-Cas9 in Biotechnology
- Nano biotechnology
- Smart Biomaterials
- White/industrial biotechnology
Recommended Journals
Article Tools
Article Usage
- Total views: 16965
- [From(publication date):
August-2015 - Mar 31, 2025] - Breakdown by view type
- HTML page views : 12200
- PDF downloads : 4765