Priority Pollutant Sample Preparation, Extraction and Clean Up From Spiked Water and Solid Matrices with Internal, Volumetric and Standard Addition for Analysis by GC and GC/EI &NICI-MS
Received: 03-Nov-2022 / Manuscript No. jabt-22-79104 / Editor assigned: 05-Nov-2022 / PreQC No. jabt-22-79104 / Reviewed: 18-Nov-2022 / QC No. jabt-22-79104 / Revised: 21-Nov-2022 / Manuscript No. jabt-22-79104 / Accepted Date: 27-Nov-2022 / Published Date: 28-Nov-2022 QI No. / jabt-22-79104
Abstract
This manual was devised as a one semester program of work for the Masters in Analytical Chemistry for one day per week. It was designed to demonstrate the analytical methodology and instrumentation utilized to proceed from the raw sample stage through sample preparation to the final analysis of reduced sample residues by capillary gas chromatography and GC/Mass Spectrometry. A variety of target analytes were employed to highlight the techniques to detect and quantify these compounds using external and internal standard addition, including selected priority pollutants , chloro-hydrocarbons (OCHs), polyaromatic hydrocarbons (PAHs) and chlorophenols. Liquid and solid matrices were employed to illustrate the analytical methodology, spiked with the target analytes to be monitored. The USEPA method 625 was adopted for liquid-liquid acid-base extraction of semi-volatile priority pollutants aimed at the demonstrating of quality assurance/control, recovery efficiency and application of derivatization to enhance detection. Solid phase extraction (SPE) of target analytes from water was utilized to illustrate the clean-up and concentration of analytes.Demonstration of on-column injection was illustrated for the profiling of n-alkanes and PAHs. The focus is the analytical methodology and procedures for presenting priority pollutant residues for analysis by GC-ECD and GC/EI or NICIMS. Representative data analyses, calibration and quantitation are not exemplified. Historic references relevant to the analytical methodology employed in the procedures for the laboratory manual are included.
Keywords
Pollutants priority; Analytical methodology, Spiked with the target analytes
Introduction This manual was devised as a one semester program of work for the Masters in Analytical Chemistry for one day per week. It was designed to demonstrate the analytical methodology and instrumentation utilized to proceed from the raw sample stage through sample preparation to the final analysis of reduced sample residues by capillary gas chromatography and GC/Mass Spectrometry. A variety of target analytes were employed to highlight the techniques to detect and quantify these compounds using external and internal standard addition, including selected priority pollutants, chloro-hydrocarbons (OCHs), polyaromatic hydrocarbons (PAHs) and chlorophenols. Liquid and solid matrices were employed to illustrate the analytical methodology, spiked with the target analytes to be monitored. The USEPA method 625 was adopted for liquid-liquid acid-base extraction of semi-volatile priority pollutants aimed at the demonstrating of quality assurance/control, recovery efficiency and application of derivatization to enhance detection. Solid phase extraction (SPE) of target analytes from water was utilized to illustrate the clean-up and concentration of analytes. Demonstration of on-column injection was illustrated for the profiling of n-alkanes and PAHs. The focus on the analytical methodology and procedures for presenting priority pollutant residues for analysis by GC-ECD and GC/EI or NICI-MS. Representative data analyses, calibration and quantitation are not exemplified. Historic references relevant to the analytical methodology employed in the procedures for the laboratory manual are included [1-7].
The Experiments Conducted Were as Follows
Experiments I/III.
Liquid-liquid acid-base extraction (LLE) from water based on USEPA method 625 for semi-volatile organic priority pollutants, targets, a. OCHs, b. PAHs, and c. chlorophenols
(GC-ECD and GC/EI and NICI-MS)
Experiment II
Solid phase extraction (SPE) from water with C18 ODS sorbent cartridges to illustrate concentration and clean-up of target analytes a. (GC-ECD with wide bore capillary column analysis or GC/NICI-MS).
Experiment III
Adaptation of USEPA method 625 for the analysis of acidic priority pollutants involving derivatization of chlorophenols using pentafluorobenzyl bromide. Target analytes c.
(GC-ECD and GC/NICI-MS)
Experiment IV
Extraction of organochlorine pesticides from solid matrix using ultrasonic-solvent extraction (USE) based on USEPA method 3550. Target analytes a. aldrin, dieldrin and endrin
GC/NICI-MS)
Experiment V
An on-column injection technique using GLC-FID for the analysis of
1. PAHs.
2. N-alkanes, C12 to C40.
3. Application of linear (programmed) retention indices.
Experiment Overview
Target compound analysis in water and soil matrices - methodology and instrumentation.
Aims
To extract, separate via clean-up as required with derivatization as appropriate, identify/characterise determine and quantify a selected range of organic priority pollutants with the use of internal and volumetric standards using both GC and GC/MS instrumentation as recommended. To illustrate a range of techniques and applications available to the analyst and chromatographer.
Scheme of work
Provided are a set of methods and procedures for sample preparation involving the extraction, separation, derivatization and work-up of the target analytes present in two matrices of a river system - water and soil/sediment. General instructions are provided for the general analytical and instrumental methods to be used; devising some of the techniques for analyte separation and clean-up may be required.
Experiment I: This experiment is based on USEPA Method 625 (Federal Register, October 1984 and subsequent modifications) and is, used here to illustrate the analyses of semi-volatile base/neutral and acidic priority pollutants present in a water sample which is representative of a real sample taken from a water course (RIVER SYSTEM). Samples A, B, C, D and E of water contain a representative group of priority pollutants comprising organochlorine compounds (OCHs), polyaromatic hydrocarbons (PAHs) and a trichlorophenol (TCP). A set of standard solutions will be required, which are used (i) to characterize the targets via their retention data and mass spectra and (ii) to determine the amounts present by GC-ECD and GC/MS. Details of reference compounds, internal and volumetric standards, for calibration with the concentration requirements are presented. Summaries of the methods for the use of calibration data employing internal and volumetric standards obtained by GC-ECD and GC/MS are described.
Experiment II: This experiment is designed to illustrate methods for the isolation, separation (and clean-up) of these analytes in water and illustrates the use of solid phase extraction (SPE) using a C18 silica absorbent.
Experiment III: The procedure for EXPERIMENT III follows USEPA method 625 as does EXPERIMENT I. In this method the analysis by GC/MS involves direct chromatography of the phenols. The method can be employed as such or after derivatizing the phenols to improve the detection and therefore the determination of the analyte which is of importance for analysis at trace levels. Analysis of acidic priority pollutants and application of derivatization of phenols using alkylation with benzyl halides is illustrated. For several reasons, including improved chromatography and increased sensitivity of detection, phenols are derivatized using a variety of reagent and the method for the use of an appropriate derivatizing reagent is given. Pentafluorobenzyl bromide (PFBBr) can be used to prepare strongly electron affinic (electron capturing) derivatives of carboxylic acids, amines, barbiturates, uracils, phenols and thiols. Both protons on a primary amine may be replaced by the PFB group.
RCOOH, RNH2 or ArOH + R’Br = RCOOR’, RNR’2 or ArOR’ + HBr
Experiment IV: Method for the extraction, separation and cleanup of target analytes in soils.
Although liquid-liquid extraction can be employed to remove target analytes from soil problems arise because of emulsion formation and interferences in the chromatography. For this reason, alternative extraction techniques have been devised and two methods are mentioned in the section following. Advice will be given as to whether both methods should be attempted depending on the time available. Clean-up techniques involving solid sorbents (column chromatography) are included under EXPERIMENT II and the experiment can be attempted with and without clean-up if there is enough time in the laboratory periods.
Extraction of semi-volatile compounds from solids
This method based on USEPA method 3550 is outlined as follows:
a. Add internal standard to 10 g of soil finely ground (<1 mm diameter) in a 100 ml flask.
b. Sonicate the sample for 15 min with 3 x 30 ml portions of an appropriate extraction solvent (DCM, pentane).
c. Centrifugation of the sample and solvent is required at each stage. An ultrasonic probe or bath is used
d. Filter and dry over sodium sulphate the combined extract and concentrate to 10 or 1 ml as required in a K-D evaporator.
Experiment V: Experiment V is for information and reference only.
Reference Compound, Internal/Volumetric Standard And Calibration Standard Requirements Calibration For GC-ECD or GC/MS
External calibration: aldrin, dieldrin, and endrin only
Concentration range for calibration, 100 μgL-1 to 1mgL-1
Analyze the standard reference solutions in this concentration range and construct calibration curves for each organochlorine pesticide using area in units of μgL-1. Analyze the concentrated extracts from spiked water samples provided by liquid-liquid extraction and solid phase extraction. Dilution of the extract may be required to obtain a concentration in the range of the calibration standards (NOTE: the upper limit of the linearity of GC-ECD detector ≤ 1 ngL-1 or 1 mgL- 1 injected). Using the data obtained from the calibration curve, the original concentrations of organochlorine pesticides in the extracts from the spiked water samples can be determined and expressed in μgL-1.
Volumetric standardization
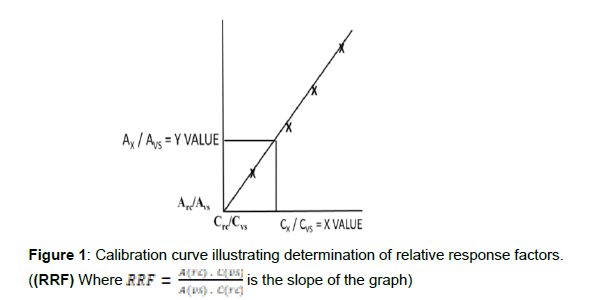
Aldrin, dieldrin, endrin and volumetric standard, decachlorobiphenyl, calibration concentrations as above with DCBP at 400 μgL-1. Construct calibration curves using the volumetric standard peak area and concentration to obtain relative response factors, RRF (see graphical presentation on page 4).
Calibration for GC/MS: The concentrations of PAHs in the concentrated extract can be determined in two ways as follows:
Concentration range for calibration, PAH reference compounds, 2 to 10 mgL-1, deuterated PAH internal and volumetric standards, 2 mgL-1
Use of internal standards: The two internal standards are to be added before extraction are d8-naphthalene and d10-phenanthrene and these are used to analyze the hydrogen analogues, h8-naphthalene and h10-phenanthrene. Since the analyte and internal standard coelute the total ion area attributable to each component cannot be obtained independently. However, because of the selectivity of the mass spectrometer specific (characteristic) ions, m/z, 128 and 136 for the naphthalene analogues and 178 and 188 for the phenanthrene analogues can be selected for quantitation. Calibration curves can be constructed by plotting area ratios for each pair of specific ions against concentration ratios to obtain relative response factors. The RRF can be used in the determination of the concentrations of the target analytes. Reference is made to the area ratios obtained for the unknown amount of target analyte with respect to internal standard which has been added initially and which is at the same concentration as that in the calibration standards. NB The use of internal standards, which have the same chemical and physical properties as the analytes should avoid problems of loss during extraction and work up and any variation in instrument response during analysis.
Use of volumetric standards
(i) PAHs: The concentrations of naphthalene, phenanthrene, anthracene and chrysene can be obtained by reference to volumetric standards added to the concentrated extract. For anthracene the volumetric standard employed is the isotopically labelled analogue which coelutes. This volumetric standard can also be used to determine phenanthrene and chrysene concentration. A comparison of the determinations using the two isotopically labelled analogues is
Note: d10-biphenyl, which is included here in support of d10- anthracene as a volumetric standard in the absence of analogue standards, can be used to determine the concentrations of naphthalene and anthracene but its retention time is too far removed from chrysene for it to be employed in this case as a volumetric standard.
(ii) Aldrin, dieldrin and endrin: An appropriate volumetric standard is decachlorobiphenyl, particularly for GC/MS in NICI mode.
Table 1. summarizes characteristic ions for semi-volatile hazardous substances list (HSL-USEPA).
| EI (positive ions) | NICI (negative ions) | |||||
|---|---|---|---|---|---|---|
| Analyte | Primary | Secondary | Primary (Secondary) | |||
| Aldrin | 66 | 263, 220 | 330 (328,332) | |||
| Dieldrin | 79 | 263, 279 | 237 (235,239); 346 (344, 348) | |||
| Endrin | 263 | 82,81 |
|
|||
| m/z for chlorine pattern, source temperature 250oC |
||||||
| Cl-phenol | 128 | 64,130 | 127 | 129 | ||
| TriCl-phenol | 196 | 198, 200 | 195 | 197, 199 | ||
| TriBr-phenol | 330 | 332, 141 | 329 | 331, 333 | ||
| PentaCl-phenol | 266 | 264,268 | 230 | 228, 232 | ||
| RO- fragment from derivatized phenol | ||||||
| Naphthalene | 128 | 129,127 | ||||
| Anthracene | 178 | 179, 176 | ||||
| Phenanthrene | 178 | 179, 176 | ||||
| Chrysene | 226 | |||||
| d8-naphthalene | 136 | 66 | ||||
| d10-biphenyl | 164 | |||||
| d10-anthracene | 188 | |||||
| d10-phenanthrene | 188 | |||||
| decaCl-biphenyl | 498 496, 500 | 498(496, 500) | ||||
Table 1: Characteristic ions for semi-volatile hazardous substances list (HSL-USEPA).
The ions listed are used to detect analytes and the primary ion (100% base mass peak) is used in quantitation by GC/MS (USEPA specified). The calibration curve to obtain response factors is illustrated below in Figure 1 for which

is the slope of the graph, and where Arc’ Crc’ Ax’ Cx’ Avs’ and Cvs are the peak areas/concentrations of target reference compound, unknown target analyte and volumetric standard, respectively. Least mean squares analysis can be employed to determine r2 RRF, intercept and Cx but only if linearity is indicated by r2 > 0.995. If the correlation coefficient is not close to the accepted value, rely only on the graphical method by extrapolation as above (or calculation of the RRF = slope from experimental curve). The unknown analyte concentration, Cx (in the concentrated extract) is obtained from the graph by extrapolation. Use the analytical data obtained for the ratio of Ax/Avs to extrapolate to the value of Cx/CVS on the x-axis as indicated above by the broken lines to the plotted line. It follows that:
Cx (extract) = (x-value)extrap. x Cvs (mg or μgL-1)
The concentration of the target analyte in the water sample prior to extraction and concentration can be determined as follows:

Where CF is the concentration factor.
The unknown concentration of the target analyte in the original water sample can also be determined using the calculated RRF data and the following equation:

i. Recoveries
Internal standards (is), for example, d8-naphthalene and d10- phenanthrene employed in these experiments, or authentic reference compounds (rc) are used in quality assurance/control to verify recoveries. In this instance, a known concentration of the standard is employed to spike into the water or soil and the area ratio (Ax/Ais or Ax/Arc) data after extraction/concentration fitted to the standard calibration curve to obtain the unknown concentration Cx as above under 2a. For the authentic reference compound:

Where the Crc and Arc are the concentration and area expected if no loss occurred during work- up of the sample analyzed, relating to the concentration of the reference compound.
Note: the area for the volumetric standard (Avs) is included in the first form of the equation above for the experimental and calibration data to compensate for any variation in response of the detector and system under the two analysis conditions. The simplified second form of the equation is only valid if the volumetric standard shows no variation in response under the two analyses conditions.
Important For the Analysis of Results Obtained
The recoveries can only be calculated for the two internal standards for the PAHs and for the tribromophenol, which are added prior to extraction and sample preparation. In addition, only single point averaging can be employed because the same concentration of internal standard has been added to each of the calibration solutions.
Experiments I and III
Environmental analysis using GC or GC/MS - Extraction methodology for, and the determination of base/neutral and acidic priority pollutants in a spiked water matrix.
Introduction
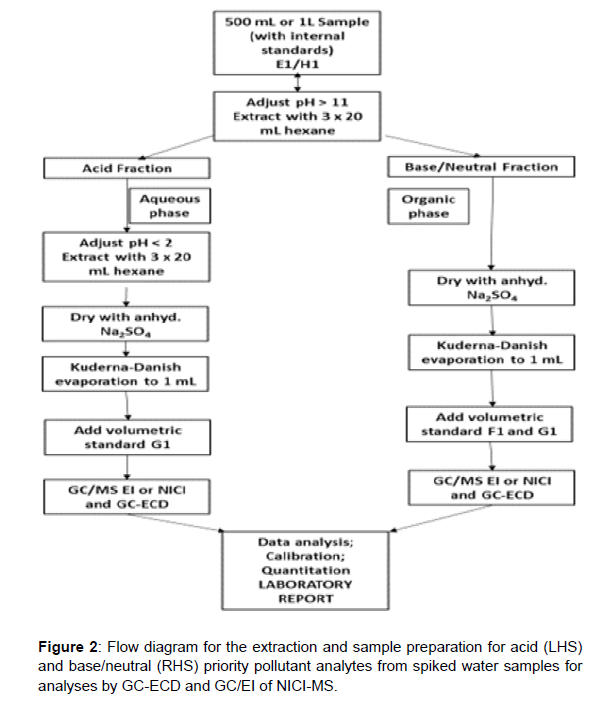
Methods for identifying and quantifying trace levels of individual organic compounds in potable water or wastewater requires extraction and a prior concentration step. The most widely used methods are liquid-liquid extraction solid sorbent extraction purge and trap and distillation methods. The methods described cover the determination of base/neutral extractable organic pollutants (OCs, PAHs, etc.) and acidic components (chlorophenols) as classified by USEPA in water/waste waters. The methods can be modified and applied to the determination of other classes or organic pollutants, such as pesticides. Spiking of water samples is required to determine recoveries of pollutants for quality assurance/control purposes. The analysis scheme for LLE applied to the water samples containing the analytes to be determined is illustrated in Figure 2.
Reagent, solvent and standards
a. Hexane or DCM
b. Concentrated NaOH/H2SO4 (base/neutral and acidic priority pollutants, respectively)
c. Sodium chloride
d. Distilled water
e. Sodium sulphate anhydrous
f. Reference standard solutions
Note all concentrations below are given in units of mg or μgL-1 in keeping with convention. The labels on the bottles containing standard solutions of reference compounds provided signify the concentrations in ppm or ppb. To convert simply replace ppm or ppb by mgL-1 or μgL-1 as appropriate.
A2. 20 mgL-1 of a mixture of naphthalene, anthracene, phenanthrene and chrysene
(NAPC) in hexane for calibration standards
E1. 2 mgL-1 of internal standards d8-naphthalene and d10- phenanthrene in acetone for spiking into samples A, B, C, D and E F1. 2 mgL-1 of volumetric standards d10-biphenyl and d10-anthracene in hexane for adding to extracts from samples A, B, C, D and E
E2. 10 mgL-1 of internal standards of d8-naphthalene and d10- phenanthrene in hexane for calibration standards
F2. 10 mgL-1 of volumetric standards d10-biphenyl and d10- anthracene in hexane for calibration standards
B2. 2 mgL-1 of aldrin, dieldrin and endrin (ADE) in hexane
G1. 400 μgL-1 of DCBP for addition to extracts from A, B, C, D and E.
G2. 4 mgL-1 of volumetric standard decachlorobiphenyl (DCBP) in hexane for calibration
C2. 2 mgL-1 of trichlorophenol (TCP) in hexane
H1. 400 μgL-1 internal standard tribromophenol (TBP) in acetone for spiking into solutions A, B, C, D and E. H2. 4 mgL-1 of internal standard TBP in hexane for calibration standards
Figure 2 illustrates the flow diagram for the extraction and sample preparation for acid (LHS) and base/neutral (RHS) priority pollutant analytes from spiked water samples for analyses or GC-ECD and GC/ EI of NICI-MS.
Apparatus and materials LLE:
a. GC-ECD or GC/EI and NICI-MS 1000 ml separatory funnel for LLE
b. Rotary evaporator,
c. Kuderna-Danish evaporator (or micro-Snyder column)
d. Pasteur pipette
e. Manifold and nitrogen cylinder gas, fume cupboard.
The spiked water samples, 500 mL, will be provided - unknown A, B, C, D and E. 1.0 ml
Solutions of 2 mgL-1 d8-naphthalene and d10-phenanthrene (E1) and 400 μgL-1 of tribromophenol
(H1) internal standards, as advised in a hexane/acetone mixed solvent, are to be added to the 500 mL samples prior to extraction and work-up of the samples.
Preparation of reference standard solution for calibration:
a. Dispense 1.0, 2.5, and 5.0 mL of 20 mgL-1 solution (A2) of NACP with 2 ml of each of 10 mgL-1 solutions (E2 and F2) of internal and volumetric standards and make up to final volume in 10 ml graduated flasks
b. Dispense 1.0, 2.5, and 4.0 ml of 2 mgL-1 of the solution (B2) of ADE, with 1.0 ml of 4 mgL-1 of solution (G2) of DCBP and make up to a final volume of 10 ml in 10 ml graduated flasks.
c. Dispense 1.0, 2.0, and 4.0 ml of 2 mgL-1 solution (C2) of TCP with 1ml of 4 μgL-1 solution (H2) of TBP and 1 ml of 4 mgL-1 solution (G2) of DCBP and make up to final volume of 10 ml as above.
Procedures for Experiments I and III
LLE Base/neutral component extraction (I)
Transfer 500 mL of the sample provided to 1000 ml separatory funnel. Add AR sodium chloride (30 g). Add internal standards - 1.0 mL of 2 mgL-1 solution (E1) of d8-naphthalene and d10-phenanthrene and 1.0 mL of 400 μgL-1 solution (H1) of TBP and adjust the pH to 11- 12 with 4 M NaOH. Add 20mL hexane and shake vigorously for 5 min. Allow the layers to separate, then drain the water layer into 1000 mL conical flask and the solvent layer into a 250 mL conical flask fitted with a filter funnel charged with AR sodium chloride.
(Caution Ensure That Pressure in the Funnel Is Released At Intervals during Shaking)
Repeat the extraction of the water layer twice with 20 mL of hexane. Combine all the solvent layers.
Important: Do Not Discard the Water Layer As It Is Required For Acid Extraction Later
a. Wash the anhydrous sodium sulphate in the filter funnel with a small volume of hexane and decant the dried solvent extract from the 250 mL conical flask to a 250 mL Buchi flask. Wash the conical flask with hexane and combine with solvent extract.
b. Concentrate to ca. 10 mL on rotary evaporator and transfer the extract to a K-D evaporation tube (or micro-Snyder column). Evaporate to the residue state - dryness.
c. Add 1.0 mL of 2 mgL-1 solution (F1) containing volumetric standards, d10-biphenyl and d10-anthracene to the residue, 1 mL of 400 μgL-1 of solution (G1) of DCBP and re- Concentrate to exactly 1 ml. (Hint: add 1 mL solution F1 first and dry to the residue stage and then add accurately, 1mL of solution of G1).
d. Store in a 3 mL vial fitted with open cap and silicone teflon-faced septum.
LLE Acid component extraction (III)
Use the procedure above as for base/neutral component extraction with the following changes and exceptions.
Stage a. adjust pH of the water layer resulting from stage c. above from 11-12 to 1-2. Under stage b. there is no internal standard TBP addition as this component has been added previously during base/ neutral extraction. Under stage c. do not add internal standards for PAHs but add only 1 ml of 400 ppb solution (G1) of DCBP.
Instrumental analysis
a. Perform analysis on GC-ECD or GC/MS as advised using the GC conditions outlined in instrumental handouts
b. Inject 1 μl aliquot of (i) calibration solutions containing authentic reference standards and internal standards and (ii) concentrated extracts
c. Quantify using RIC or selected ions characteristic of the target analytes and standards.
Calculation (US-EPA method)
a. Obtain response factors for each of the analytes graphically, see below.
b. Calculate the amounts in μg/L using the relationship outlined previously or that below using the relationship outlined previously.
c. Calculate the recovery efficiency (%) by comparing with amounts of internal standards, d8-naphthalene, d10-phenanthrene, and tribromophenol in the extracts and in calibration reference solutions.
d. For concentration and relative response factor determinations for low and high-level water samples the adapted equation from USEPA method 625 can be used alternatively, if desired as follows:

Ax = Extracted ion current profile (EICP) area at the characteristic
m/z of the analyte. GC/MS or GC-ECD peak area.
Avs = EICP area at the m/z of the specified volumetric standard
Ivs = amount of volumetric standard
Vo = volume of water extracted (mL)
Vi = volume of extract injected (μL)
Vt = volume of total extract (1 or 2 mL)
Obtain the response factors for the target analytes from the calibration data for the reference standard solutions (1, 5 and 10 mgL-1) using the slope from the plot of Ars /Avs : Crs/Cvs
Where Crs and Ars are the concentration and area of characteristic ion for the reference standard for the target analyte, with Avs and Cvs for the volumetric standard, respectively.
Experiment IIA
SPE Base/neutral component extraction
Solid phase/sorbent techniques can be used for (i) extraction/ concentration (SPE) from an aqueous matrix as described in Experiment IIA and (ii) clean-up of a concentrated solvent extract (adsorption-elution chromatography), Experiment IIB. The former technique involves isolation and concentration of ADE from aqueous samples A, B, C, D or E using C18 ODS cartridges and a simple apparatus can be used. A vacuum manifold system is employed, which can accommodate up to 10 cartridges, simultaneously.
General background
The general method for isolation of base/neutral and acidic components from an aqueous matrix employs the reversed phase liquid chromatography mode as follows:
a. Solid sorbent/analyte non-polar (np)
b. Liquid matrix polar (p)
Under these conditions, np (solid phase), np (isolate or analyte) interactions are strongest and the most polar analytes elute first. Increasing the solvent polarity increases the retention (k’, partition ratio) of the analytes. C18 ODS silica is a good choice of solid sorbent for the extraction because the analytes are non- polar and the liquid matrix is very polar. No significant amounts of matrix interferences exist that are also non-polar, and a relatively non-selective extraction is desired as all organochlorine components are to be extracted. The use of a highly selective detector (ECD or NICI- MS) allows for a less selective sorbent extraction. The addition of methanol to the water matrix improves the solubility of OCHs and helps maintain a wetted condition as the large volume of water is passed through.
Procedure
Use the extraction apparatus as advised comprising a manifold (connected to a vacuum pump) fitted with a reservoir - 250 ml separating funnel containing the water matrix modified with methanol. The cartridges should be conditioned as suggested prior to passing the water matrix through. The flow rate of elution should not be greater 10 mL min-1 and can be restricted by the tap on each exit line of the vacuum manifold. The flow rate can be checked during conditioning of the sorbent with the water/methanol mixed solvent prior to loading the sample.
a. Analytes, non-polar priority pollutants, ADE, soluble only in non-polar solvents.
b. Matrix 200 mL of polar, aqueous matrix from samples A, B, C, D and E
c. Sorbent C18 ODS silica cartridge, 500 mg
d. Extraction procedure
e. Sorbent conditioning
A. hexane;
B. methanol/water (1:100)
1. Sample treatment
Add 2 ml methanol to 200 ml of water matrix from sample A, B, C, D and E and pass through the sorbent
2. Analyte elution: hexane
*Conditioning procedure and loading
A. Hexane 5 ml and dry for 5 min.
B. Methanol/water (1:100) 5 ml.
A. Apply sample and dry for 5 min.
B. Elute with hexane, 5 ml into a small tube and evaporate to the residue state
A. Add 1 mL of a 400 μgL-1 solution of internal standard DCBP (G1).
Carry out the analysis as for Experiment I and compare the results. Note that the matrix volume, 200 mL is less than that used for LLE.
Experiment IIB
Important Note: Only Carry Out This Experiment If There Is Enough Time Available
Experiment IIB involves a clean-up technique which makes use of normal phase LC mode in that
1. polar (silica or florisil)
2. extract non-polar (hydrocarbon)- polar (hydrocarbon)
3. analyte polar function (active)
Thus P’ (analyte) = P’ (sorbent) ≠ P’(solvent) where P’ is the polarity index for each component. Ideally the clean-up technique can be used to separate non-polar analytes (ADE), polarizable analytes (PAHs) from polar analytes (TCP and its internal standard - TBP) present in the concentrated extract, i.e. if the LLE (Experiment 1) had been conducted at pH 2 only. Here, the acid extract can be used to demonstrate the extraction/clean-up to isolate polar analytes, TCP. Pass 0.5 mL of the acid extract through conditioned silica or florisil cartridge (with 5 ml hexane). Elute with a suitable polar solvent. Analyze the clean-up extract after blowing down to 1 ml using GC-ECD or GC/NICI-MS and determine the concentration of the TCP as for the organochlorine components, ADE, above.
Experiment III
Adaptation of USEPA Method 625 for the Analysis of Acidic Priority Pollutants Involving the Derivatization of Phenols Using Pentafluorobenzyl Bromide
It has been reported that pentafluorobenzyl bromide can be used to derivatize a range of phenols at concentrations down to ca. 5 pg for analysis by GC-ECD. The method is used for acidic priority pollutants, including trichlorophenol (TCP) and pentachlorophenol (PCP). The chromatography of phenols without derivatization is aggravated by the presence of active (acidic) hydrogen, which leads to severe tailing on many columns due to the presence of active surfaces (SiOH) or impurities in the stationary phase (SP).
Chromatography of phenols without derivatization
US-EPA method 625 recommends direct analysis of acidic components – phenols, i.e., without derivatization. However, for optimization of the chromatography it is recommended that PCP, one of the PPs, is analyzed to determine the tailing factor, which is explained below. Observe the chromatographic peak profile for PCP analyzed directly. Tailing factors can be obtained from the peaks by drawing a horizontal line at 10% of the peak height from the base cutting the peak at points A and C, dropping a perpendicular from the apex of the peak to this line at B and determining the ratio of BC/AB at this 10% line. A tailing factor of ≥ 3/1 is unacceptable for US-EPA contract analyses and the column would need to be replaced or deactivated.
Chromatography of phenols with derivatization
Derivatization leads to enhancement of the chromatography, higher response at the peak and a correspondingly greater sensitivity and lower detection limit. Furthermore, if the group added is highly electron affinic as with PFB then the molecule will exhibit enhanced GC-ECD and GC/NICI-MS responses.
ROH + PFBBr -> ROPFB + HBr ; ROPFB + e- -> RO- + PFB m/z
(M-181)- observed GC-ECD or GC/NICI-MS detection
ROPFB + e- -> ROPFB+ + 2e- ; ROPFB+ -> RO = PFB+
m/z, 181 observed GC/EI-MS detection
Method
This method using acetonitrile as the reaction medium and ethyl acetate or hexane for the sample injection requires high purity reagents to avoid contamination by impurities and interference in the chromatography. All glassware should be washed with AR grade solvents.
PFBBr – The amount of derivatizing agent is dispensed directly from neat PFBBr (Caution- lachrymator)
Reaction Protocol
Derivatization of trichlorophenol in extracts and standards by alkylation with pentafluorobenzylbromide
Prepare the following solution:
• Analyte residue 0.5 mL extract evaporated by nitrogen blowdown
• Acetonitrile- 60 μL
Add the following
• PFBBr- 10 μL
• Triethylamine- 10 μL
1. Leave the solution at room temperature for 15 min and then add 0.5 mL ETOAc, mix thoroughly and add 0.5 mL hexane.
2. Leave the solution at room temperature ca. 15 min while a white precipitate forms and then wash the organic layer with 2 x 1 mL 0.5 M HCl.
3. Dry the organic layer using a small amount of anhydrous sodium sulphate, evaporate slowly under a stream of nitrogen gas, and dissolve the residue in 1 mL HPLC grade hexane.
This method also specifies a clean-up procedure using a silica sorbent, which is necessary for a complex mixture/range of phenols but which can be omitted for a simple mixture.
Note: For derivatized calibration standards use 0.5 mL solutions of 200 and 400 μgL-1 of TCP with 400 μgL-1 TBP.
Analysis
Conduct the analysis using GC/NICI-MS and compare with data obtained using GC/EI-MS if time permits. Determine the amount by reference to the single duplicated derivatized standard.
Furthermore, a comparison can be made with an extract and standards for underivatized phenols, again, if there is enough time available.
Results
Determine the amount in the extract and in the original spiked volume from Experiment 1 obtained by acid extraction. Use the results to obtain the % recovery of the target phenols with respect to the internal standard (surrogate) assuming that the losses are identical.s
GC-ECD: Use the peak areas relative to TBP in the determination of the amounts.
a. For underivatized phenols use ions specified in the page listing characteristic ions for quantitation again relative to TBP.
b. Note: that m/z 181 is observed by EI-MS for all derivatives of phenols due to PFB+. This ion although a feature of all derivatized phenols (or other compounds, such as, carboxylic acids) is not a characteristic ion of the phenol and therefore is not diagnostic.
GC/NICI-MS: The (M-181)- negative ion observed by NICI-MS would, however, be specific because it is the characteristic RO- ion of the particular target phenol and therefore is more diagnostic.
Experiment IV
Extraction of Organochlorine Compounds and Pyrethroids from Sediment/Soil samples
Introduction
If possible, samples should be extracted without being dried because the drying process can lead to the loss of pesticides and other organic materials. If the sediment cannot be extracted at once, it should be removed stored at low temperature in a deep freeze. Sediment may contain sulphides and carbonates, which must be removed prior to extraction of the OCs from the matrix. There are two procedures for extracting OCHs from sediments, either agitating the sediment and hexane solvent with ultra-sound or repeated extraction using a Soxhlet extractor (steam distillation extraction SDE can also be employed, handout if available). A check on the efficiency of extraction is made by adding internal (surrogate, if available) standards, which are extracted and quantified. However, this does not necessarily reflect the extraction efficiency of the method because the OCHs in the sample may more tightly bind to the sediment particles than the added standards.
Preliminary analysis
The amount of OCHs in the sediment needs to be related to the amount of dry matter and to the total organic content of the sample. These parameters can be determined separately by the moisture content by drying a weighed amount of the sediment in an oven at 105o C and by the total organic content using ignition in a muffle furnace or chemical oxidation with chromic acid.
The following protocol describes the extraction of OCHs from the sediment with ultra-sonics.
Protocol Extraction of organochlorine compounds from sediments
The materials and apparatus required are as follows.
• Glacial acetic acid
• Deionized water, hexane extracted
• Propan-2-ol'
• 250 mL conical flask
• Ultrasonic bath
• Mechanical shaker
• 100 mL centrifuge tubes
• Centrifuge if required
Method
Centrifuge the sample immediately or once it has been thawed out and pipette off the excess water with a Pasteur pipette.
1. Weigh out accurately about 5 g of wet or dry sediment into a 100 mL prewashed conical flask. Remove the sulphides and carbonates by adding 5mL of glacial acetic acid, 10 mL of hexane extracted deionized water and 15 mL propan-2-ol. Shake well and vent off the gases.
2. Using a micro-litre syringe add the internal standards and spike (pesticide or PAH) [surrogate or deuterated PAH].
3. Clamp the unstoppered flask in an ultrasonic bath and agitate with ultrasound for about 30 min.
4. Add 15 mL hexane, stopper the flask and shake in a mechanical shaker for 5 min.
5. Using a Pasteur pipette transfer the hexane extract and the emulsion that will have formed into a 100 mL centrifuge tube.
6. Balance the tube with another tube containing water and centrifuge at 1500 rpm for 15 min.
7. Filter through a number 4 porosity sintered glass crucible and the washings from the flask provided by a small volume of hexane.
8. Remove the hexane layer with a pipette and dispense into a stoppered tube.
9. Repeat the hexane extraction from step 5 twice more and add the extracts to the tube. Discard the sediment.
10. The sulphur compounds in the extract can be removed if required.
11. Dry the hexane extract with sodium sulphate in a column
12. Add 1 mL deuterated volumetric standard (for PAH) or decachlorobiphenyl in hexane.
13. Evaporate to 1 mL using a K-D evaporator and nitrogen blowdown or a micro Snyder column.
14. Analyze by GC-ECD and GC/MS using 1 μL injection and GC conditions as specified in the general guidance notes.
a. Compare with suitable calibration standards at appropriate concentration.
b. Determine the concentration of pesticide or PAH (as specified) in the sample, μg/kg and% recovery if instructions are given to spike the soil sample under point 3 above.
Table 2 summarizes the USEPA specified contract required detection limits (CDRL) for priority pollutants in water and soil/ sediment. The level detectable and therefore the minimum detection limit is matrix dependent.
| Semi-volatile | CAS No | Low water, µg/L | Low soil, µg/kg |
|---|---|---|---|
| Aldrin | 309-002 | 0.05 | 8 |
| Dieldrin | 60-57-1 | 0.1 | 16 |
| Endrin | 72-20-8 | 0.1 | 16 |
| Naphthalene | 91-20-3 | 10 | 330 |
| Anthracene | 120-12-7 | 10 | " |
| Pyrene | 129-00-0 | 10 | " |
| Chrysene | 218-01-9 | 10.01 | 330 |
Table 2: The USEPA specified contract required detection limits (CDRL) for priority pollutants in water and soil/sediment.
Example
Amount detected by GC/EI-MS after extraction from 30 g soil, aldrin CDRL = 8 μg/kg; 0.008 μg/g; 0.24 μg/30 g; 8 ppb After extraction and concentration to 1 mL
amount in extract = 0.24 μg/mL, 1 μL injected into GC/MS,
amount in 1 μL = 0.24 ng or 240 ppb (CF x 30)
Note: The amounts detectable in water are lower because of the higher concentration factor (CF) than for soil ; 1 litre of water is taken compared with 30 g soil. The CDRL for Aldrin in water is in fact 160 times lower than that for soil. Note also that there is the need for extremely low detection limits in potable (drinking) water.
Experiment V
Gas Chromatographic Analysis of Polyaromatic Hydrocarbons (PAHs) using n-alkane homologous series and linear retention indices
Introduction
For temperature programmed GC a homologous series, such as n-alkanes, exhibit a chromatographic profile indicating a regular elution pattern. This type of profile is typical of other series the members of which increase in RMM by a CH2 group (14 amu), e.g., methyl esters of fatty acids, alkyl ketones, etc.
For a homologous series of compounds with the same functional groups, which differ only in the length of the alkyl chain (carbon number) ΔH, increases by an approximately constant increment with each additional carbon atom.
log po = a + b.n and ΔHv = c + d.n
Where po is the vapour pressure, ΔHv is the enthalpy of vaporisation of the pure solute, n is functional the carbon number, and a, b, and d are constants, which depend on the type of functional group. The vapour pressure of a solute above a solution, such as a liquid stationary phase (SP), is determined by ΔHs the enthalpy of vaporisation, which is not the same as for the pure solute due to molecular interactions, for the solute with molecules of the SP. Hence, it is also determined by the activity coefficient given by
log yi = (ΔHs - ΔHv)/RT + constant
Correspondingly, the boiling point of the members of homologous series increase by a constant value.
Under isothermal conditions a plot of log retention volume, time or k’ against Cn (for n > 4) is linear for a homologous series. The Kovat’s retention index (Iy) system is based primarily on the n-alkane homologous series being given values of 100 x carbon number, i.e., for n- hexane Iy =600. The values for this series are independent of the nature of the SP and all other compounds or series have I values relative to the values for n-alkanes. Shifts in I on different phases, considered relative to the most non-polar SP (squalane or dimethyl silicone gum), can be used to characterise SPs (polarity index) and used to identify compounds from the character of their functional groups and thus polarity (ref: Rohrschneider and McReynolds constants for SP characterisation). For temperature programmed conditions there is linear increase in retention time, tr, with Cn which provides the basis for the linear retention index system and the values for n-alkanes are again set at 100 x Cn.
Linear retention index, I = 100z + 100 {(t)rx - (t)rz / (t)rz+1 - (t)rz} where t is the retention time for solute x, and alkanes with carbon number, z and z+1, which solute x elutes between.
Note: The Kovat’s retention index is determined from the chromatography conducted under isothermal conditions. Under these conditions the relationship between retention time (or partition ratio) and carbon number is logarithmic.
GC Conditions and Experiment
The experiment to be demonstrated and conducted involves the use of cold-on column injection (COC) injection which allows application of the whole sample to the column in the liquid state by employing a capillary-needled syringe (outer diam. 0.17 mm for a 0.32 mm i.d. column). Secondary cooling is required to enable the solution to be applied to the column without evaporation of the solution avoiding evaporation of the solvent. The initial temperature of the column is held slightly above the boiling point of the solvent for a specified time (solvent dwell or delay time) to allow the solvent to be removed. The temperature is then ramped at a specified rate to elute each component according to its vapour pressure and b.pt. With COC injection no discrimination against high b.pt. solutes, i.e., high carbon number n-alkanes (> C30) occurs as for split or splitless injection, which involves flash vaporisation from a heated chamber. This mode of injection provides the most efficient transfer of solutes to the column and potentially is the most appropriate system for quantitative determination of a wide volatility range of solutes (semivolatile organic compounds). The column employed is a 12 or 25 m x 0.32 mm FSOT with a non-polar SP, dimethyl siloxane based, film thickness, df 0.1 μm.
The GC conditions and temperature programme are typically as follows:
• Initial temperature 70o
• Initial time 0 min
• Programme rate 15oC/min
• Final temperature 300oC
• Final time 4 min
Assignment
1. Plot the total retention time, tr, for n-alkanes against 100 x Cn to obtain the graphical relationship from which the linear retention indices for other solutes can be determined.
2. Use the retention data for PAHs to obtain Iy for each PAH. Note a similar but limited plot can be obtained for the series of PAHs using carbon number appropriate to each PAH, e.g., A 10, C 14, D 16 and E 18 (Note: biphenyl is not a member of this series).
3. Comment on the use of retention indices to characterise stationary phases and functionality of organic compounds.
References
- Bishop DF (1980) Report EPA-600012-80-196 Avail NTS.
- Method Team (2016) Method 625.1, December 2016.: Base/Neutrals and Acids by GC/MS. United States Environmental Protection Agency
- SW-846 Test Method 3550C: Ultrasonic Extraction
- Junk G A, Richard JJ, Fritz JS, Calder GV (1974) Use of macroreticular resins in the analysis of water for trace organic contaminants. J Chromatogr 99:745-762.
- Bellar TA, Lichtenberg JJ (1974) Determining Volatile Organics at Microgram- per-Litre Levels by Gas Chromatography. Journal of the American Water Works Association 66:739-744.
- Lee, Hing-Biu, Chau, Alfred SY (1983) Analysis of Pesticide Residues by Chemical Derivatization. VII. Chromatographic Properties of Pentafluorobenzyl Ether Derivatives of Thirty-two Phenols1. J AOAC 66: 1029–1038.
- Lee, Hing-Biu, Weng, Li-Da, Chau, et al. (1984) Chemical Derivatization Analysis of Pesticide Residues. IX. Analysis of Phenol and 21 Chlorinated Phenols in Natural Waters by Formation of Pentafluorobenzyl Ether Derivatives. J AOAC 67:1086-1091.
Indexed at, Google Scholar, Crossref
Citation: Baugh PJ (2022) Priority Pollutant Sample Preparation, Extraction and Clean Up From Spiked Water and Solid Matrices with Internal, Volumetric and Standard Addition for Analysis by GC and GC/EI &NICI-MS. J Anal Bioanal Tech 13: 483.
Copyright: © 2022 Baugh PJ. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Open Access Journals
Article Usage
- Total views: 1971
- [From(publication date): 0-2022 - Oct 30, 2025]
- Breakdown by view type
- HTML page views: 1516
- PDF downloads: 455