PRF in Oral Surgery: A Literature Review
Received: 05-Oct-2016 / Accepted Date: 23-Nov-2016 / Published Date: 01-Dec-2016
Abstract
The research of adjuvant surgical to promote the healing is a challenge. A lot of processes were done especially the PRF (platelet rich fibrin ) that owes its action to its slow polymerisation which permits to pick up the growth factors inside the fibrin mesh. These factors and due to a slow release, permit a local stimulation of the healing time as well. Nowdays, with the use of the PRF, the oral surgery undergoes a lot of controversies. The clinical observations are in advance in regard to the scientific evidence. In spite of the encouraging results, research are to be deepen to get a complet scientific validity.
Keywords: Platelet-rich fibrin; Dentistry; Literature review
79046Introduction
The oral surgery is confronted to a diversification of interventions that leads to new requirements both for the patient and the practitioner as well. Also, the improvement of the operative suites and the decrease of the healing time. In fact the mechanismes that control the healing are complisated and some aspects remain unknown [1]. But a well understanding of the growth factors role helped to elaborate new bioactive materials capable of guiding and promoting the healing [2]. On the basis, platelet concentrates were used in the prevention and the bleeding treatment,especially in the case of the thrombopénies, leukemia. Initially, the first device which was known to have intersting properties on the healing made fibrin adhesives in the 70s [3]. However, because the risks of transmission linked to sanguine products, the research leads to the concentrated platelet autologous. A lot of procedures were created: the first generation, the PRP “Platelet rich plasma” then another family of platelet concentrate appeared “Platelet rich fibrin” (PRF) based on a matrix of fibrin [1,3]. The potentials applications of PRF are numerous which gives new perspectives of treatment in oral surgery. This work suggests discussing the role and contribution of PRF in the Oral surgery based on literature Data.
What is PRF?
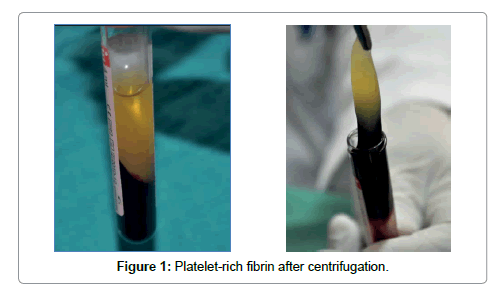
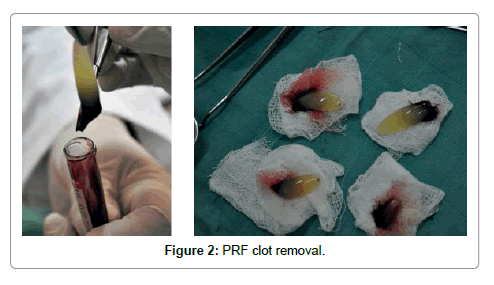
Platelet-rich fibrin (PRF) described by Choukroun et al. [1] is a second-generation platelet concentrate which contains platelets and growth factors in the form of fibrin membranes prepared from the patient’s own blood free of any anticoagulant or other artificial biochemical modifications [1,4]. PRF prolongs the effects of typical physiologic wound healing. This provides a condensed network of fibrin that is saturated with cytokines, growth factors during 1 to 4. PRF speeds up the healing process and also optimizes bone grafting results [5,6]. It is capable of generating both soft tissue and bone and can be used in conjunction with either a bone substitute or alone [7-10]. The PRF procedure is a simple one beginning with drawing a patient’s blood and placing it in a centrifuge for 10 minutes without the addition of an anticoagulant. During the centrifuge process, the blood coagulates and separates into three distinct layers. The bottom layer is a red blood cell (RBC) layer that is removed and discarded while the top layer encompasses a cell free layer that is also unused. The middle layer is a mesh network which contains the majority of the platelets and fibrin (Figures 1 and 2) [1]. Once seperated from the clot, the PRF may be withdrawed. This layer can be compressed into a membrane or shaped into a plug depending on what treatment is needed [1].
PRF and Periodontal Regeneration
The aim of periodontal therapy is to arrest and control the periodontal infection and to regenerate all tissues of the periodontium (periodontal ligament, bone, cementum, and connective tissue) [11]. Currently, the local application of growth factors, has been investigated for use in the promotion of periodontal regeneration and healing. These agents act by augmenting the anabolic bone formation, angiogenesis, cementogenesis, osteoblast differentiation, mitosis, chemotaxis, and other processes that improve the healing environment. The use of PRF in the treatment of intrabony defects has shown significant clinical benefits when compared with open flap debridement alone [12-15]. Yuchao showed that the use of PRF as the sole grafting material seems to be an effective modality of regenerative treatment for periodontal intrabony defects [16]. Thorat had compared the clinical and radiological effectiveness of autologous PRF gel in the treatment of intra-bony defects of chronic periodontitis patients with conventional periodontal flap surgery alone [12]. He showed a significant reduction in probing depth and clinical attachment level gain both groups (test and control group) when compared with baseline and 9 months. However, there was more probing depth reduction (4.56 ± 0.37) and gain of clinical attachment (3.69 ± 0.44) in the PRF treated group. The percentage of intra bony defect fill in the PRF group (46.92%) was higher than the conventionally treated subjects (28.66%); suggesting that the various growth factors present in the PRF may enhance regeneration [13]. Also, Panda showed that the use of PRF can be effective as a sole regenerative material, in combination with open flap debridement [15]. Lekovic et al. [13] evaluated the effectiveness of PRF membrane in promoting clinical signs of periodontal regeneration in human intra-bony defects. They suggest that treatment of intra-bony defects with PRF results in significant improvements of pocket depth, clinical attachment level and defect compared with conventionally treatement [13]. Pradeep concluded that PRF and PRP were superior in the treatment of intra bony defects than open flap debridement alone [14]. PRF has great potential for surgical wound healing and can be used in surgical treatment of intra-bony defects. Further studies are necessary to assess the histology of the regenerated tissue while using PRF.
PRF and Sinus Lift
A sinus floor elevation is a technique to increase the residual bone height of the posterior edentulous maxilla [17]. Sinus augmentation with autogenously bone grafts by the lateral window technique was reported by Boyne and James in the 1980s and developed by Tatum [18]. Today, the protocol has evolved a lot not only on technical aspect but also by the launch of biomaterials. Various bone substitutes are usable in this clinical situation: autogenous bone, graft, xenogeneic, allogeneic, and some artificial materials. Recently, the possibility of sinus-lift without any grafted material was reported; authors have shown that a full sinus-lift can be performed using the lateral approach with PRF as sole filling material [19-23]. PRF can be used in two ways, either as fragments mixed with different bone substitutes or as a sole filling material [21,22,24-26]. In Mazor [20] and Simonpieri [21] studies, one or two PRF membranes were placed on the sinus membrane and osteotomy window as a sole filling material and no grafting material was used to fill the created space. Implants were placed spontaneously with sinus lift and serve as tent pegs. Tent pegs technique based on guided bone regeneration as implants are placed immediately with sinus lift [21,22,24]. Tajima placed also PRF clots, no membranes, as a sole filling material [25]. No complications were observed during the healing period. While only Simon Pieri [21] study provided a long term follow up (2-6 years). Mazor [20] and Tajima [25] studies provided only a 6 month follow up period [23-25]. This analysis revealed that all studies [23-25] were case series with no control group to prove benefits gained from the use of PRF to fill sinus instead of natural blood clot.
The PRF fragments can be used with different bone substitutes. Choukroun et al. [27] studied the combination of PRF fragments with demineralized freeze dried bone allograft DFDBA; They showed an equivalent new bone formation for PRF/DFDBA mixture after 4 months and DFDBA alone after 8 months.
This analysis revealed that the use of PRF with DFDBA as filing material in sinus lift showed optimistic results. It accelarated bone regeneration, reduce maturation time of DFDBA and allow implant placement after only 4 months rather than 8 months of healing.
However, the use of PRF with DFDB doesn’t provide any additive value for the volume stability of the graft and implant survival [27].
The new bone formationin of choukroun study was lower than that reported by Kolerman study which used mineralized freeze-dried bone allograft (FDBA) [28].
Inchingolo [29], Zhang [30], Tatullo [31] and Bolukbasi [32] studies used PRF fragments with xenograft mixture. In Inchingolo et al. study implants were placed immediately, PRF/xenograft mixture was used as a filling material in all sinuses and PRF membranes were placed on the sinus membrane and osteotomy window. They reported an average increase in the peri-implant bone density of 31% after 6 months [29].
Zhang [30], Tatullo [31] and Bolukbasi [32] studies used PRF/ xenograft mixture for the test group and xenograft alone for the control group with implants placed at the second stage surgery. Zhang [30] and Tatullo [31] reported presence of mineralized tissue adequate in amount and density, well integrated with the residual bone, in all cases. Bolukbasi et al. showed statistically signifiance lower resorption for PRF/Bio-oss group at areas of implant placement [32]. Only limited randomized controlled clinical trials evaluate the use of PRF in sinus floor elevation as a sole filling material or with bone substitutes. Further studies are needed to validate this treatment strategies [33].
PRF and Regeneration of Peri-Implant Bone Defects
Platelet concentrates may not be relevant to improve osseo integration in normal conditions, but they may help for the regeneration of peri-implant bone defects [34]. Three specific situations can be encountered. The first situation concerns conceins the peri-implantitis also called deosseo-integration. The second are provoked during implant placement, when the initial bone volume for implantation is not large enough for the support of implants [34]. The last kinds of peri-implant bone defects can be encountered during an immediate post-avulsion or post-extraction implantation procedure [35]. There have been few reports about a graft using PRF alone for peri-implant bone defects [36]. Lee et al. demonstrated, in animal model, that periimplant defect sized 3.0 × 5.0 mm (width × length) was successfully repaired by the application of PRF alone in the bony defect [37]. Only limited in vitro studies have been carried out on the effects of PRF on regeneration of peri-implant bone defects. There is a need for further studies to determine the behavior of PRF applied for use in criticalsized bone defects in humans.
PRF and Gingival Recession
The root coverage procedures aim at covering the exposed surface to enchance esthetics, relieve hypersensitivity as well as difficulties to maintain an optimal bucco-dental hygiene.
Coronally advanced flap procedure, with subepithelial connective tissue is the most predictive plastic procedure. Recently, in order to improve the efficiency of the root coverage treatments and reduce the morbidity of the techniques (second surgical donor site…), various alternative are used such as the platelet rich fibrin (PRF) replacing the connective tissue graft. According to Aroca [38] PRF membrane increased gain in width of keratinized gingiva at the test sites at 6 months compared to the modified coronally advanced flap alone. In Jankovic study, the use of PRF membrane in gingival recession treatment provided acceptable clinical results at 6 months compared to connective tissue graft (CTG) treated gingival recessions. No difference could be found between PRF and CTG procedures in gingival recession’s therapy, except for greater gain in keratinized tissue [39].
Kumar [40] demonstrated that the autogenous platelet concentrate graft (PCG) or subepithelial connective tissue graft (SCTG), covered by a coronally positioned flap, were effective in the treatment of shallow gingival recession defects (≥ 2 mm) with significant root coverage (87% and 80% for SCTG and PCG, respectively) at 12 months postoperatively. The clinical implications and advantages of PRF membrane as graft material are related to the avoidance of a donor site surgical procedure and a major decrease in patient discomfort during the early wound healing period [40]. PRF can be considered as a viable cost effective option. Further studies are necessary to assess the histology of the regenerated tissue.
Conclusion
Dental treatment options are expanding and new techniques are constantly being developed. Present treatment modalities can assist with the stimulation of tissue formation after dental surgical procedures, leading to various results. The general concept of bone regeneration is to promote the simultaneous regeneration of a bone volume and the gingival tissue above through the use of PRF. The placement of PRF provides enhanced healing following specific dental procedures using the patient’s donor tissue. PRF is an advantageous technique that provides optimal results when used in conjunction with many dental procedures. Further research are needed to validate these treatment strategies in evidence-based clinical practice.
Conflicts of Interest
The authors report no conflicts of interest related to this review.
References
- Dohan DM, Choukroun J, Diss A, Dohan SL, DoÂhan AJ, et al. (2006) Platelet-rich fiÂbrin (PRF): a second-generation platelet conÂcentrate, part I: technological concepts and evolution. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 101: e37-e44.
- Gassling VL, Açil Y, Springer IN, Hubert N, Wiltfang J (2009) Platelet-rich plasma and platelet-rich fibrin in human cell culture. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 108: 48-55.
- Kiran NK, Mukunda KS, Tilak Raj TN (2011) PlateÂlet concentrates: A promising innovation in dentistry. J Dent Sci Res 2: 50-61.
- Gupta V, Bains BK, Singh GP, Mathur A, Bains R (2011) Regenerative potential of platelet rich fibrin in dentistry: Literature review. Asian J Oral Health Allied Sci 1: 22-28.
- Kawase T, Kamiya M, Kobayashi M, Tanaka T, Okuda K, et al. (2015) The heat-comÂpression technique for the conversion of plateÂlet-rich fibrin preparation to a barrier mem Platelet-rich fibrin. Int J ClinExp Med 8: 7922-7929.
- Wu CL, Lee SS, Tsai CH, Lu KH, Zhao JH, et al. (2012) Platelet-rich fibrin increases cell atÂtachment, proliferation and collagen-related protein expression of human osteoblasts. Aust Dent 57: 207-212.
- Saluja H, Dehane V, Mahindra U (2011) Platelet-Rich fibrin: A second generation platelet conÂcentrate and a new friend of oral and maxilloÂfacial surgeons. Ann Maxillo Fac Surg 1: 53-57.
- BölükbaÃ…?i N, Ersanli S, KeklikoÄ?lu N, BaÃ…?eÄ?mez C, Ozdemir T (2015) Sinus augmentation with plateÂlet-rich fibrin in combination with bovine bone graft versus bovine bone graft in combination with collagen membrane. J Oral Implantol 41: 586-595.
- Joseph VR, Sam G, Amol NV (2014) Clinical evaluÂation of autologous platelet rich fibrin in horiÂzontal alveolar bony defects. J Clin Diagn Res 8: 43-47.
- Kim TH, Kim SH, Sándor GK, Kim YD (2014) ComÂparison of platelet-rich plasma (PRP), platelet-rich fibrin (PRF), and concentrated growth facÂtor (CGF) in rabbit-skull defect healing. Arch Oral Biol 59: 550-558.
- Cortellini P, Tonetti MS, Lang NP, Lindhe J (2008) Regenerative periodontal therapy. Clinical Periodontology and Implant Dentistry (5th edn.). Forlaget Munksgaard, Copenhagen. pp: 902–954.
- Thorat M, Pradeep AR, Pallavi B (2011) Clinical effect of autologous platelet rich fibrin in the treatment of intra-bony defects: a controlled clinical trial. J Clin Periodontol 38: 925–932
- Lekovic V, Milinkovic I, Aleksic Z, Jankovic P, Kenney EB, et al. (2012) Platelet-rich fibrin and bovine porous bone mineral vs. platelet-rich fibrin in the treatment of intrabony periodontal defects. J Periodontal Res 47: 409–417.
- Pradeep AR, Rao NS, Agarwal E, Bajaj P, Kumari M, et al. (2012) Comparative evaluation of autologous platelet-rich fibrin and platelet-rich plasma in the treatment of 3-wall intrabony defects in chronic periodontitis: a randomized controlled clinical trial. J Periodontol 83: 1499–1507.
- Panda S, Sankari M, Satpathy A, Jaykumar D, Mozzati M, et al. (2016) Adjunctive effect of autologus Platelet-Rich Fibrin to barrier membrane in the treatment of periodontal intrabony defects. J Craniofac Surg 27: 691-696.
- Yu-Chao C, Kuo-Chin W, Jiing-Huei Z (2011) Clinical application of platelet-rich fibrin as the sole grafting material in periodontal intrabony defects. J Dent Sci 6: 181-188.
- Ridell A, Gröndahl K, Sennerby L (2009) Placement of Brånemark implants in the maxillarytube region: Anatomical considerations, surgical technique and long term results. Cli Oral Implants Res 20: 94-98.
- Renouard F, Nisand D (2005) Short implants in the severely resorbed maxilla: A 2-year retrospective study. Clin Implant Dent Relat Res 7: S104-S110.
- Choukroun J, Diss A, Simonpieri A, Girard MO, Schoeffler C, et al. (2006) Platelet-rich fibrin (PRF): a second generation platelet concentrate. Part V: histologic evaluations of PRF effects on bone allograft maturation in sinus lift. Oral Oral Med Oral Pathol Oral Radiol Endod 101: 299-303.
- Mazor Z, Horowitz RA, Del Corso M, Prasad HS, Rohrer MD, et al. (2009) Sinus floor augmentation with simultaneous implant placement using Choukroun's platelet-rich fibrin as the sole grafting material: a radiologic and histologic study at 6 months. J Periodontol 80: 2056-2064.
- Simonpieri A, Choukroun J, Del Corso M, Sammartino G, Dohan Ehrenfest DM (2011) Simultaneous sinus-lift and implantation using micro threaded implants and leukocyte- and platelet rich fibrin as sole grafting material: a six year experience. Implant Dent 20: 2-12.
- Kim BJ, Kwon TK, Baek HS, Hwang DS, Kim CH, et al. (2012) A comparative study of the effectiveness of sinus bone grafting with recombinant human bone morphogenetic protein 2-coated tricalcium phosphate and platelet-rich fibrin-mixed tricalcium phosphate in rabbits. Oral Surg Oral Med Oral Pathol Oral Radiol 113: 583-592.
- Zhang Y, Tangl S, Huber CD, Lin Y, Qiu L, et al. (2012) Effects of Choukroun's platelet-rich fibrin on bone regeneration in combination with deproteinizedbovinebone mineral in maxillary sinus augmentation: a histological and histomorphometricstudy. J Craniomaxillofac Surg 40: 321-328.
- Ali S, Bakry SA, Abd-Elhakam (2015) Platelet-rich fibrin in maxillary sinus augmentation: A systematic review. J Oral Implantol 41: 746-753.
- Tajima N, Ohba S, Sawase T, Asahina I (2013) Evaluation of sinus floor augmentation with simultaneous implant placement using platelet-rich fibrin as sole grafting material. Int J Oral Maxillofac Implants 28: 77-83.
- Chiapasco M, Casentini P, Zaniboni M (2009) Bone augmentation procedures in implant dentistry. Int J Oral Maxillofac Implants 24: 237-259.
- Choukroun J, Diss A, Simonpieri A, Girard MO, Schoeffler C, et al. (2006) Platelet-rich fibrin (PRF): A second generation platelet concentrate. Part V: Histologic evaluations of PRF effects onbone allograft maturation in sinus lift. Oral Surg Oral Med Oral Pathol Oral Radiol Endod Mar 101: 299-303.
- Kolerman R, Tal H, Moses O (2008) Histomorphometric analysis of newly formed bone after maxillary sinus floor augmentation using ground cortical bone allograft and internal collagen membrane. J Periodontol 79: 2104-2111.
- Inchingolo F, Tatullo M, Marrelli M, Inchingolo AM, Scacco S, et al. (2010) Trial with platelet-rich fibrin and Bio-Oss used as grafting materials in the treatment of the severe maxillar bone atrophy: clinical and radiological evaluations. Eur Rev Med Pharmacol Sci 14: 1075-1084
- Zhang Y, Tangl S, Huber CD, Lin Y, Qiu L, et al. (2012) Effects of Choukroun’s platelet-rich fibrin on bone regeneration in combination with deproteinized bovine bone mineral in maxillary sinus augmentation: A histological and histomorphometric study. J Craniomaxillofac Surg 40: 321-328.
- Tatullo M, Marrelli M, Cassetta M, Pacifici A, Stefanelli LV, et al. (2012) Platelet Rich Fibrin (P.R.F.) in reconstructive surgery of atrophied maxillary bones: clinical and histological evaluations. Int J Med Sci 9: 872-880.
- Bolukbasi N, Ersanli S, Keklikoglu N, Basegmez C, Ozdemir T (2015) Sinus augmentation with platelet-rich fibrin in combination with bovine bone graft versus bovine bone graft in combination with collagen membrane. J Oral Implantol 41: 586-595.
- Simonpieri A, Corso MD, Vervelle A, Jimbo R (2012) Current knowledge and perspectives for the use of platelet-rich plasma (PRP) and platelet-rich fibrin (PRF) in oral and maxillofacial surgery part 2: bone graft, implant and reconstructive surgery. Curr Pharm Biotechnol 13: 1231-1256.
- Mouhyi J, Dohan E, Albrektsson T (2012) The peri-implantitis: implantsurfaces, microstructure, and physico-chemical aspects. Clin Implant Dent Relat Res 14: 170-183.
- Dohan E, Vazquez L (2008) Pulling out, extraction or avulsion? Implant Dent 17: 4.
- Jang ES, Park JW, Kweon H, Lee KG, Kang SW, et al. (2010) Restoration of peri-implant defects in immediate implant installations by Choukroun platelet-rich fibrin and silk fibroin powder combination graft. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 109: 831-836.
- Lee JW, Kim SG, Kim JY, Lee YC, Choi JY, et al. (2012) Restoration of a peri-implant defect by platelet-rich fibrin. Oral Surg Oral Med Oral Pathol Oral Radiol 113: 459-463.
- Aroca S, Keglevich T, Barbieri B, Geera I, Etienne D (2009) Clinical evaluation of a modified coronally advanced flap alone or in combination with a platelet rich fibrin membrane for the treatment of adjacent multiple gingival recessions: a 6 month study. J Periodontol 80: 244-2452.
- Jankovic S, Aleksic Z, Klokkevold P, Lekovic V, Dimitrijevic B, et al. (2012) Use of platelet-rich fibrin membrane following treatment of gingival recession: a randomized clinical trial. Int J Periodontics Restorative Dent 32: 41-50.
- Kumar G, Murthy K (2013) A comparative evaluation of sub epithelial connective tissue graft (SCTG) versus platelet concentrate graft (PCG) in the treatment of gingival recession using coronally advanced flap technique: a 12-month study. J Indian Soc Periodontol 17: 771-776.
Citation: Hamdoun R, Ennibi OK, Ismaili Z (2016) PRF in Oral Surgery: A Literature Review. J Med Imp Surg 1: 110.
Copyright: © 2016 Hamdoun R, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Conferences
42nd Global Conference on Nursing Care & Patient Safety
Toronto, CanadaRecommended Journals
Open Access Journals
Article Usage
- Total views: 15979
- [From(publication date): 0-2016 - Apr 04, 2025]
- Breakdown by view type
- HTML page views: 14566
- PDF downloads: 1413