Production of Omega 3 Fatty Acids and Phycoremediation of Dairy Industrial Effluents from Ambattur Region, Tamil Nadu, India
Received: 12-Jul-2018 / Accepted Date: 11-Sep-2018 / Published Date: 14-Sep-2018 DOI: 10.4172/2155-6199.1000453
Keywords: Phycoremediation; Dairy industry; Waste water effluent; Microalage
Introduction
The present era of industrialization and urbanization has resulted in consumption of naturally available water resources in vast quantities. The water pollution caused due to dumping of the toxic effluent into the water bodies has increased the rate of environmental pollution. Dairy industry is one of the major industries having economic importance among the agro-based industries. Among the milk producing countries, India has attained the first rank in milk production and is noted as one of the major contributors of waste water production. It is estimated that about 110 million tonnes of milk and about 275 million tonnes of waste water are being generated annually from Indian dairy industry [1].
Tamil Nadu is one among the leading milk producing States and heads the list by having Co-operative societies in more than 50% of revenue villages. There are 11,839 Milk Producers Cooperative societies with 22.95 lakh milk producers in Tamil Nadu. It is proposed to achieve the sales target of 25 LLPD by March 2016, which includes 13.50 LLPD in Chennai metro by Tamil Nadu Cooperative Milk Producers’ Federation and 11.50 LLPD in other districts by District Unions in a phased manner. The dairy industrial waste water contain high BOD and COD content and also high levels of dissolved or suspended solids including fats, protein, oil and grease, nutrients such as ammonia or minerals or phosphates that require proper attention before allowing it to be disposed into the water bodies.
Phycoremediation is the use of algae for removal or biotransformation of pollutants from waste water. Over the last few decades efforts have been used as biological tertiary treatment of secondary effluents [2,3]. Unicellular green algae such as Chlorella and Scenedesmus species have been widely used in waste water treatments as they are known for its faster growth and high nutrient removal capabilities. The waste water is generally regardless a suitable growth medium for the microalgae.
In addition to these the microalgae plays an important role in the tertiary treatment of waste water treatment. Microalgae can assimilate a considerable amount of nutrients because they require high amounts of nitrogen and phosphorus. Heterotrophic growth of microalgae always eliminates the requirement for light and hence offers the possibility of increasing algal density and productivities. Culturing of microalgae in the effluent can produce valuable biomass while they remove organic solvent and minerals. Cultivation of micro algae in dairy effluent offers several advantages such as 1) Biomass production for the utilization of organic carbon, nitrogen and minerals without the need for any additional nutrients 2) Reduction of COD and BOD of the effluent 3) Oxygenation of treated effluent 4) Possibility to extract high value products like lipids proteins and carbohydrate for fuel, pharmaceutical/nutraceutical and chemical industries.
Omega-3 (ω-3) fatty acids are polyunsaturated fatty acids (PUFAs) and essential components for the growth of higher eukaryotes. Nutritionally, eicosapentaenoic acid (EPA, 20:5) and docosahexaenoic acid (DHA, 22:6) are the most important fatty acids belonging to this group of bioactive compounds. Omega 3 fatty acids are specific group of poly unsaturated fatty acids where the first double bond is located between the third and fourth carbon atom counting from the methyl end of the fatty acid [4].
In this study the undertaken aims were to isolate the algal strains which were present in dairy industrial wastewaters sites and the strains were selected on the basis of growth rate and biomass for the treatment of wastewater and production of omega 3 fatty acids. The final objective of this study was to evaluate treated dairy wastewater for Omega 3 fatty acids production by cultivating consortium.
Materials and Methods
In the present study, samples were collected from dairy industrial effluents from Ambattur region, Tamil Nadu, India from the primary effluent treatment wells. The number of samples collected was around 10 L from each 3 wells roughly 30 L was collected from the dairy industrial effluent and the period of sampling collected twice during December 2016 and January 2017.
More than 24 algal strains were identified from the collection sites. The collected samples were subjected to microscopic studies by simple wet mount preparation. One drop of algal sample was placed in a clean microscopic slide and put a cover slip on it. This slide was observed under low power (10X), High dry power (45X) and oil immersion (100X). based on which the dominant species were selected. Chlorella vulgaris and Scenedesmus quadricauda were dominant in the collection sites so we used to carried out my research work in these two dominant species grown in appropriate medium along with the consortiums of these algae by altering pH slightly. The parameter analysis for both raw and treated effluent by using microalgae were estimated by the following method adapted from APHA standard methods.
Collection of dairy industry effluent waste water was collected from industry in two different months. The Physicochemical analysis of collected dairy waste water effluents were performed using Atomic force microscopy (AFM) for both control and treated samples. The Isolation of Microalgae was performed both in Solid and Liquid Bold Basal Medium (BBM). The morphological identification of Microalgae was identified using standard monographs and the microscopic view has been confirmed by Prof. S. Elumalai, Department of Biotechnology, University of Madras, Guindy Campus, Chennai – 600 025 [5].
The growth rate of the experiments on growth of Chlorella vulgaris and Scenedesmus quadricauda at optimum temperature 28°C, 0.06 g urea and different light:dark illustration, like light condition 12:12 and dark condition 6:18. Determination of growth rate was done Spectrophotometer Hitachi U 2900. Optical wavelength of 680 nm was determined for the cultures and the results range from 677 nm to 684 nm. The wavelength of 680 nm was confirmed by literature. Growth rate (K) and generation time (G) was calculated by the equations 1 and 2
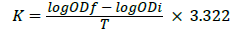
ODf: final optical density,
ODi: initial optical density,
T: time in days.
The pigment estimation of total Chlorophyll a and Chlorophyll b were estimated by Jeffrey and Humphery [6] method with the absorbance values at 664, 647 nm and carotene content were absorbed by 450 nm were recorded. Bio-Chemical analysis such as Carbohydrate [7], Protein [8] and Lipid [9] were also determined. GC-MS and FT-IR was extracted for Omega 3 fatty acids.
Results
Feasibility study was conducted by growing various Microalgae in the industrial effluents were studied from collected Microalgae. The algae employed for the study was Chlorella vulgaris , Scenedesmus quadricuda , and its consortium (Chlorella vulgaris and Scenedesmus quadricuda ) used for the dairy waste water treatment. The Experimental culture was plated frequently and observed microscopically to confirm the viability and were grown in culture flasks for further treatment.
Chlorella vulgaris shows good biomass production than Scenedesmus quadricuda whereas the consortium showed enough biomass to treat the effluents. The physio-chemical analysis of the raw effluent along with the microalgal treated samples from Dairy industry effluent. On reaching the exponential phase the culture was transferred with the different Concentrations of the effluent in the ratio of 100:400, 200: 300, 250: 250, 300:200 and 400:100 respectively.
Among the physio-chemical parameters studied in dairy industrial effluents treatment TDS, TSS, BOD, COD, Nitrogen, Phosphorus, Magnesium, Chloride, Sulphate, Sulphide, Nitrate, Nitrite, Calcium, Sodium, Potassium and Trace metals such as Copper, Manganese, Chromium showed reduced concentrations significantly; whereas pH and dissolved oxygen showed increased concentration after the treatment with microalgae and its consortium.
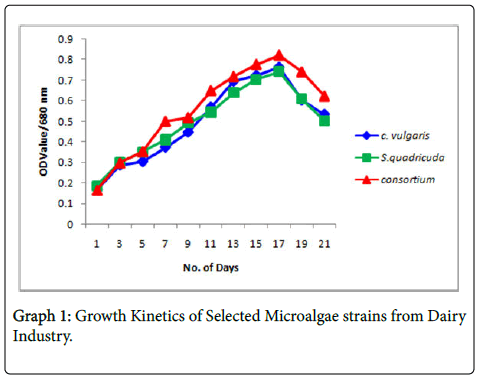
Growth Kinetics
The algae employed for the study was Chlorella vulgaris , Scenedesmus quadricuda and its consortium. They were cultured in a conical flask and the growth measurement was recorded at 680 nm using Spectroscopy analysis and the results was recorded.
Chlorella vulgaris shows good biomass production than Scenedesmus quadricuda whereas the consortium showed enough biomass to treat the effluents (Graph 1).
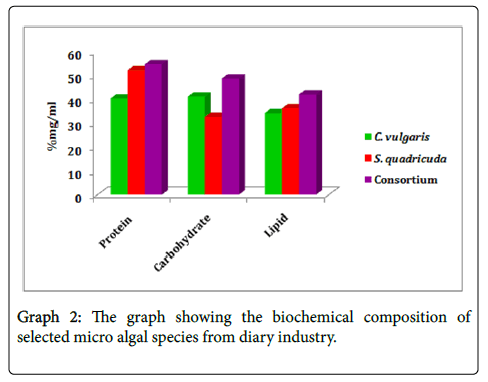
Biochemical composition
Estimation of dry biomass: The selected Micro algal strain of Chlorella vulgaris , Scenedesmus quadricuda and consortium showed the biomass productivity of 512.88 ± 14.03 mg dry weight L-1 232.50 ± 4.52 mg dry weight L-1 and consortium after 21 days of incubation.
Estimation of protein: The percentage of the protein content in all three selected strains ranged from 40-54% of dry matter. The highest percent of the protein was measured in consortium (54.6 ± 0.70%) of the dry weight of biomass followed by Scenedesmus quadricuda of (52.1 ± 0.62%) and lowest in the Chlorella vulgaris (42.07 ± 0.70%).
Estimation of carbohydrate: The carbohydrate content was quantitatively elevated high in Consortium of micro algal samples 48.6 μg ml-1. In which, the microalgae Chlorella vulgaris and Scenedesmus quadricuda engulfs about 41.04 μg/ml-1 and 32.6 μg ml-1 respectively. The carbohydrate content was increased about 2.05% and 0.67% in the microalgal Consortium followed by Chlorella vulgaris and Scenedesmus quadricuda of treated microalgae.
Estimation of lipid: The maximum lipid content was observed in the Consortium of (42.01 ± 4.40%) followed by strain Scenedesmus quadricuda (36.14 ± 2.58%) whereas the strain Chlorella vulgaris (34.12 ± 1.2%) recorded low lipid content. Our results suggest that the members of the class Chlorophyceae accumulated higher lipid content than the Trebouxiophyceae members (Graph 2).
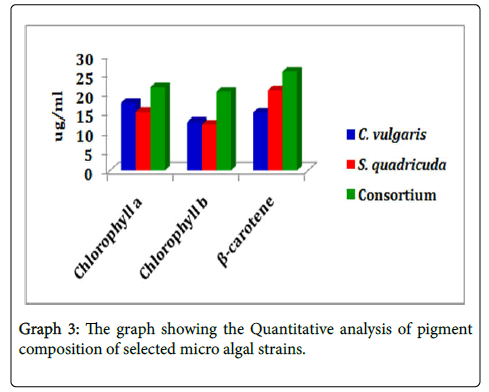
Estimation of chlorophyll a and chlorophyll b: The predominant photosynthetic pigment chlorophyll a was quantitatively high in Consortium of (21.57 μg ml-1) followed by in the micro algal strain Chlorella vulgaris (17.48 μg ml-1) when compared to Scenedesmus quadricuda (15.09 μg ml-1). The Chlorophyll a content was elevated more in consortium and low in the case of Scenedesmus quadricuda .
The same way Chlorophyll b content was found high in the consortium with 20.36 μg ml-1when compared with other two strains (12.63 μg ml-1) and Scenedesmus quadricuda (11.95 μg ml-1). The three micro algal strains except Scenedesmus quadricuda quantitatively yield more content of chlorophyll b which shows the increase in the percentage of chlorophyll b content in consortium and Chlorella vulgaris.
Estimation of carotene: The carotene content was accumulated high in treated micro algal samples. The group of micro algae consortium shows (20.67 mg ml-1) which was high when compared with the other strains Scenedesmus quadricuda bags about (20.75 mg ml-1) of carotene and Chlorella vulgaris (14.93 mg ml-1). The percentage of accumulation of carotene was increased in consortium and Chlorella vulgaris and which was very low in Chlorella vulgaris (Graph 3).
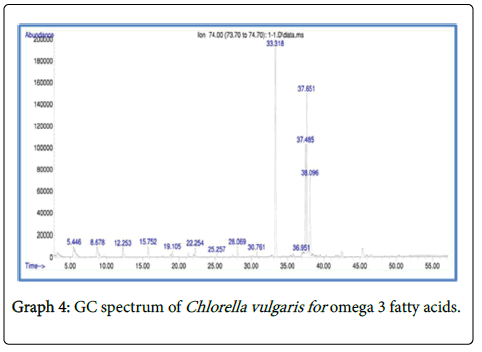
Analysis of fatty acid methyl esters (FAME) by gas chromatography and mass spectrometry (GC-MS)
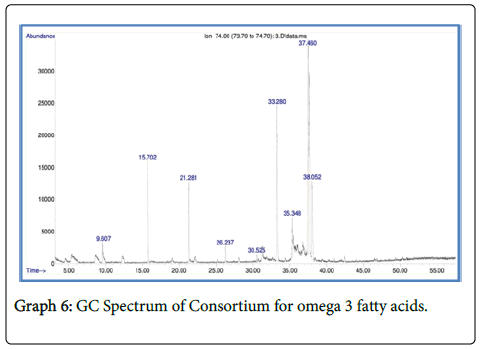
The total fatty acid methyl esters (FAME) obtained in the selected microalgalstrain was about 34.12% of the total lipid content in Chlorella vulgaris , 36.14% in Scenedesmus quadricuda and 42.01% in consortium.
The fatty acid methyl esters of Chlorella vulgaris and Scenedesmus quadricuda and consortium showed the presence of Hexadecanoic acid methyl ester, 6-Octadecenoic acid, methyl ester (Z) and Methyl stearate with high proximity (Graphs 4-6).
Fourier transforms infra red spectrometric (FT-IR) analysis of FAME
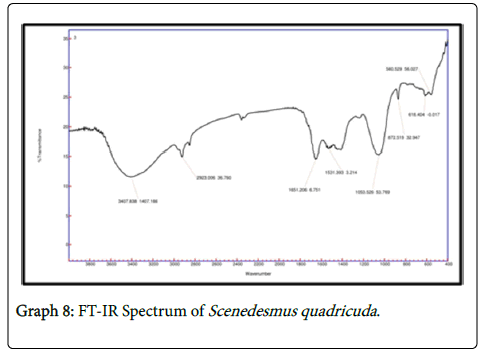
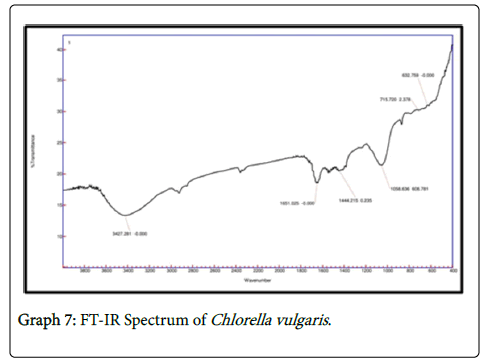
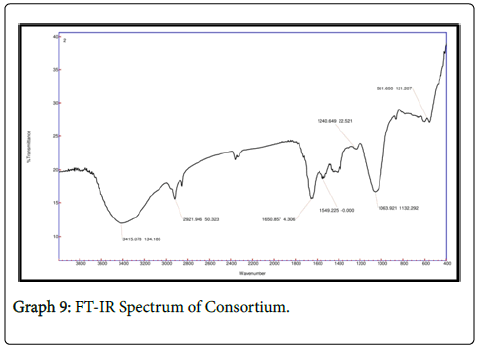
It is observed that the FTIR spectrum of effluent treated by chlorella showed peaks at 1058 cm-1 which is due to the C-N stretch indicating aliphatic amines and the peak at 715 cm-1 attributes to N-H wag suggesting the presence of 1 and 2 amines. In FTIR spectrum of 1650 cm-1 indicates the presence of 1 amines and the peak at 1549 cm-1 indicates the asymmetrical stretching of nitro compounds. The peak value of 1240 cm-1 indicates the aliphatic amines stretching vibration. In FTIR spectrum of consortia the peak value of 1531 cm-1 suggests asymmetrical stretching f nitro compounds. The peak value of 1053 indicates C-N stretch indicating the presence of aliphatic amines whereas the peak 872 cm-1 indicates the presence of NH wag of 1 and 2 amines. The intensity of amines stretchning vibrations caused due to the presence of ammonium ions has decreased which indicates the presence of various nitro groups in the treated sample indicating the major removal of ammoniacal nitrogen through the conversion of amino groups to nitro groups (Graphs 7-9).
Discussion
In our present study, the waste water sample was collected from dairy industrial effluents from Ambattur region, Tamil Nadu, India. The wastewater samples were collected using one litre plastic bottles from collection tank, aeration tank, clarifier and UASB. Ajim [10] has reported the source for the collection of wastewater sample throughout the present study was Gokul Dairy, Kolhapur. The methodology involved in collection of samples at different units. The process in treatment of dairy industrial effluent consists of one and more process such as equalization, Neutralization, Physical and Biological Treatment.
Jegan in his work he reported that it was virtually observed that among the different photoperiod taken for this study, 24 hours of continuous light condition is blatantly utilized for the proliferation of the three selected microalgae. But among the three microalgae, Chlorella vulgaris alone obtained elevated growth when compared with other two microalgae Acutodesmus nygaardii and Scenedesmus armatus where the growth rate was lower. A reduction of the O.D. after 17 days indicates the consistency of growth of Chlorella vulgaris and Scenedesmus quadricuda species and in consortia and also of the depletion in nutrient content mainly phosphate and ammonia in the medium resulting in reduction of OD.
The algae considered for the study was Scenedesmus quadricuda, Chlorella vulgaris and its consortium. The samples were cultured in a conical flask and the growth measurement was recorded at 680 nm using Spectroscopy analysis.
The rapidly growing microalgae Chlorella cells were exhibited by a higher protein and low carbohydrate content, so the rapidly growing cells of natural day light at 25-30°C showed higher amount of protein [11].
The fatty acid methyl esters include Propanoic acid C27:0 (22.60%); Propanoic acid C27:0 (15.65%); 9-octadecenoic acid C38:0 (15.21%) and Carotenoic acid C27:0 (10.8%). The GC-MS analysis of the microalgal sample Chlorella vulgaris showed the presence of Z, E-2- Methyl-3,13- octadecadien-1-ol, cyclopentanecarboxylic acid, octadecyl ester, Docosanedioic acid dimethyl ester, 7,10,13- hexadecatrienoic acid which confirms the presence of omega 3 fatty acids in these compounds.
It is observed that the FTIR spectrum of effluent treated by Chlorella showed peaks at 1058 cm-1 which is due to the C-N stretch indicating aliphatic amines and the peak at 715 cm-1 attributes to N-H wag suggesting the presence of 1 and 2 amines. Characteristic functional groups contributing to the formation of absorption bands at specific wave-numbers. In response to Cd stress, there was a general decrease in the protein and carbohydrate content indicated by a decrease in the intensity of absorption bands especially in the 1800 to 800 cm−1 region [12].
Conclusion
Microalgae production is a promising way to treat and utilize wastewaters. Omega-3, are essential in human nutrition since they play an important role in the organism and prevent several diseases. Omega-3 fatty acids are one of the most valuable products from microalgae; they are likely to be the “game-changer” towards largescale economical microalgae cultivation that will catalyze the production of other important algal bioproducts. Heterotrophic microalgae have been used for the production of omega-3 fatty acids, in particular DHA. However, as the primary producers of PUFAs, the use of autotrophic microalgae for large-scale production of omega-3 fatty acids has recently attracted a lot of interest.
Acknowledgement
We thank The Head, Department of Biotechnology, University of Madras, Chennai for his support and encouragement.
References
- Kushwaha JP, Srivastava VC, Mall ID (2011) An overview of various technologies for the treatment of dairy wastewaters. Crit Rev Food Sci Nutr 51: 442-452.
- Oswald W, Gotaas HB (1957) Photosynthesis in sewage treatment. Trans Am Soc Civ Eng 122: 73-105.
- De la Noue J, Laliberte G, Proulx D (1992) Algae and wastewater. J Appl Phycol 4: 247-254.
- Ryckebosch E, Bruneel C, Muylaert K, Foubert I (2012) Microalgae as an alternative source of omega-3 long chain polyunsaturated fatty acids. Lipid Technol 24: 128-130.
- Elumalai S, Saravanan GK, Rajesh G, Sangeetha T, Roop SD (2014) Biochemical and pigment analysis on phycoremediated microalgal biomass. Golden Research Thoughts Vol: 3.
- Jeffrey SW, Humphrey GF (1975) New spectrophotometric equations for determining chlorophyll a, b c1 and c2 in higher plants, algae and natural phytoplankton. Biochem Physiol Pflanz 167: 191-194.
- Dubois M, Gilles KA, Hamilton JK, Rebers PT, Smith F (1956) Colorimetric method for determination of sugars and related substances. Analytical Chemistry 28: 350-356.
- Bradford M (1976) A Rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry 72: 248-254.
- Folch J, Lees M, Sloane SGH (1957) A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 226: 497-509.
- Ajim SS (2015) Effluent Treatment Plant of Dairy Wastewater – A Performance Evaluation. International Research Journal of Engineering and Technology 2: 837-840.
- Sayegh FAQ, Montagnes DJS (2011) Temperature shifts induce intraspecific variation in microalgal production and biochemical composition. Bioresour Technol 102: 3007-3013.
- Dumas P, Miller L (2003) The use of synchrotron infrared microspectroscopy in biological and biomedical investigations. Vib Spectrosc 32: 3-21.
Citation: Arjun A, Saravanan GK, Banupriya R, Elumalai S (2018) Production of Omega 3 Fatty Acids and Phycoremediation of Dairy Industrial Effluents from Ambattur Region, Tamil Nadu, India. J Bioremediat Biodegrad 9: 453. DOI: 10.4172/2155-6199.1000453
Copyright: © 2018 Arjun A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 3519
- [From(publication date): 0-2018 - Feb 28, 2025]
- Breakdown by view type
- HTML page views: 2828
- PDF downloads: 691