Processes Responsible for Global Freshwater Loss
Received: 22-Feb-2018 / Accepted Date: 06-Apr-2018 / Published Date: 10-Apr-2018 DOI: 10.4172/2155-9910.1000251
Abstract
This review summarizes earlier observations related to the changes of ocean as the largest osmotic system. The global warming and the melting of freshwater reserves (polar glaciers, ice sheets and permanent sno) provided evidence for the sea level rise and dilution of seawater contradicting the unsustainable geochemical theory of steady-state ocean system. The osmolarity of blood of terrestrial vertebrates (~0.3 Os) is known to reflect the osmolarity of the primordial ocean at the time of their migration to land during the Devonian period some 400 million years ago. The osmotic concentration of the present day ocean (1.09 Osm) is now more than three times higher than that of the blood of land vertebrates referred to as the ’salinity gap’. The recent dilution tendency is opposed by other processes that suggest a long-term salination of ocean rather than the decrease of the osmolarity of terrestrial vertebrates. The salinity increase of ocean is likely to be related to the global loss of freshwater raising questions about the extent of water deficit and how the global loss of fresh water could be developed. Beside salination other factors of freshwater loss include photohydrolysis of water, biological oxidation of food molecules, and escape of hydrogen to the space. This study also specifies cooperating processes that limit the extent of the dilution caused by global warming. The melting of ice and snow of the available freshwater reservoirs could not support more than 50 m sea level rise. Data of earlier sea level rises up to 200 to 400 m in the past 500 million years have been used to estimate the global freshwater loss and its contribution to the salination of ocean.
Keywords: Dilute solutions; Osmolarity gap; Seawater level rises; Salination processes; Freshwater deficit
Major Factors of Global Water Loss
The bioelements of the CHNOPS group are found in the 1st-3rd periods of the periodic table with carbon (C) as the key component of life forms on Earth. Three cycles, namely the carbon cycle, water cycle and the nitrogen cycle make the life on our planet sustainable. These cycles are recycling carbon, nitrogen and water. The global carbon cycle consists of four major reservoirs, the atmosphere, the terrestrial ecosystem (plants and soils responsible for photosynthesis), ocean and the sediments. The carbon cycle was supposed to be stable without fossil fuels, cement and land use [1]. Nitrogen is converted in the biogeochemical nitrogen cycle to multiple forms of chemical structures circulating among the atmosphere, terrestrial and marine subcycles. The water (or hydrological) cycle deals with the movement of water on, below and over the surface of the Earth. Whereas the mass of water remained relatively constant over geological ages, the volume of the three major water bodies, namely freshwater reservoirs (permanent snow and ice), saline water (primarily ocean) and atmospheric water vapour is variable depending on climatic changes. Changes in water cycle resulted in global warming and the melting of freshwater reserves as well as salinity increase of ocean during ice ages. This review deals with the increasing ’salinity gap’ causing the increase of salt concentration of sea and the gradual loss of water in freshwater reservoirs and in the atmosphere.
Major reasons of salination of sea and the estimation of global water deficit are discussed in this study. Water here means freshwater to distinguish it from brackish, salty, seawater and from polluted and undrinkable water. Estimates of water loss come from calculations based on earlier high (up to 300-400 m) seawater level elevations during dilution periods, which could not be obtained again due to the reduction of freshwater reservoirs.
Four major factors are considered to cause freshwater loss on Earth that impact other water bodies including seawater. These factors include:
• Long-term salination of sea
• Photohydrolysis of water by irradiation
• Freshwater loss related to biological oxidation from the metabolism of food molecules
• Loss of freshwater through the escape of hydrogen to the space.
Long-term salination of sea
The uniform osmolarity of blood in terrestrial vertebrates (~0.3 Osm) reflects the osmolarity of an ancient stage, namely the concentration of the primordial ocean at the time of migration of vertebrates to land [2,3]. The osmotic concentration of the present day ocean (1.09 Osm) is now more than three times higher than that of the blood of land vertebrates referred to as ‘salinity gap’. In the 1990s the idea of long-term concentration process of seawater was objected by the geochemical theory of a steady state ocean system and rejected due the lack of direct evidence for any change in its salinity. The acceptance of the steady-state ocean theory gave the false illusion of an inexhaustible compensational power that would counteract any chemical influence. The intellectual challenge of the salinity paradox inspired nearly 120 years ago the ’salt clock’ method that was assumed to determine the age of Earth based on the continuous salinity intake of ocean by the water cycle. Although the ’salt clock’ method did not work, it led to the realization that the 3-salination of sea, could be a much longer lasting process than hypothesized [4-6]. As direct evidence of salinity increase was still missing, experiments related to the osmolarity of the inner environment of terrestrial vertebrates were expected to prove that it was not the reduced osmolarity of blood serum of land vertebrates, but the salination of seawater that caused the increasing salinity gap [7]. Among the possible causes responsible for the salinity changes of sea, the following processes were discussed [8]:
• Continental drift and outpouring lava increase the surface of the seabed.
• The formation of snow ice in the Polar Regions, cause freshwater deficit, its melting causes sea level rise.
• Weathering and denudation carry away the surface of land. The material is deposited in oceans building sedimentary rocks the dissolved salt content of which increases the salinity of seawater.
• The hydrologic cycle is based on evaporation from sea, evapotranspiration from soil and vegetation, and precipitation. One pathway is the flow of freshwater that rivers carry constantly as diluted salt to the ocean.
• Anomalies in geochemical balance and sea-to-sea variations in chemical composition, even if very small, may cause a long-term change but could appear in short-term as a steady-state system.
• Chemical pollution contributed by man was formerly neglected but is becoming of increasing concern. Environmental pollution is related to the principle of randomness. Biological organisms are highly organized, but the price of this organization is paid by the increasing disorganization (randomness) of the outer environment (milieu exterior). The major contributor to disorganization is mankind. This aspect of loss of freshwater and focus in the next chapter will be placed on the loss of water caused by the hydrolysis of water.
Photohydrolysis of water by irradiation
Atmospheric photodissociation: Penetrating photon components present in the visible and ultraviolet light, in x-rays and gamma irradiation can induce among many other reactions the photohydrolysis of water. Hydrogen atoms and molecules generated by photolysis from water vapour are relatively distant from surface of the Earth. These are smallest volatile components of air and are much less attracted by gravitation than the heavier oxygen atoms. Volatile, reductive gases of Earth, primarily hydrogen, helium and biogenic methane are trickling away to the space and cause irreversible oxidation [9]. That freshwater can escape as water vapor to the outer space was confirmed by the isotope composition and volume of Earth’s early oceans [10].
Photosynthesis: One of the major causes of increasing atmospheric oxygen is photosynthesis in a complex process which converts carbon dioxide, water and some inorganic salts into carbohydrates (mainly cellulose and sugars) in the green pigments of chlorophyll in plants, algae, certain bacteria utilizing the energy of the sun. During photosynthesis, the energy of visible light is sufficient to split water to hydrogen and oxygen atoms. Among the many evolutionary processes, the loss of water is related to the gradual increase of oxygen in the atmosphere. The constantly increasing amount of oxygen in the atmosphere reached by now about 21%. This tendency is unlikely change in the future confirming the notion that the green Earth is turning to an oxidized red planet similarly to Mars.
Freshwater loss related to the metabolism of food molecules
Hydrolytic conversion of oxidation energy of carbon to reductive power: The heating of coke at high temperature (1000°C) in the presence of steam generates a gaseous mixture consisting of an equimolar mixture of carbon monoxide (CO) and hydrogen gas (H2):
C(s) + H2O(g) → CO(g) + H2(g), (s) = solid, (g) = gaseous form
Carbon monoxide and H2 are combustible gases. Such direct hydrolytic reaction does not occur in cells at high temperature.
Lump sugar made from sucrose can be burned directly at high temperature to form carbon dioxide and water:
T°
C12H22O11 + 12O2 → 12CO2 + 11H2O
One could also use glucose for direct combustion:
T°
C6H12O6 + 6O2 → 6CO2 + 6H2O
Metabolic hydrolysis of water: Similarly to the hydrolytic oxidation of carbon, the oxidation of glucose in the presence of oxygen does not occur in cells at high temperature. At lower physiological temperature cells utilize glucose. In the presence of oxygen, glucose is metabolized in the cytosol of cells in the first stage of cellular respiration known as glycolysis and subdivided into 10 steps from α-D-glucose to pyruvate. The second stage of cellular respiration is known as the citric acid cycle (tricarboxylic acid cycle, Krebs cycle). Reactions of the Krebs cycle take place in the matrix of mitochondria, in the steps of the Krebs cycle the carbon atoms of acetate (in acetly-CoA) are subjected to hydrolytic oxyditain producing CO2 and generating reductive equivalents (NADH + H+ and FADH2) indicated with [H]. The third stage of cellular respiration takes place in the mitochondrial electron transport chain that is coupled to oxidative phosphorylation in eukaryotes and generates ATP.
There are two hydrolytic reactions in the citrate cycle, one in the succinyl-CoA → succinate reaction and the second one during the fumarate → malate conversion. The hydrolytic reactions in the citrate cycle can be summarized as
CH3COO- + H+ + 2H2O → 2CO2 + 8[H]
The hydrogen atoms come from the acetate itself (4[H]) and the rest of them is of hydrolytic origin (4[H]). The reductive equivalents (8[H]) are oxidized back to water in the terminal oxidation, but in the overall reaction, no net energy is gained. The summarized reaction:
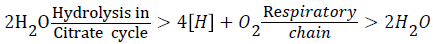
would suggest that during the oxidation of acetate water is oxidized directly to O2, which is certainly not the case, rather a secondary alcohol is formed first and oxygen becomes part of the carbonyl and carboxyl group as the oxidation of the secondary carbon atoms proceeds. These reactions have been described earlier [11].
The conversion of oxidative energy to reductive power by hydration takes place in a similar manner in the β-oxidation of fatty acids in a series of reactions [12] summarized as_
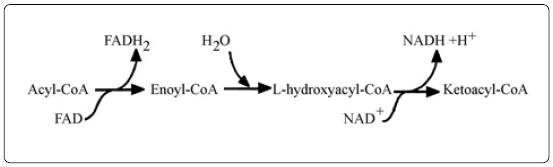
The hydrolytic reactions in amino acid metabolism are summarized in the scheme below [13].
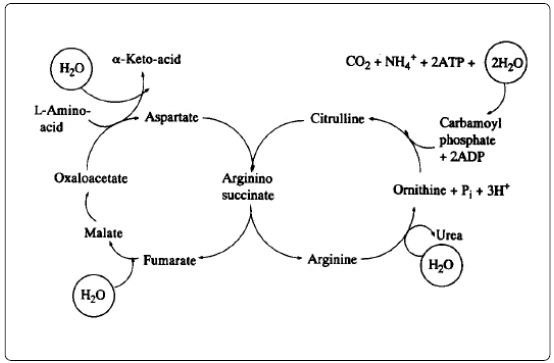
The sum of these hydrolytic reactions involved in the metabolism of amino can be given briefly as:
α-amino acid + NAD(P+) + H2O → α-ketoacid + NH4+ + NAD(P)H + H+
Production of biogas: Corresponding to the vast size of the biomass, a huge amount of H gas is released but not all captured and utilized as biochemical energy with a significant portion released into the upper part of the atmosphere from the biogas. Of the two basic factors recognized by Lavoisier involved in cellular respiration, the oxidation of carbon to carbon dioxide, its relationship with hydrolytic reactions and importance has not been dealt with and not described in biochemistry textbooks. The importance of the energetic conversion is to utilize the oxidation energy of the unoxidized carbon atom of acetate to carbon dioxide through the hydrolysis of two molecules of water to produce four hydrogen ions and two oxygen atoms.
A large amount of hydrogen is produced by the intestinal bacteria in rodents and mammals including humans [14]. There are several other processes to obtain hydrogen from water such as thermal, thermochemical, photochemical and biochemical reactions which did not find so far industrial applications [15]. There is no registered information of how much biogas is burned directly and how much is utilized for electricity and heat production. Most of the biogas is still burned in a flare.
Loss of freshwater through the escape of hydrogen to the space
From the viewpoint of water loss, the fate of hydrogen atoms generated by atmospheric photodissociation is of primary importance. Released reactive so-called ’in statu nascendi’ hydrogen atoms form H2 and are the lightest molecules (2.015 g/mol) among atmospheric gases. Other components of air are heavier and due to their similar molecular weights: nitrogen 28.0134, oxygen 31.9988, carbon monoxide 28.0101 g/l and gravity, form a relatively stable mixture (28.966 g/l). Water as vapour, steam or clouds (18.01585 g/l) is lighter than air and depending on the thickness of its layers appear in the upper atmosphere. The remaining so-called trace gases also referred to as greenhouse gases are carbon dioxide, methane, nitrous oxide and ozone. Carbon dioxide has a higher molecular weight (44.0095 g/mol) and without mixing tends to sediment. The instability and constant interconversion of ozone results in two regions of the atmosphere. The ozone (47.9982 g/mol) as a layer (~90%) is present in the lower stratosphere 20-30 km above the Earth seasonally and geographically varying its thickness. The rest (~10%) constantly undergoes the ozone cycle present in the lower region of the troposphere ranging between 5-15 km height above the Earth surface. Occasionally high ’smog ozone’ episodes may develop in urban and rural areas.
Biogases: The breakdown of organic matter from raw materials of different origin (agriculture, manure, municipal, plant, sewage, green or food waste, etc.) in the absence of oxygen generates a mixture of gases of different composition. Combustible components of biogases and volatiles are methane, carbon monoxide, hydrogen and hydrogen sulfide. Hydrogen is the simplest, lightest and most abundant element in the universe and the third most abundant on Earth. Among the different mechanisms describing the atmospheric escape of gases, the Jeans Escape is the best known [16]. The low molecular weight hydrogen gas could have escaped from the lower atmosphere early in the Earth’s history and is still escaping to the outer space at higher altitudes most of it after the ionic photolysis of water vapour. Gas giants (e.g. Jupiter and Saturn) are likely to attract hydrogen emitted by the Earth. The loss of hydrogen comes ultimately from water.
How much freshwater is being lost?
The highest average sea level elevation (320 m) in the past 500 million years was taken from the Haq’s (Exxon’s) (400 m) and Hallam’s (240 m) values [17,18]. The melting of an estimated 80% (~20 × 106 km3) of the recently available freshwater reserves (25 × 106 km3=100%) would cause not more than 50 m seawater level rise. Data of the calibration curve are summarized in Figure 1A [19] showing the sea levels versus the volumetric changes relative to the recent zero level. Earlier lowest (-130 m) and highest (+320 m) seawater levels are indicated by the red lines in Figure 1B. High levels and volumes originating from earlier melting periods coincide with the development of terrestrial vertebrates (~400 Mya). The freshwater reservoir used for the 320 m sea level elevation was an estimated 130 × 106 km3. The calibration curve also indicates that the freshwater reserves gradually diminished and are now only (~25 × 106 km3). Taking the small proportion of freshwater (now 2.5% of sea) the volumetric decrease of seawater in the past 500 Mya could not have caused more than 10% salination, underscoring the importance of other processes in the salination of ocean. Based on the recent low sea level and freshwater resupply it is concluded that previous high (200-400 m) sea level elevations will not occur in the future [19].
Figure 1: Fluctuation of freshwater reserves based on sea level rises and falls in the past 500 million years. A) Seawater volumes belonging to different seawater level elevations. a) radii relative to the Earth’s mean radius denoted R⊕ [17]; given as the distance from the Earth's centre to its surface, estimated to be 6,371.008 km [20]. b) volumes of seawater in the absence of oceanic crust, c) volumetric increase of seawater levels in the presence of oceanic crust under the seawater (+30%), d) sea level rises. B) Calibration curve: seawater level rise versus seawater volume increase. Red lines indicate the lowest (-130 m) (~20,000 years ago) and highest (320m) (~500 Mya) seawater levels. Modified with permission [19].
Conclusion
The recent global melting of ice and snow with its temporary dilution effect on seawater revealed the unsustainability of the geochemical constancy of the sea. Salinity changes were supported by Raoult’s law applied to the ocean as the largest global dilute solution [8]. Salinity fluctuations of sea over geological ages represent a dynamic osmolyte system versus the theory of general geochemical balance that denies salinity changes. Salinity oscillations involve a) glacial periods favouring salination of seawater and accumulation of freshwater reservoirs and b) interglacial periods of dilutions characterized by the melting of accumulated ice and snow. The freshwater deficiency and the widening of the osmotic gap between the ionic concentration of land vertebrates (0.3 Osm) and seawater (1.09 Osm) are reflected by the salination of ocean. Global environmental changes disprove geochemical constancy and require explanation to the global deficit of freshwater. This brief study summarized the reasons leading to the salination of sea and the gradual exhaustion of freshwater reservoirs. Salination of ocean and loss of freshwater impose more serious threat to biodiversity and global life than global warming.
References
- Prentice IC, Farquhar GD, Fasham MJR, Goulden ML, Heimann M, et al. (2001) The carbon cycle and atmospheric carbon dioxide. In: Climate Change 2001: The scientific basis. Cambridge University Press 185-237.
- Smith HW (1943) Lectures on the kidney, Porter Lectures IX, University of Kansas Press, Lawrence.
- Joly J (1899) An estimate the geological age of the Earth. Trans Royal Soc Dublin 2: 23-66.
- Burchfield JD (1998) The age of the Earth and the invention of geological time. Geological Society, London, Special Publications 143: 137-143.
- Dalrymple GB (2001) The age of the Earth in the twentieth century: a problem (mostly) solved. Special Publications, Geological Society of London 190: 205-221.
- Banfalvi G (1989) Long-term concentration of seawater. The Rolex Awards for Enterprise Montres Rolex S.A, 1211 Geneva, Switzerland Ref. 5-C 12238.
- Banfalvi G (1991a) Evolution of osmolyte systems. Biochem Education 19: 136-139.
- Catling DC, Zahnle KJ, Mckay C (2001) Biogenic methane, hydrogen escape, and the irreversible oxidation of early Earth. Science 293: 839-843.
- Pope EC, Bird DK, Rosing MT (2012) Isotope composition and volume of Earth's early oceans. Proc Natl Acad Sci 109: 4371-4376.
- Banfalvi G (1991b) Conversion of oxidative energy to reductive power in the citrate cycle. Biochem Education 19: 24-26
- Banfalvi G (1992a) Conversion of oxidative energy to reductive power: II. Contribution of hydration to the reductive energy in the β-oxidation of fatty acids. Biochem Education 20: 105-106.
- Banfalvi G (1992b) Conversion of oxidation energy to reductive power: Ill Hydrolytic energy conservation in amino acid metabolism. Biochem Education 20: 216-218.
- Ohno K, Ito M, Ichihara M, Ito M (2012) Molecular hydrogen as an emerging therapeutic medical gas for neurodegenerative and other diseases. Oxid. Med Cell Longev 1-11.
- Häussinger P, Lohmüller R, Watson AM (2011) Hydrogen, 1. Properties and occurrence. In Ullmann’s Encyclopedia of Industrial Chemistry, Wiley-VCH.
- Gargaud M (2011) Encyclopedia of Astrology, Vol. 3. Springer Science & Business Media 879.
- Cornell S (2008) 542 Million years of sea level change: Exxon’s sea level reconstruction. Penn State's Coll Earth Miner Sci 890.
- Hallam A (1983) Early and mid-Jurassic molluscan biogeography and the establishment of the central Atlantic seaway. Palaeogeogr Palaeoclimatol Palaeoecol 43: 181-193.
- Banfalvi G (2017) Global loss of freshwater and salination of sea. J Marine Sci Res Dev 7: 1-5.
- Williams DR (2004) Earth Fact Sheet. NASA Official. Grayzeck E (2016) last updated.
Citation: Banfalvi G (2018) Processes Responsible for Global Freshwater Loss. J Marine Sci Res Dev 8: 251. DOI: 10.4172/2155-9910.1000251
Copyright: © 2018 Banfalvi G. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 4085
- [From(publication date): 0-2018 - Mar 31, 2025]
- Breakdown by view type
- HTML page views: 3274
- PDF downloads: 811

