Preventing Carcinogenesis with Compounds that Inhibit Cytochrome P450 1A1 and 1B1
Received: 12-Mar-2018 / Accepted Date: 21-Mar-2018 / Published Date: 29-Mar-2018 DOI: 10.4172/2168-9652.1000230
Abstract
Cytochrome P450 1A1 and 1B1 convert polycyclic aromatic hydrocarbons to carcinogenic compounds in extrahepatic tissues. In 1997, resveratrol, a naturally occurring stilbene found in grapes and red wine, was found to inhibit the growth of tumors and that one potential mechanism was by inhibiting cytochrome P450 1A1 and 1B1. This prompted further research on various stilbene derivatives as chemopreventive agents. It has recently been discovered that aryl morpholino triazenes represent a new class of cytochrome P450 1A1 and 1B1 inhibitors opening up the field to explore another type of potential chemopreventive agent.
Keywords: Triazenes; Chemopreventive; Stilbenes; Cytochrome P450 1A1; Cytochrome P450 1B1
Abbreviations
NCI: National Cancer Institute; CYP1A1: Cytochrome P450 1A1; CYP1B1: Cytochrome P450 1B1; PAHs: Polycyclic Aromatic Hydrocarbons; HMG CoA reductase: 3-Hydroxy- 3-Methyl-Glutaryl-Coenzyme A reductase
Introduction
The National Cancer Institute estimates that there were 14 million new cases of cancer and 8.2 million cancer deaths worldwide in 2012, the most recent year for which we have global cancer statistics. Additionally, the NCI projects that the incidence of cancer will increase 50% and the deaths from cancer will increase 60% by 2030, making cancer one of the leading causes of death across the globe [1]. It has thus become increasingly important for scientists and medical professionals to find more effective ways of preventing and treating various types of cancer to increase people’s life spans and increase their quality of life by sparing them the pain of going through cancer treatments such as surgery, chemotherapy and radiation and the resulting recovery periods.
Tumor formation in a variety of tissues can be stimulated by the hyperactivity and/or overexpression of a variety of different proteins including those involved in signal transduction pathways such as epidermal growth factor [2,3] and RAS [4], those involved in inflammation like cyclooxygenase 1 and 2 [5,6] and other proteins such as HMG CoA reductase [7] and cytochrome P450 1A1 and 1B1 [8- 10]. In addition, tumors can also form because of the hypoactivity of proteins involved in apoptosis or tumor suppression such as p53 [11,12].
The focus of this review will be on the role of cytochrome P450 1A1 and 1B1 in cancer and the variety of compounds that have been discovered to inhibit these enzymes showing potential chemopreventive activity.
Cytochrome P450 1A1 and 1B1
Cytochrome P450 is a superfamily of heme containing enzymes that oxidize other compounds and function within various electron transfer chains. In fact 57 human cytochrome 450 genes and 58 pseudogenes have been identified by the Human Genome Project [13]. CYP1A1 and CYP1B1 are two members of this superfamily of enzymes, both of which are extrahepatic enzymes that catalyze the mono-oxidation of a variety of different endogenous compounds. For example, some CYP1A1 substrates include Vitamin D [14], 17β-estradiol, melatonin and arachidonic acid [15], while CYP1B1 acts upon 17β-estradiol [16]. Both enzymes also catalyze reactions with a variety of xenobiotic compounds such as PAHs [10,15,17], which are ubiquitous in our environment and can be found in soot, emissions from forest fires, fossil fuel processing, cigarette smoke, automobile exhaust, charred meat cooked over an open flame, etc. [18-20].
CYP1A1 and CYP1B1 have been the focus of many recent research studies because of their ability to generate carcinogenic compounds from procarcinogens like PAHs [10,15,21-23]. First the PAHs are oxidized by CYP1A1 and CYP1B1 to epoxides, which are converted to diols by epoxide hydroxylase and finally into epoxydiols by CYP1A1 and CYP1B1 [8,10,23,24]. The epoxydiols can then modify the amine groups in adenosine or guanosine residues in DNA leading to errors during replication [19,25,26]. If the mutations are located in genes associated with signal transduction or other pathways that either stimulate or inhibit cell division this can cause the uncontrolled cell division that leads to cancer. As a result, many research groups are intent on finding compounds that can inhibit these two enzymes as a means of preventing cancer.
While CYP1A1 and CYP1B1 only share 38% homology between them, the active sites in both enzymes are narrow, flat and hydrophobic, which are a good fit for planar, aromatic compounds such as PAHs [15,17] and many of the early compounds used to probe and study the active site: α-napthoflavone, 7-ethoxycoumarin, 7-ethoxyresorufin and ellipticine [17,27]. In addition, many of these compounds have been shown, through x-ray crystallography or molecular docking studies, to interact with a variety of phenylalanine residues in the active site of both enzymes through hydrophobic and π-π interactions [15,17,28,29].
Resveratrol and other stilbenes
Interest in CYP1A1 and CYP1B1 exploded in the early 2000s when in 1997 it was discovered that resveratrol, a compound derived from grapes and found in significant amounts in red wine, was a chemopreventive agent against promyelocytic leukemia (HL-60) cells, a mouse skin cancer model and other cells and tissues and this chemopreventive activity was further verified by others [6,30-32]. In 1999, Chun et al. [33] discovered that resveratrol inhibited CYP1A1 and that this was one likely mechanism for inhibiting the initiation and development of cancer based upon studies showing this enzyme’s involvement in tumor formation as previously stated. Subsequently, resveratrol was also discovered to inhibit CYP1B1 providing another possible mechanism by which resveratrol exerts its anti-cancer effects [34].
Because resveratrol is a type of stilbene, various stilbene derivatives were shown to either prevent or reduce tumor formation in a variety of cell lines, tissues and animal models such as colon cancer, breast cancer cells and human SK-Mel-28 melanoma cells [30,32,35-39]. Furthermore a variety of these stilbenes were proven to be more effective than resveratrol at inhibiting CYP1A1 and CYP1B1 (Table 1). In particular, the methoxystilbenes have been some of the most potent inhibitors of both enzymes with some being more selective towards one enzyme over the other. Resveratrol has an IC50 of 23 μM with respect to CYP1A1 and an IC50 of 1.4 μM with respect to CYP1B1 [40]. The monomethoxy substituted stilbenes, rhapontigenin and 3,5-dihydroxy-4’-methoxystilbene, inhibit CYP1A1 with an IC50 of 0.4 μM each while rhapontigenin has an IC50 of 9 μM and 3,5-dihydroxy- 4’-methoxystilbene has an IC50 of 1 μM both with respect to CYP1B1 [41]. Thus, both monomethoxy substituted stilbenes inhibit CYP1A1 more strongly than resveratrol and CYP1B1. While rhapontigenin is less effective at inhibiting CYP1B1 than resveratrol, 3,5-dihydroxy-4’- methoxystilbene is more effective than resveratrol.
| Stilbenes | CYP1A1 | CYP1B1 |
|---|---|---|
| IC50 | IC50 | |
| µM | µM | |
| Resveratrol | 23a | 1.4a |
| Rhapontigenin | 0.4b | 9b |
| 3,5-dihydroxy-4’-methoxystilbene | 0.4b | 1b |
| 3,4’-dimethoxy-5-hydroxystilbene | 0.1b | 0.1b |
| 3,4’,5-trimethoxystilbene | 0.6b | 0.4b |
| 3,4,2’-trimethoxy-trans-stilbene | 0.36c | 0.0040c |
| 3,4,2’5’-tetramethoxystilbene | 0.5c | 0.62c |
| 2,2’,4,6’-tetramethoxystilbene | 0.170d | 0.002d |
| 2,4,3’,5’-tetramethoxystilbene | 0.300e | 0.006e |
| 3,5-dimethoxy-2’-thiophenylstilbene | 0.061e | 0.011e |
Table 1: IC50 values for various stilbenes
adata taken from [40]
bdata taken from [41]
cdata taken from [42]
ddata taken from [43]
edata taken from [44].
Furthermore, many dimethoxy, trimethoxy and tetramethoxy substituted stilbenes are more effective than resveratrol at inhibiting CYP1A1 and CYP1B1 and are about equally effective at inhibiting both of these enzymes. For example, 3,4’-dimethoxy-5- hydroxystilbene has an IC50 of 0.1 μM with respect to CYP1A1 and CYP1B1, while 3,4’,5-trimethoxystilbene has an IC50 of 0.6 μM with respect to CYP1A1 and 0.4 μM with respect to CYP1B1 [41]. Also, 3,4,2’,5’-tetramethoxystilbene has an IC50 of 0.5 μM with respect to CYP1A1 and an IC50 of 0.62 μM with respect to CYP1B1 [42].
On the other hand, a trimethoxy substituted stilbene, 3,4,2’-trimethoxy-trans-stilbene and two tetramethoxy substituted stilbenes, 2,4,3’,5’-tetramethoxystilbene and 2,2’,4,6’-tetramethoxystilbene were highly potent CYP1B1 inhibitors: IC50 of 4.0 nM, 6 nM and 2 nM respectively. Furthermore they were both more selective towards CYP1B1 over CYP1A1 (IC50 of 360 nM, 300 nM and 170 nM respectively) [42-44]. Thus, it seems that some stilbenes with a greater number of methoxy substituted groups are even more potent inhibitors of CYP1B1. Therefore, while several monomethoxystilbenes are more selective inhibitors of CYP1A1 over CYP1B1, at least one trimethoxystilbene and two tetramethoxystilbenes are more selective inhibitors of CYP1B1 over CYP1A1.
Overall, Chun et al. [43] showed that adding methyl groups to hydroxyl groups to form the methoxy groups lowered the IC50 of 2,4,3’,5’-tetramethoxystilbene by 1000 fold towards CYP1B1 as compared to oxyresveratrol. He hypothesized that this was because it increased the lipophilicity of this stilbene, which made it bind more tightly to the hydrophobic environment in the active site of the enzyme. Thus it would follow that increasing the number of methoxy groups on the aryl rings would also increase its’ lipophilicity and could potentially increase its’ ability to inhibit CYP1B1. In addition, many of these multi methoxylated stilbenes have cytotoxic effects on various cancer cells and it is possible that their ability to inhibit CYP1B1 as well as CYP1A1 is one mechanism by which they do so. One caveat to this is that Mikstacka et al. [42] demonstrated that two of the pentamethoxystilbenes tested inhibited the enzymes less than the tetra, tri and di methoxystilbenes tested indicating that other factors also play a role in the ability of stilbenes to inhibit these enzymes.
Furthermore, the substitution pattern of methoxy groups on the aryl rings also influences the ability of stilbenes to bind and inhibit CYP1A1 and CYP1B1, as seen in Table 1, by comparing 3,4,2’-trimethoxy-transstilbene which has an IC50 of 4.0 nM with 3,4’,5-trim ethoxystilbene which has an IC50 of 600 nM. Previously Mikstaka et al. [42] concluded that the substitution pattern on the aryl rings affects the inhibition of both enzymes and Kim et al. [44] suggested that simply the presence of a substituent on the 2 position of the stilbene skeleton plays a critical role in the ability of a stilbene to distinguish between CYP1A1 and CYP1B1.
Finally, substituents other than methoxy groups may also enhance a stilbene’s ability to inhibit CYP1A1 and/or CYP1B1. For example, Kim et al. [44] tested the addition of thiol, furanyl, pyridyl and fluoro groups added to the aryl ring and found that some of these compounds, such as 3,5-dimethoxy-2’-thiophenylstilbene, exhibited an IC50 of 61 nM with CYP1A1 and 11 nM with CYP1B1, which is on par with the multiple methoxy substituted stilbenes mentioned above.
Thus, the literature on stilbene research and CYP1A1 and CYP1B1 inhibitors has shown us that the planarity of the compound and the location and type of substituents on the aryl ring play an important role in determining the effectiveness of the inhibitors for these enzymes and are likely important in designing other inhibitors of these enzymes as will be discussed in the next section.
Triazenes
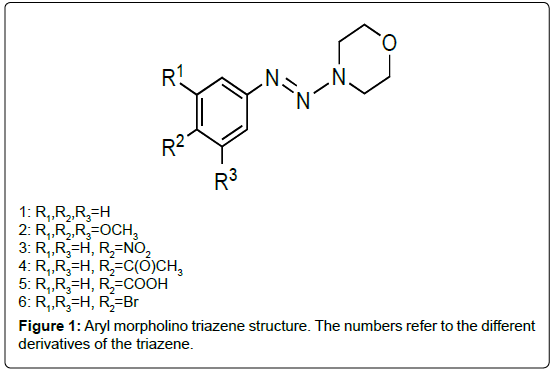
My collaborator, Ralph Isovitsch and I became interested in triazenes as potential CYP1A1 and CYP1B1 inhibitors because we were initially exploring various stilbene derivatives to test their potency in inhibiting these enzymes and the method for making the stilbenes was a two-step synthesis in which the aryl morpholino triazene was the intermediate compound. These aryl morpholino triazenes consist of an aryl ring connected to three consecutive nitrogens (triazene unit) and a morpholine ring. Because these triazenes have some planar characteristics due to the aryl group and triazene unit, I wondered if this class of compound might fit into each enzyme’s active site and thus be a good inhibitor of CYP1A1 and CYP1B1.
Somewhat similar to stilbenes, a methoxy triazene, compound 2 (Figure 1 for triazene structures), was a more potent inhibitor of CYP1A1 versus those substituted with nitro (compound 3), acetyl (compound 4), carboxy (compound 5) or bromo (compound 6) with an IC50 of 10 μM and a good inhibitor of CYP1B1, relative to other triazenes tested, with an IC50 of 18 μM (Table 2). Also, similar to stilbenes, some triazenes were specific to inhibiting one enzyme over the other. The most potent CYP1B1 inhibitors were the triazenes substituted with nitro and bromo groups, which had IC50 values of 2 μM and 7 μM respectively whereas their IC50 values for inhibiting CYP1A1 were 115 μM and 63 μM respectively. Furthermore, there was one triazene, the acetyl substituted triazene, that was more selective for CYP1A1 (IC50=82 μM) than CYP1B1 which showed no inhibition towards this latter enzyme. These results indicated that aryl morpholino triazenes are a new class of CYP1A1 and CYP1B1 inhibitors [45].
| Compounds | 1A1 | 1B1 |
|---|---|---|
| IC50 | IC50 | |
| µM | µM | |
| Compound 1 (Triazene) | 120 | 163 |
| Compound 2 | 19 | 18 |
| Compound 3 | 115 | 2 |
| Compound 4 | 82 | NDb |
| Compound 5 | 167 | 400 |
| Compound 6 | 63 | 7 |
Table 2: IC50 values for compounds 1-6 with CYP1A1 and BYP1B1a .
The aryl morpholino triazenes are likely able to inhibit the CYP1A1 and CYP1B1 enzymes in part because the aryl group is planar, which should fit well into the active sites of both enzymes, but also because the triazene unit has a π electron system that could potentially form π-π interactions with the aromatic ring in Phe 224 in CYP1A1 [15] and Phe 231 in CYP1B1 [17] similar to α-naphthoflavone, or with other phenylalanine residues that were identified by Gonzalez et al. [29]. Because the unsubstituted aryl morpholino triazene, compound 1, has some inhibitory activity against both enzymes, this might be the case.
In addition, the methoxy substituted triazene, compound 2, was the most potent inhibitor against CYP1A1 and one of the more potent inhibitors of CYP1B1 likely because the methoxy groups substituted on the aryl ring made compound 2 even more hydrophobic compared to the unsubstituted aryl ring or to other substituted groups and possibly because the electron donating property of the methoxy group could have made the π electron system on the triazene unit even more robust [45].
However, because the morpholine ring is conformationally flexible, this makes the entire molecule less planar than stilbenes. As a result, we are working on testing more planar triazene compounds with a variety of different substituents on the aryl ring to determine if they might inhibit CYP1A1 and CYP1B1 even more strongly than the aryl morpholino triazenes and thus be even better candidates as cancer preventive agents.
In the future, comparing substitution patterns in the triazene compounds will also be an important area of study to determine if, like the stilbenes discussed above, the position to which various substituents are attached on the aryl unit influences the ability of the triazenes to inhibit these enzymes.
Other CYP1A1 and CYP1B1 inhibitors
Many other compounds, most of which contain one or more aromatic rings like stilbenes and the aryl morpholino triazenes, are also CYP1A1 and CYP1B1 inhibitors. These include the flavonoids, naphthoquinones, anthraquinones, coumarins, alkaloids, non-tumor inducing polycyclic aromatic hydrocarbons and a variety of other naturally occurring compounds [46]. Many members in the above family of compounds also show chemopreventive activity such as several coumarin derivatives, imperatorin and bergamottin and several flavonoids including naringenin, apigenin, quercetin and hesperidin [47]. This suggests that many anti-cancer compounds may exert their effects through inhibiting CYP1A1 and CYP1B1.
Conclusion
In conclusion, many CYP1A1 and CYP1B1 inhibitors have also been shown to prevent and/or inhibit the progression of a variety of cancers. Thus, it has become important to identify new classes of compounds that can inhibit these enzymes in the hope of discovering even more potent inhibitors and thus more potent chemopreventive agents. We recently identified aryl morpholino triazenes as a new class of compounds that inhibit CYP1A1 and CYP1B1 and we are currently working on a more planar type of triazene with a variety of substituents on the aryl ring to determine if these compounds will be even better inhibitors. We also hope to eventually work on substitution patterns on the aryl ring to determine how much that influences the ability of the triazenes to inhibit these enzymes. It will then become important to determine if these compounds can inhibit cancer formation in vitro and ultimately in vivo.
References
- Roskowski R (2014) The ErbB/HER family of protein-tyrosine kinases and cancer. Pharmacol Res79: 34-74.
- Cohen RB (2003) Epidermal growth factor receptor as a therapeutic target in colorectal cancer. Clin Colorectal Cancer 2: 246-251.
- Malumbres M, Barbacid M (2003) RAS oncogenes: The first 30 years. Nat Rev Cancer 3: 459-465.
- Lin W, Li Z (2017) Blueberries inhibit cyclooxygenase-1 and cyclooxygenase-2 activity in human epithelial ovarian cancer. Oncol Lett 13: 4897-4904.
- Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, et al. (1997) Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science 275: 218-220.
- Efimova EV, Ricco N, Labay E, Mauceri HJ, Flor AC, et al. (2018) HMG-CoA reducatase inhibition delays DAN repair and promotes senescence after tumor irradiation. Mol Cancer Ther 17: 407-418.
- Androutsopoulos VP, Tsatsakis AM, Spandidos DA (2009) Cytochrome P450 CYP1A1: wider roles in cancer progression and prevention. BMC Cancer 9: 187.
- Halberg RB, Larsen MC, Elmergreen TL, Ko AY, Irving AA, et al. (2008) Cyp 1b1 exerts opposing effects on intestinal tumorigenesis via exogenous and endogenous substrates. Cancer Res 68: 7394-7402.
- Shimada T, Fujii-Kuriyama Y (2004) Metabolic activation of polycyclic aromatic hydrocarbons to carcinogens by cytochromes P450 1A1 and 1B1. Cancer Sci 95: 1-6.
- Ding L, Getz G, Wheeler DA, Mardis ER, Mclellen MD, et al. (2008) Somatic mutations affect key pathways in lung adenocarcinoma. Nature 455: 1069-1075.
- Yachida S, White CM, Naito Y, Zhong Y, Brosnan JA, et al. (2012) Clinical significance of the genetic landscape of pancreatic cancer and implications for identification of potential long term survivors. Clin Cancer Res 18: 6339-6347.
- Nelson DR (2011) Progress in tracing the evolutionary paths of cytochrome P450. Biochim Biophys Acta 1814: 14-18.
- Gupta RP, He YA, Patrick KS, Halpert JR, Bell NH (2005) CYP3A4 is a vitamin D-24- and 25-hydroxylase: Analysis of structure function by site-directed mutagenesis. J Clin Endocrin Metab 90:1210-1219.
- Walsh AA, Szklarz GD, Scott EE (2013) Human cytochrome P450 1A1 structure and utility in understanding drug and xenobiotic metabolism. J Biol Chem 288: 12932-12943.
- Tsuchiya Y, Nakajima M, Kyo S, Kanaya T, Inoue M, et al. (2004) Human CYP1B1 is regulated by estradiol via estrogen receptor. Cancer Res 64: 3119-3125.
- Wang A, Savas U, Stout CD, Johnson ER (2011) Structural characterization of the complex between a-naphthoflavone and human cytochrome P450 1B1. J Biol Chem 286: 5736-5743.
- Courter LA, Musafia-Jeknic T, Fischer K, Bildfell R, Giovanini J, et al. (2007) Urban dust particulate matter alters PAH-induced carcinogenesis by inhibition of CYP1A1 and CYP1B1. Toxicol Sci 95: 63-73.
- Baird WM, Hooven LA, Mahadevan B (2005) Carcinogenic polycyclic aromatic hydrocarbons-DNA adducts and mechanism of action. Environ Mol Mutagen 45: 106-114.
- Phillips DH (1999) Polycyclic aromatic hydrocarbons in the diet. Mutat Res 443: 139-147.
- Rendic S, Guengerich FP (2012) Contributions of human enzymes in carcinogenic metabolism. Chem Res Toxicol 25: 1316-1383.
- Shimada T, Hayes CL, Yamazaki H, Amin S, Hecht SS, et al. (1996) Activation of chemically diverse procarcinogens by human cytrochrome P-450 1B1. Cancer Res 56: 2979-2984.
- Xue W, Warshawsky D (2005) Metabolic activation of polycyclic and heterocyclic aromatic hydrocarbons and DNA damage: A review. Toxicol Appl Pharmacol 206: 73-93.
- Shimada T, Oda Y, Gillam EMJ, Guengerich FP, Inoue K (2001) Metabolic activation of polycyclic aromatic hydrocarbons and other procarcinogens by cytochromes P450 1A1 and P450 1B1 allelic variants and other human cytochromes P450 in Salmonella typhimurium NM2009. Drug Metab Dispos 29: 1176-1182.
- Takemura H, Nagayoshi H, Matsuda T, Sakakibara H, Morita M, et al. (2010) Inhibitory effects of chrysoeriol on DNA adduct formation with benzo[a]pyrene in MCF-7 breast cancer cells. Toxicology 274: 42-48.
- Ruan Q, Gelhaus SL, Penning TM, Harvey RG, Blair IA (2007) Aldo-keto reductase and cytochrome P450-dependent formation of benzo[a]pyrene-derived DNA adducts in human bronchoalveolar cells. Chem Res Toxicol 20: 424-431.
- Tassaneeyakul W, Birkett DJ, Veronese ME, McManus ME, Tukey RH, et al. (1993) Specificity of substrate and inhibitor probes for human cytochromes P450 1A1 and 1A2. J Pharmacol Exp Ther 265: 401-407.
- Sridhar J, Goyal N, Liu J, Foroozesh M (2017) Review of ligand specificity factors for CYP1A subfamily enzymes from molecular modeling studies reported to-date. Molecules 22: 1143.
- Gonzalez J, Marchand-Geneste N, Giraudel JL, Shimada T (2012) Docking and QSAR comparative studies of polycyclic aromatic hydrocarbons and other procarcinogen interactions with cytochromes P450 1A1 and 1B1. SAR QSAR Environ Res 23: 87-109.
- Fulda S (2010) Resveratrol and derivatives for the prevention and treatment of cancer. Drug Disc Today 15: 757-765.
- Lu R, Serrero G (1999) Resveratrol, a natural product derived from grape, exhibits antiestrogenic activity and inhibits the growth of human breast cancer cells. J Cell Physiol 179: 297-304.
- Larrosa M, Tomas-Barberan FA, Espin JC (2003) Grape polyphenol resveratrol and the related molecule 4-hydroxystilbene induce growth inhibition, apoptosis, S-phase arrest and upregulation of cyclins A, E and B1 in human SK-Mel-28 melanoma cells. J Agric Food Chem 51: 4576-4584.
- Chun YJ, Kim MY, Guengerich FP (1999) Resveratrol is a selective human cytrochrome P450 1A1 inhibitor. Biochem Biophys Res Commun 262: 20-24.
- Guengerich FP, Chun YJ, Kim D, Gillam EMJ, Shimada T (2003) Cytochrome P450 1B1: A target for inhibition in anticarcinogenesis strategies. Mutat Res 523-524: 173-182.
- Pan MH, Lin CL, Tsai JH, Ho CT, Chen WJ (2010) 3,5,3;,4’,5’-pentamethoxystilbene (MR-5), a synthetically methoxylated analogue of resveratrol, inhibits growth and induces G1 cell cycle arrest of human breast carcinoma MCF-7 cells. J Agric Food Chem 58: 226-234.
- Pires de Lima D, Rotta R, Beatriz A, Marques MR, Montenegro RC, et al. (2009) Synthesis and biological evaluation of cytotoxic properties of stilbene-based resveratrol analogs. Eur J Med Chem 44: 701-707.
- Rimando AM, Suh N (2008) Biological/chemopreventive activity of stilbenes and their effect on colon cancer. Planta Med 74: 1635-1643.
- Murias M, Jager W, Handler N, Erker T, Horvath Z, et al. (2005) Antioxidant, prooxidant and cytotoxic activity of hydroxylated resveratrol analogues: structure-activity relationship. Biochem Pharmacol 69: 903-912.
- Lee SK, Nam KA, Hoe YH, Min HY, Kim EY, et al. (2003) Synthesis and evaluation of cytotoxicity of stilbene analogues. Arch Pharm Res 26: 253-257.
- Mikstacka R, Przybylska D, Rimando AM, Baer-Dubowska W (2007) Inhibition of human recombinant cytochromes P450 CYP1A1 and CYP1B1 by trans-resveratrol methyl ethers. Mol Nutr Food Res 51: 517-524.
- Chun YJ, Ryu SY, Jeong TC, Kim MY (2001) Mechanism-based inhibition of human cytochrome P450 1A1 by rhapontigenin. Drug Metab Dispos 29: 389-393.
- Mikstacka R, Wierzchowski M, Dutkiewicz Z, Gerlara-Korzanska A, Korzanski A, et al. (2014) 3,4,2’-trimethoxy-trans-stilbene- a potent CYP1B1 inhibitor. Med Chem Commun 5: 496-501.
- Chun YJ, Oh YK, Kim BJ, Kim D, Kim SS, et al. (2009) Potent inhibition of human cytochrome P450 1B1 by tetramethoxystilbene. Toxicol Lett 189: 84-89.
- Kim S, Ko H, Park JE, Jung S, Lee SK, et al. (2002) Design, synthesis and discovery of novel trans-stilbene analogues as potent and selective human cytochrome P450 1B1 inhibitors. J Med Chem 45: 160-164.
- Lee D, Perez P, Jackson W, Chin T, Galbreath M (2016) Aryl morpholino triazenes inhibit cytochrome P450 1A1 and 1B1. Bioorg Med Chem Lett 26: 3243-3247.
- Liu J, Sridhar J, Foroozesh M (2013) Cytochrome P450 family 1 inhibitors and structure-activity relationships. Molecules 18: 14470-14495.
- Shimada T (2017) Inhibition of carcinogenic-activating cytochrome P450 enzymes by xenobiotic chemicals in relation to antimutagenicity and anticarcinogenicity. Toxicol Res 33: 79-96.
Citation: Iimoto D (2018) Preventing Carcinogenesis with Compounds that Inhibit Cytochrome P450 1A1 and 1B1. Biochem Physiol 7: 230. DOI: 10.4172/2168-9652.1000230
Copyright: ©2018 Iimoto D. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 3863
- [From(publication date): 0-2018 - Dec 27, 2024]
- Breakdown by view type
- HTML page views: 3156
- PDF downloads: 707