Research Article Open Access
Production and Characterization of Streptokinase Enzyme by Using Streptococcus mutans Strain in Liquid State Fermentation through Corn Steep Liquor (CSL) Substrate
| Abdul Ghaffar*#, Bilal Ahmed*#, Bushra Munir, Rana Faisal and Zahid Mahmood | |
| Department of Applied Chemistry and Biochemistry, Main Allama Iqbal Road, Government College University, Faisalabad, Pakistan | |
| #Authors contributed equally | |
| Corresponding Authors : | Abdul Ghaffar Department of Applied Chemistry and Biochemistry Government College University, Faisalabad, Pakistan Tel: +92 41 9200037 E-mail: aghaffaruaf@yahoo.com |
| Bilal Ahmed Department of Applied Chemistry and Biochemistry Government College University Faisalabad, Pakistan E-mail: bilalahmed814@gmail.com |
|
| Received September 04, 2015; Accepted September 22, 2015; Published September 29, 2015 | |
| Citation: Ghaffar A, Ahmed B, Munir B, Faisal R, Mahmood Z (2015) Production and Characterization of Streptokinase Enzyme by Using Streptococcus mutans Strain in Liquid State Fermentation through Corn Steep Liquor (CSL) Substrate. Biochem Physiol 4:178. doi: 10.4172/2168-9652.1000178 | |
| Copyright: © 2015 Ghaffar A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
| Related article at Pubmed, Scholar Google | |
Visit for more related articles at Biochemistry & Physiology: Open Access
Abstract
Background: Enzymes play a very important and central role in the chemical reactions, occurring in biological systems. The study of enzymes has an immense practical importance. They have high catalytic power, many times greater than the synthetic catalysts. Vascular blockage can cause serious consequences leading to death by a thrombus (blood clot) developed in the circulatory system. Streptokinase is an extracellular protein Trans-located by many strains of beta-hemolytic Streptococci. By converting the plasminogen, to fibrin lytic enzyme, plasmin, it favor’s the blood clot lysis.
Results: The main objective of this research was the production of streptokinase in liquid state fermentation using CSL substances as substrate from Streptococcus species which was grown on blood agar media. Streptococcus mutans was selected for streptokinase production using corn steep liquor as substrate. Corn steep liquor (CSL) was applied in different concentrations ranging from 0.1% to 0.8% in liquid state fermentation culture medium and fibrin clot lysis method was used for enzyme assay.
Conclusion: Among different concentrations of CSL 0.3% was selected as optimum concentration, 44% blood lysis was observed by CSL in liquid state fermentation culture medium for streptokinase production.
| Keywords |
| Corn steep liquor; Streptokinase; Streptococcus mutans; Agar media; Fermentation |
| Introduction |
| Enzymes play a very important and central role in the chemical reactions, occurring in biological systems. The study of enzymes has an immense practical importance. They have high catalytic power, many times greater than the synthetic catalysts [1]. Vascular blockage can cause serious consequences leading to death by a thrombus (blood clot) developed in the circulatory system [2]. In earlier days, treatment of thromboembolic vascular diseases was relied on the use of anti-coagulants, to inhibit the development of fibrin clot. Fibrin lyses can occur in vivo by conversion of plasminogen to plasmin [3]. A blood clot or thrombus consists of blood cells occluded in matrix of the protein fibrin. Enzyme mediated dissolution of the fibrin clot is known as thrombolysis or fibrinolysis. In mammalian circulation, the enzyme responsible for fibrinolysis is plasmin, a trypsin-like protease [4]. Accounts of cardiovascular diseases have become the leading cause of death in the world [5]. Many blood clot-dissolving agents, such as streptokinase, urokinase and tissue plasminogen activator (t-PA), have been utilized in clinical treatments for cardiovascular diseases [6]. Microbes are preferred source for enzyme production [7]. It is now the leading fibrino-lytic agent in the treatment of thromboembolic conditions [8]. Streptokinase is an extracellular protein Trans located by many strains of beta-hemolytic Streptococci [9]. By converting the plasminogen, to fibrin lytic enzyme, plasmin, it favor’s the blood clot lysis. [10]. Streptokinase is produced by several members of different groups of Streptococci [11] such as Streptococcus equisimilis, Streptococcus mutans, Streptococcus feacalis, Streptococcus uberis, etc. Among these microorganisms Streptococcus mutans are easy to achieve because it can be easily obtained from dental plaque. |
| Streptokinase is composed of 414 amino acids including N-terminal single peptide amino acid, which is broken down at some stage in secretion yield. The mature streptokinase protein containing 414 amino acids possesses 47,000 Da molecular mass [12]. Three domains are present in streptokinase and these domains are alpha, beta and smart residue [13]. Due to streptokinase-plasminogen complex and the resultant streptokinase-plasmin complex the free plasminogen is converted into plasmin and by anti-plasminogen shows resistance to inactivation [14]. Because Streptococcal infections are common, detectable levels of antibodies against streptokinase occur in most populations [15]. Fed-batch cultures have been widely used for recombinant protein production by Escherichia coli [16]. |
| Objectives of the research were: |
| i. To isolate the Streptococcus strain from blood culture |
| ii. To produce streptokinase enzyme from Streptococcus strain |
| iii. To compare enzyme activity of streptokinase enzyme secreted by Streptococcus on different concentration of CSL. |
| Materials and Methods |
| All the experimental work was performed in Enzyme Biotechnology Lab, Department of Applied Chemistry and Biochemistry, Government College University, Faisalabad, Pakistan. |
| Microorganism |
| Streptococcus mutans was used as test organism that was obtained from Enzyme Biotechnology Lab, Department of Chemistry & Biochemistry, Government College University Faisalabad, Pakistan. |
| Growth media |
| The selected strain Streptococcus mutans used in the present study was grown on blood agar media (Table 1). |
| It was autoclaved at 121°C for 15 min at 15 lbs and cooled the media to 45-50°C. Then aseptically added 50 mL of sterile delibrinated blood and mixed thoroughly and then dispensed into plates. |
| Nutrient agar media |
| The selected strain Streptococcus mutans used in the present study was maintained on nutrient agar media (Table 2). |
| Production of streptokinase |
| Inoculum preparation: The selected strain was examined accurately by cultivation in blood agar media. Inoculum medium (50 mL) was prepared in 250 mL flask according to composition given in Table 3. Its pH was adjusted to 7 and was autoclaved for 15 minutes at 121°C. A loop full culture of Streptococcus mutans was transferred aseptically into the flask. It was then incubated on orbital shaker (120 rpm) for 24 hours at 35oC. |
| Fermentation media: For the production of streptokinase, Streptococcus mutans was grown on liquid state fermentation culture media using different substrates at 37°C 120 rpm and for 24 hours at pH 7. |
| Substrate optimization |
| For substrate optimization pre-optimized fermentation media was used in which Corn Steep Liquor (CSL) Different concentrations e.g., 0.1%, 0.2%, 0.3%, 0.4%, 0.5%, 0.6%, 0.7%, 0.8% of substrate was used in the fermentation media for streptokinase production. |
| Streptokinase production using CSL (corn steep liquor) as substrate: For the determination of optimum concentration value, 0.1% - 0.8% CSL was used as substrate (Table 4) in liquid state fermentation culture media at 37oC and 120 rpm for 24 hours at pH 7. Then streptokinase enzyme was isolated by harvesting of fermentation media and was used for enzyme assay to calculate the optimum concentration of Corn Steep Liquor (CSL) substrate. |
| Enzyme assay |
| Plasminogen of humans and other animals are the only known protein substrates for streptokinase [17]. Streptokinase assay rely on its ability to activate plasminogen to plasmin. Plasmin hydrolyzes an indicator substrate and the extent to hydrolysis over a given period is related back to the concentration of streptokinase. The indicator substrates for plasmin include the fibrin clot, casein, other proteins and various synthetic esters. Fibrin clot lysis method was used for the enzyme assay [18]. In fibrin clot lysis method, 0.5 ml blood was taken from human volunteer and it was allowed to clot. Then 0.25 ml enzyme suspension was applied on this blood and %age lysis of blood was noted within 10 minutes. The %age lysis of blood showed enzyme activity. |
| Comparison of streptokinase activity of optimum substrate concentrations with standard |
| Experimentally determined optimum substrate concentrations were used for streptokinase production and enzyme assay. Then, the experimentally determined enzymatic activities of optimum substrate concentrations were compared with a Standard streptokinase enzyme. The standard streptokinase enzyme was obtained from FIC (Faisalabad Institue of Cardiology), Faisalabad, Pakistan. |
| Statistical analysis |
| Mean and Analysis of Variance (ANOVA) techniques were used for statistical analysis. For the comparison of enzymatic activities of optimum substrate concentration Mean was used. To determine the significance of the results of the enzymatic activities and comparison of optimum substrate concentrations with standard analysis of variance (ANOVA) was used. |
| Results and Discussion |
| Although several organisms have been reported to produce streptokinase, however, Streptococcus mutans is an important organism used for industrial production. Streptokinase is a clinically important and cost effective plasminogen activator but its use is not risk free. The potent agent is derived from β- hemolytic Streptococci and is consequently associated with risk of anaphylaxis. The risk is dependent upon level of patients inoculating anti-streptokinase antibodies. Streptokinase acts by stimulating the conversion of plasminogen to plasmin. It is a proteolytic enzyme which is able to disrupt fibrin stability and production. The half-life of streptokinase is 6 hours. Plasmin resistant, long life variants of protein engineered streptokinase have been produced in protease deficient recombinant Bacillus subtilis WB 600 [19]. |
| Streptokinase production |
| Producing microorganism: The selected test organism Streptococcus mutans was grown on blood agar media. Clear zones were observed around the colonies of the Streptococcus mutans. These clear zones on the blood agar media confirmed the growth of the selected microorganism, e.g., Streptococcus mutans. Furthermore, these colonies of the Streptococcus mutans were maintained on nutrient agar media. Yellowish growth of the colonies of the test organism was observed on nutrient agar media [20]. |
| Fermentation: Literature reports that group A hemolytic Streptococci commonly require complex and rich media supplemented with various nutritional factors for growth [20]. So, fermentation media containing glucose, yeast extract, KH2PO4, CaCO3 was used. Furthermore, in order to fulfill the nutrional requirements, CSL, used in their optimized concentrations as substrate at 37°C and 120 rpm for 24 hours at pH 7 in fermentation media for streptokinase production from Streptococcus mutans. Streptokinase was isolated from Streptococcus mutans culture by centrifugation at 10,000 rpm for 20 minutes at 0oC. Since enzyme is extracellular supernatant. So, it was stored at -20oC [21]. |
| Assaying streptokinase: Characterization of the streptokinase production by fermentation and assessment of the product require methods for assaying the streptokinase. In fibrin plate method, a zone of lysis produced on a fibrin film in a petri dish was measured and related to the concentration of the fibrino-lytic protein. I used fibrin clot lysis method because it is an easy and simple method to determine enzyme activity. |
| Substrate optimization |
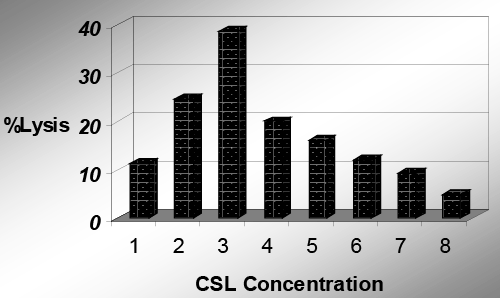
| Corn steep liquor (CSL) as substrate: The selected Streptococcus strain was grown in liquid state fermentation using different concentration of corn steep liquor (CSL) ranging from 0.1% to 0.8% as substrate. The observed streptokinase enzymatic activity was 11.33, 24.66, 38.66, 20, 16, 12, 9.33 and 4.66 percent at 0.1, 0.2, 0.3, 0.4, 0.5, 0.6, 0.7 and 0.8 percent concentrations of corn steep liquor, respectively (Table 5). |
| Maximum streptokinase enzymatic activity was shown at 0.3% concentration of corn steep liquor in liquid state fermentation. So, 0.3% CSL concentration was selected as the optimum concentration. Further increase in substrate concentration decreased the enzyme production. Figure 1 indicates the streptokinase enzymatic activity using corn steep liquor (CSL) as substrate in liquid state fermentation culture medium. According to Dubey et al., [22] the fibrino-lytic activity of streptokinase obtained from Streptococcus species using 8% CSL and fibrin plate method was 32%, but in contrast, in our results the enzymatic activity was better and more economical and only 0.3% CSL to give 38.66% fibrino-lytic activity. |
| Summary |
| The main objective of this research was the production of streptokinase in liquid state fermentation using CSL substances as substrate from Streptococcus species which was grown on blood agar media. Streptococcus mutans was selected for streptokinase production using corn steep liquor as substrate. |
| Conclusion |
| Corn steep liquor (CSL) was applied in different concentrations ranging from 0.1% to 0.8% in liquid state fermentation culture medium and fibrin clot lysis method was used for enzyme assay. Among different concentrations of CSL 0.3% was selected as optimum concentration, 44% blood lysis was observed by CSL in liquid state fermentation culture medium for streptokinase production. |
| For comparison standard streptokinase enzyme was used and 56% fibrino-lytic activity was shown by standard. By comparing it was concluded that CSL showed the maximum and highest enzymatic activity of streptokinase at 0.3% concentration in liquid state fermentation. Therefore, from this research work study it is concluded that streptokinase enzyme with better fibrin lytic activity in liquid state fermentation can be produced by using optimum concentrations of CSL substrate with its optimum concentrations such as 0.3% for CSL, at commercial level so that it can fulfill the needs of streptokinase for health and medicinal use in our country. |
| Authors Contribution |
| First two authors contributed equally, Bilal Ahmed and Bushra Munir contribute for experimental work of the research and Rana Faisal and Zahid Mahmood perform editing and grammatical corrections and Dr. Abdul Ghaffar supervise the research work. |
| Acknowledgements |
| The authors wish to thank the Institute for the Promotion of Innovation through Science and Technology in Pakistan (GCUF) for financial support in the framework of the Ph.D. grant of Bilal Ahmed. The authors wish to thank Dr. Zahid Mahmood for valuable comments on the manuscript and edits which has been improved by his helpful suggestions. |
References
|
Tables and Figures at a glance
| Table 1 | Table 2 | Table 3 |
| Table 4 | Table 5 | Table 6 |
Figures at a glance
 |
| Figure 1 |
Relevant Topics
- Analytical Biochemistry
- Applied Biochemistry
- Carbohydrate Biochemistry
- Cellular Biochemistry
- Clinical_Biochemistry
- Comparative Biochemistry
- Environmental Biochemistry
- Forensic Biochemistry
- Lipid Biochemistry
- Medical_Biochemistry
- Metabolomics
- Nutritional Biochemistry
- Pesticide Biochemistry
- Process Biochemistry
- Protein_Biochemistry
- Single-Cell Biochemistry
- Soil_Biochemistry
Recommended Journals
- Biosensor Journals
- Cellular Biology Journal
- Journal of Biochemistry and Microbial Toxicology
- Journal of Biochemistry and Cell Biology
- Journal of Biological and Medical Sciences
- Journal of Cell Biology & Immunology
- Journal of Cellular and Molecular Pharmacology
- Journal of Chemical Biology & Therapeutics
- Journal of Phytochemicistry And Biochemistry
Article Tools
Article Usage
- Total views: 23405
- [From(publication date):
December-2015 - Apr 26, 2025] - Breakdown by view type
- HTML page views : 18312
- PDF downloads : 5093
