Primary Abdominal Wall Clear Cell Carcinoma: Case Report and Review of Literature
Received: 16-Sep-2021 / Accepted Date: 30-Sep-2021 / Published Date: 07-Oct-2021 DOI: 10.4172/2476-2024.1000020
Abstract
Background: Endometriosis occurring in a surgical scar is well recognized and occurs mainly in patients with a history of hysterectomy or Caesarean section. Scar endometriosis, as well as endometriosis at other sites, can undergo malignant change. Clear cell carcinoma of the endometrium is a very rare and highly malignant neoplasm that accounts for less than 5% of endometrial carcinoma.
Case presentation: We report a very rare case of clear cell adenocarcinoma arising from endometriosis of the abdominal scar. We must pay more attention on the following points in the surgical treatment of clear cell carcinoma: (1) Extensively resect tumors as far as possible; (2) Surgical treatment of clear cell carcinoma would easily result in poor wound healing especially in the patients receiving chemotherapy or radiotherapy because of extensive soft tissue stripping .
Conclusion: The patient died only 25 months after she was first diagnosed with the cancer.
Lessons: This case highlights the difficulties in preoperative diagnosis as well as the poor prognosis of these tumors. Accurate diagnosis of a lump within a scar is important to define the prognosis and treatment. These therapeutic principles are increasingly being applied to patients presenting with tumors greater than 5 cm and negative lymph nodes or even smaller tumors, who are considered to have operable disease and a better outcome. Early recognition and prompt treatment can be essential to these patients' survival.
Keywords: Endometriosis; Clear cell carcinoma; Case report; Abdominal wall; Caesarean section
Abbreviations
PET: Positron-Emission Tomography; FDG: Fluorodeoxy Glucose; SUV max: Maximum Standard Uptake Value; CT Scan: Computed Tomography Scanner; Ki67: Antigen Ki-67; Napsin A: Napsin A Aspartic Peptidase; KRAS: KRAS Protooncogene; BRAF: B-Raf Proto-oncogene Serine/Threonine Kinase; ERBB2: erb-b2 Receptor Tyrosine Kinase 2; CTNNB1: Catenin beta 1; PTEN: Phosphatase and Tensin Homolog; PIK3CA: Phosphatidylinositol-4,5-bisphosphate 3-kinase Catalytic Subunit alpha; ARID1A: AT-rich Interaction domain 1A; PPP2R1A: Protein Phosphatase 2 Scaffold Subunit alpha;Ca125: Cancer Antigen 125; Pax-8: Paired Box Gene 8; TP53: Tumor Protein 53; Ki67: Ki67 Antigen; PR: Progesterone Receptor; ER: Estrogen Receptor; HIFU: High-Intensity Focused Ultrasound; CS: Caesarean Section
Background
Abdominal wall endometriosis is one of the major extra pelvic sites and usually is highly related to abdominal surgeries and obstetric and gynecological procedures like a cesarean section, hysterectomy, amniocentesis, tubal ligation, appendectomies, umbilical hernioplasties and laparoscopic trocar tracts. Endometriosis occurring in surgical abdominal scar has been mainly documented after caesarean section (CS) or hysterectomy (0.03% upto 0.4%), and its malignant transformation is very rare: clear cell histology accounts for only 4.5% of extra gonadal endometriosis-associated malignancies, while representing the most common histotype in case of parietal localization [1,2].
We report a very rare case of clear cell adenocarcinoma arising from endometriosis of the scar. However, ovarian cancer is known to develop in 0.3%-1.6% of women with endometriosis, and endometriosis is observed in 4%-29% of patients with ovarian cancer, which suggest the association between endometriosis and ovarian cancer [3]. The majority of carcinomas derived from endometriosis are of endometrioid or clear cell histologic type, in some series accounting for 70% of the subtypes [4].
This suggests that addition of bevacizumab to paclitaxel plus carboplatin for patients with clear cell carcinoma could be a very important treatment strategy [5].
Case Report
Study participants
Here, we report a 43-year-old woman, gravida1, para1 with a history of uneventful caesarean delivery presented with increasing pain and a rapid growing mass in her cesarean section scar. She had no major health problems and her only surgery was a cesarean section for complete placenta previa during her first pregnancy in 2003.
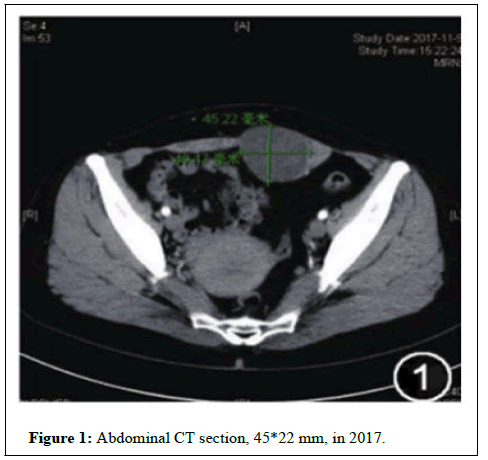
She complained of pain and swelling at the lower end of her abdominal scar during menstruation which started shortly after the surgery. She had not taken hormonal therapy. On physical examination, a large firm mass was palpated in the lower abdominal wall in the region underlying the incision in November 2017. Plain scan and enhanced scan of abdominal CT showed a 49*45 mm lowdensity shadow in the left rectus muscle (Figure 1). Serum carbohydrate antigen 125 was 21.6 Ku/L.
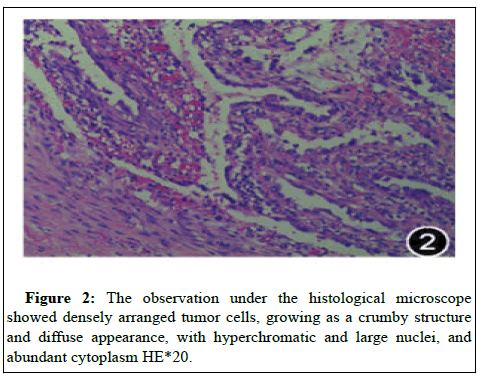
Based on these findings, we made a preoperative diagnosis of scar endometriosis. During the dissection of anterior abdominal wall, lot of fibrosis was observed with a mass of pale colored very adherent to surrounding structures on left rectus sheath involving the rectus muscle as well, it was excised and sent for histopathological analysis. The pathology from specimen showed adenocarcinoma in situ with immunohistochemistry: cytokeratin ++++, Ca125+++, Pax-8+++, TP53+, Ki67 expression 30%+, PR (-), ER (-) and Napsin A (+).
Findings were consistent with clear cell carcinoma of ovarian epithelial cell origin, with endometriosis lesions noted in close proximity of the clear cell adenocarcinoma (Figure 2). Positron emission tomography-computed tomography (PET-CT) examination suggested soft tissue nodules in the left rectus. Increased cortical glucose metabolism was detected by PET-CT studies. The maximum standardized uptake value (SUV max) of the lesion was 3.2 g/ml. Fusion transversal image of the PET/CT showing a soft tissue lesion with moderately increased glucose metabolism (maximum standardized uptake value (SUV max) 9.7 g/ml 47 min post-injection of 175 MBq (FDG) in the left rectus.
The patient underwent hysterectomy and bilateral adnexectomy with pelvic lymph node dissection in December 2017. She was treated with chemotherapy, but the cancer recurred locally In January 2018, and the patient subsequently received multiple agents secondary to progression. The induction chemotherapy consists of four cycles of paclitaxel 135 mg/m2 and carboplatin (area under the curve=6) every 3 three weeks, by continuous intravenous infusion on days 2 to 4 every 3 weeks (Figure 3). After 4 cycles, the patient was reluctant to continue the chemotherapy.
High-intensity focused ultrasound (HIFU) continues to receive increased attention as a therapeutic tool in the treatment of cancer and other tissue abnormalities. She received HIFU, but the tumor continued to grow. Radiotherapy was performed with a total dose of 45Gy plus Anlotinib and the tumor shrank remarkably in November 2018.
A month after radiotherapy, she developed severe intestinal obstruction and had to stop treatment. A physical examination revealed a hard right abdominal mass, and a subsequent CT scan detected a large abdominal tumor, which was suspected to be a clear cell carcinoma in May 2019. The patient underwent abdominal wall tumor resection once again and she continued to take the targeted drug Anlotinib orally. The goal of surgery has to be to achieve complete excision of the tumor with wide (negative) margins (>=2 cm) using frozen section pathology analysis during surgery. Abdominal wall reconstruction was successfully carried out by placing a double-layer synthetic mesh (Vicryl) in a tension-free manner, and the skin was closed over it.
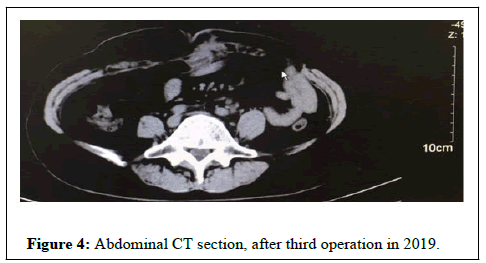
Even though the patient continued with treatment, the tumor recurred at the left surgical site and the tumor size increased (Figure 4). She underwent a third operation.
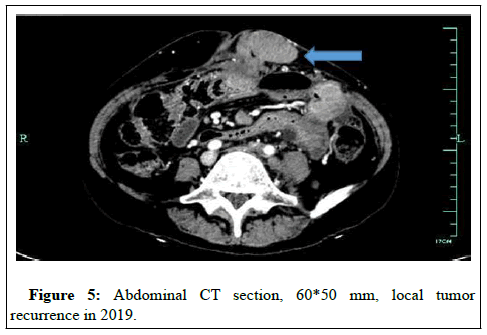
She got a fever; temperature reached highest values of 39. She was admitted to the hospital with a diagnosis of adhesive intestinal obstruction in November 2019. CT scan showed a hypermetabolic abdominal wall mass (60*50 mm), non-hypermetabolic enlarged right pelvic lymph node with no evidence of distant metastases (Figure 5).
Since the patient was rapidly deteriorating palliative care was given. She was admitted to hospital and surgically treated but died of adhesive intestinal obstruction 22 months after the clear cell carcinoma diagnosis.
Discussion
In 1925, Sampson described the first case of malignant transformation of ovarian endometriosis, and defined three criteria for the diagnosis of malignant transformation of endometriosis: (1) Demonstration of both neoplastic tissue and endometriosis within the tumor, (2) histological appearance resembling endometrial stroma surrounding characteristic glands, and (3) no other primary tumor site [6].
However, a mass in the scar tissue with symptoms of cyclic pain synchronously with menses and a positive history of cesarean section may be nearly pathognomonic. Patient in this study met Sampson’s criteria.
Dissemination of endometrial tissue during a cesarean section or uterine surgery is biologically plausible because of the opportunity to inoculate the abdominal wall with endometrial cells from a hysterotomy [7-9].
As shown in , the histological characteristics of malignant transformation in endometriosis of the abdominal wall are primarily represented by clear cell carcinoma. Thirty-five cases of Abdominal Wall Clear Cell Carcinoma have been recorded from 1986 to 2019 . In all cases, patients underwent a cesarean section. Abdominal wall endometriosis describes the involvement of ectopic endometrial tissues superficial to the peritoneum of the abdominal wall, including lesions secondary to a surgical incision and spontaneous lesions [8,10]. The use of a tissue retrieval bag might help to prevent endometriosis implants.
In the case series , the average age was 46.8 years (range 37-60). The interval from cesarean section to the onset of abdominal wall endometriosis was 16.1 years (range 1-37). The median duration of lower abdominal pain lasted for 10 years since cesarean section, and abdominal endometriosis may have already developed during that period. These patients, with surgical birth history (cesarean section) and pelvic endometriosis diagnosed for over 10 years, presented with progressive abdominal pain.
The large number of patients (72%) with the occurrence of symptoms, the advancement of tumors in our patients, metastases to lymph nodes, and one positive margin are probably connected with their short survival period. Patients with positive tumor margins and lymph nodes after tumor resection could be used as independent adverse prognostic factors.
In the present study, univariate analysis suggested that patients with tumors larger than 5 cm had a shorter survival time when compared with patients that had tumors smaller than 5 cm.
On the other hand, some cases were reported to experience relatively longer disease-free and overall survival (longer than 30 months) [11-16]; while recognizing that a more thorough molecular characterization could hopefully help define prognosis of this very rare condition, it has to be acknowledged that cases with better outcome presented with masses ranging between 4 and 9 cm, thus suggesting that a prompt recognition and treatment could make the difference.
In our case, abdominal CT can show a mass lesion involving the abdominal wall, a dilated small bowel loop with hyper enhancing and thickened wall, the involvement of the surrounding tissue. It is extremely difficult to detect early because of its deep location and lack of obvious clinical signs in its early stages. All patients underwent standardized preoperative assessment: routine blood tests and tumor markers CEA and CA19-9 determination, abdominal ultrasound (US), computed assisted tomography (CT) scan, and when needed, Magnetic Resonance Imaging or positron emission tomography (PET-CT). An abdominal CT scan revealed thickening of the abdominal wall and enlargement of the abdominal lymph nodes, but no evidence of distant metastasis in 2019.
Many epidemiologic studies supported a link between endometriosis and invasive epithelial ovarian cancer based on high prevalence and incidence of epithelial ovarian cancer and endometriosis. Unlike epithelial cells, endometriotic stromal cells are mutation free but contain widespread epigenetic defects that alter gene expression and induce a progesterone-resistant and intensely inflammatory environment, driven by estrogen via estrogen receptorbeta. The resulting increased estrogenic action in the stroma drives inflammation and sends paracrine signals to neighboring epithelial cells to enhance proliferation. Clear cell carcinoma of the endometrium is a rare type of endometrial cancer generally associated with an aggressive clinical behavior.
They are generally indolent, present in stage I (tumor confined to the ovary) and are characterized by specific mutations, including KRAS, BRAF, ERBB2, CTNNB1, PTEN PIK3CA, ARID1A, and PPPR1A, which target specific cell signaling pathways. Due to the extremely low incidence of clear cell carcinoma, it is unlikely that randomized clinical trials can be feasible to establish the standard treatment of this disease. Even though an additional abdominal wall tumor resection was performed after 1.5 year from the initial operation, there are still remaining local tumor lesions and they are gradually enlarging in size on a serial follow up CT. She received chemotherapy, including platinum-based regimens as adjuvant therapy, or at the time of disease progression. The patient underwent four courses of tri-weekly chemotherapy with carboplatin and paclitaxel.
Conclusion
In our case, the clear cell carcinoma showed a very aggressive behavior with rapid local recurrence from which the patient survived only 24 months after diagnosis. We present a case of clear cell carcinoma directly arising from scar endometriosis after a cesarean section and review all 35 cases reported. The treating physician should keep in mind abdominal wall endometriosis as a possible cause of post cesarean section scar-related masses. Treatment of cancer patients with conventional therapies (chemotherapy, hormonal therapy, and radiation and Targeted therapy) respond initially well and experience prolonged tumor-free survival. After targeted therapy and radiotherapy, the tumor appeared to shrink significantly. Better understanding of the molecular mechanisms of tumor angiogenesis, especially in clear cell carcinoma, has led to the development of multiple targeted therapies to inhibit key effectors: vascular endothelial growth factor (VEGF), VEGF receptor.
Acknowledgments
None.
Funding
Wenling key subject group of oncology.
Availability of Data and Materials
All data (imaging, pathology, immunohistochemistry, and procedures) are expected to be available.
Authors’ Contributions
J J Zhai is involved in the management of the patient and wrote the case report. C H Chen was instrumental in imaging characterization. R B Ying was instrumental in imaging characterization.
H Y Feng participated in the management of the patient and was instrumental in manuscript writing. All authors read and agreed to the final manuscript.
Competing Interests
The authors state that they have no competing interests.
Consent for publication
We have obtained consent to publish from the permissible relative (husband) of the patient since she passed away before the manuscript planning and writing.
Ethics approval and consent to participate
According to our institutional ethical committee, no explicit protocol is required for a retrospective collection of data.
References
- Ferrandina G, Palluzzi E, Fanfani F, Gentileschi S, Valentini AL, et al. (2016) Endometriosis-associated clear cell carcinoma arising in caesarean section scar: A case report and review of the literature. World J Surg Oncol 14: 300.
- Van Gorp T, Amant F, Neven P, Vergote I, Moerman P (2004) Endometriosis and the development of malignant tumours of the pelvis. A review of literature. Best practice & research Clin Obstet Gynecol 18: 349-371.
- Kim HS, Kim TH, Chung HH, Song YS (2014) Risk and prognosis of ovarian cancer in women with endometriosis: A meta-analysis. Br J Cancer 110: 1878-1890.
- Fargas Fà bregas F, Cusidó Guimferrer M, Tresserra Casas F, Baulies Caballero S, Fábregas Xauradó R (2014) Malignant transformation of abdominal wall endometriosis with lymph node metastasis: Case report and review of literature. Gynecol Oncol Case Rep 8: 10-13.
- Komiyama S, Kato K, Inokuchi Y, Takano H, Matsumoto T, et al (2019) Bevacizumab combined with platinum-taxane chemotherapy as first-line treatment for advanced ovarian cancer: A prospective observational study of safety and efficacy in Japanese patients (JGOG3022 trial). Int J Clin Oncol 24: 103-114.
- Sampson JA (1925) Endometrial carcinoma of the ovary, arising in endometrial tissue in that organ. Archives of Surgery 10: 1-72.
- Horton JD, Dezee KJ, Ahnfeldt EP, Wagner M (2008) Abdominal wall endometriosis: A surgeon's perspective and review of 445 cases. Am J Surg 196: 207-212.
- Ecker AM, Donnellan NM, Shepherd JP, Lee TT (2014) Abdominal wall endometriosis: 12 years of experience at a large academic institution. Am J Obstet Gynecol 211: e361-365.
- Zhang P, Sun Y, Zhang C, Yang Y, Zhang L, et al. (2019) Cesarean scar endometriosis: Presentation of 198 cases and literature review. BMC Womens Health 19: 14.
- Taburiaux L, Pluchino N, Petignat P, Wenger JM (2015) Endometriosis-associated abdominal wall cancer: A poor prognosis? Int J Gynecol Cancer 25: 1633-1638.
- Hitti IF, Glasberg SS, Lubicz S (1990) Clear cell carcinoma arising in extraovarian endometriosis: Report of three cases and review of the literature. Gynecol Oncol 39: 314-320.
- Miller DM, Schouls JJ, Ehlen TG (1998) Clear cell carcinoma arising in extragonadal endometriosis in a caesarean section scar during pregnancy. Gynecol Oncol 70: 127-130.
- Mert I, Semaan A, Kim S, Ali-Fehmi R, Morris RT (2012) Clear cell carcinoma arising in the abdominal wall: Two case reports and literature review. Am J Obstet Gynecol 207: e7-9.
- Sosa-Durán EE, Aboharp-Hasan Z, Mendoza-Morales RC, GarcÃa-RodrÃguez FM, Jiménez-Villanueva X, et al. (2016) Clear cell adenocarcinoma arising from abdominal wall endometriosis. Cirugia y cirujanos 84: 245-249.
- Marques C, Silva TS, Dias MF (2017) Clear cell carcinoma arising from abdominal wall endometriosis-Brief report and review of the literature. Gynecol Oncol Rep 20: 78-80.
- Lai YL, Hsu HC, Kuo KT, Chen YL, Chen CA, et al. (2019) Clear cell carcinoma of the abdominal wall as a rare complication of general obstetric and gynecologic surgeries: 15 years of experience at a large academic institution. Int J Environ Res Public Health 16.
- Schnieber D, Wagner-Kolb D (1986) Malignant transformation of extragenital endometriosis. Geburtshilfe und Frauenheilkunde 46: 658-659.
- Park SW, Hong SM, Wu HG, Ha SW (1999) Clear cell carcinoma arising in a Cesarean section scar endometriosis: A case report. J Korean Med Sci 14: 217-219.
- Ishida GM, Motoyama T, Watanabe T, Emura I (2003) Clear cell carcinoma arising in a cesarean section scar. Report of a case with fine needle aspiration cytology. Acta cytologica 47: 1095-1098.
- Sergent F, Baron M, Le Cornec JB, Scotté M, Mace P, et al. (2006) Malignant transformation of abdominal wall endometriosis: a new case report. J Gynecol Obstet Biol Reprod (Paris) 35: 186-190.
- Alberto VO, Lynch M, Labbei FN, Jeffers M (2006) Primary abdominal wall clear cell carcinoma arising in a Caesarean section scar endometriosis. Ir J Med Sci 175: 69-71.
- Razzouk K, Roman H, Chanavaz-Lacheray I, Scotté M, Verspyck E, et al. (2007) Mixed clear cell and endometrioid carcinoma arising in parietal endometriosis. Gynecol Obstet Invest 63: 140-142.
- Rust MM, Susa J, Naylor R, Cavuoti D (2008) Clear cell carcinoma in a background of endometriosis. Case report of a finding in a midline abdominal scar 5 years after a total abdominal hysterectomy. Acta cytologica 52: 475-480.
- Bats AS, Zafrani Y, Pautier P, Duvillard P, Morice P (2008) Malignant transformation of abdominal wall endometriosis to clear cell carcinoma: Case report and review of the literature. Fertility and sterility 90: e1113-1196.
- Williams C, Petignat P, Belisle A, Drouin P (2009) Primary abdominal wall clear cell carcinoma: Case report and review of literature. Anticancer Res 29: 1591-1593.
- Bourdel N, Durand M, Gimbergues P, Dauplat J, Canis M (2010) Exclusive nodal recurrence after treatment of degenerated parietal endometriosis. Fertility and sterility 93: e2071-2076.
- Yan Y, Li L, Guo J, Zheng Y, Liu Q (2011) Malignant transformation of an endometriotic lesion derived from an abdominal wall scar. Int J Gynaecol Obstet 115: 202-203.
- Li X, Yang J, Cao D, Lang J, Chen J, et al. (2012) Clear-cell carcinoma of the abdominal wall after cesarean delivery. Obstet Gynecol 120: 445-448.
- Shalin SC, Haws AL, Carter DG, Zarrin-Khameh N (2012) Clear cell adenocarcinoma arising from endometriosis in abdominal wall cesarean section scar: A case report and review of the literature. J Cutan Pathol 39: 1035-1041.
- Ijichi S, Mori T, Suganuma I, Yamamoto T, Matsushima H, et al. (2014) Clear cell carcinoma arising from cesarean section scar endometriosis: Case report and review of the literature. Case Rep Obstet Gynecol 2014: 642483.
- Heller DS, Houck K, Lee ES, Granick MS (2014) Clear cell adenocarcinoma of the abdominal wall: A case report. J Reprod Med 59: 330-332.
- Aust S, Tiringer D, Grimm C, Stani J, Langer M (2015) Therapy of a clear cell adenocarcinoma of unknown primary arising in the abdominal wall after cesarean section and after hysterectomy. Wiener klinische Wochenschrift 127: 62-64.
- Liu H, Leng J, Lang J, Cui Q (2014) Clear cell carcinoma arising from abdominal wall endometriosis: A unique case with bladder and lymph node metastasis. World J Surg Oncol 12: 51.
- Graur F, Mois E, Elisei R, Furcea L, Dragota M, et al. (2017) Malignant endometriosis of the abdominal wall. Annali italiani di chirurgia 6.
- Gentile JKA, Migliore R, Kistenmacker FJN, Oliveira MM, Garcia RB, et al. (2018) Malignant transformation of abdominal wall endometriosis to clear cell carcinoma: Case report. Sao Paulo Med J 136: 586-590.
- Yoshida S, Onogi A, Kuwahara M, Uchiyama T, Kobayashi H (2018) Clear cell adenocarcinoma arising from endometriosis in the groin: Wide resection and reconstruction with a fascia lata tensor muscle skin flap. Case Rep Obstet Gynecol 2018: 2139595.
- Tsuruga T, Hirata T, Akiyama I, Matsumoto Y, Oda K, et al. (2019) Mixed endometrioid and clear cell carcinoma arising from laparoscopic trocar site endometriosis. J Obstet Gynaecol 45: 1613-1618.
Citation: Zhai JJ, Feng HY, Ying RB, Chen CH (2021) Primary Abdominal Wall Clear Cell Carcinoma: Case Report and Review of Literature. Diagn Pathol Open S6: 020. DOI: 10.4172/2476-2024.1000020
Copyright: © 2021 Zhai JJ, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 2320
- [From(publication date): 0-2021 - Nov 29, 2025]
- Breakdown by view type
- HTML page views: 1577
- PDF downloads: 743