Prevalence of Malaria and Associated Factors among Febrile Patients Visiting Kalala Health Center in Haro Limmu Woreda, East Wollega Zone, Western Ethiopia, 2016
Received: 13-Dec-2018 / Accepted Date: 17-Dec-2018 / Published Date: 27-Dec-2018 DOI: 10.4172/2161-1165.1000365
Abstract
Background: Malaria is caused by protozoan parasites that belong to the Genus Plasmodium that are transmitted to human via the bite of infected female Anopheles mosquito. 34(75%) of the land mass of Ethiopia is malarious with two-thirds of the country’s population at risk of acquiring infection. The distribution and transmission of malaria in Ethiopia varies from place to place. Risk of malaria is highest in the western lowlands of Oromia, Amhara, Tigray and almost the entire regions of Gambella and Benishangul Gumuz regions.
Objective: To determine prevalence of malaria and associated factors among febrile patients visiting Kalala health center, Haro Limmu Woreda.
Methods: A cross-sectional study was carried out among 316 febrile patients visiting Kalala health center, from October 15 to November 15, 2016. Face to face interview was conducted using pre-tested structured questioner. Blood sample was collected and thick and thin smears were prepared and examined microscopically for the presence of malaria parasite. Data generated from the study was analyzed using SPSS statistical software version 20.0.
Results: From all study participants, 184(58.2%) were male and 132(41.8%) were female with mean age of 29.48(SD ± 16.12) years and 36(10.0%) were 5 years. From febrile cases visited Kalala health center 49.4% were positive for malaria, in which 54.5%, 15.8% and 6.6% were infected with Plasmodium falciparum, Plasmodium vivax and mixed respectively. Being female, rural residence, age group 25 to 34 and 45 to 54, lack of access to radio, not using ITNs, occupation, educational status and not taking anti malaria drug were significantly associated with malaria.
Conclusion and Recommendation: The prevalence of malaria in study area is high with dominancy of Plasmodium falciparum which needs attention of community and governments to tackle at grass root level by increasing level of knowledge and practice towards malaria prevention and control strategy.
Keywords: Kalala Health Center; Malaria; Prevalence; Plasmodium species
Introduction
The word malaria is derived from two Italian words, ‘mal’ and ‘aria’ which mean bad air. They called it so because they thought that the disease is caused by bad air [1].
Malaria remains a global health threat putting an estimated 3.3 billion people at risk of malaria of which 1.2 billion are at high risk. In high risk areas more than one malaria cases occur per 1,000 populations [2]. The latest estimates of World health organization showed that 214 million new cases of malaria worldwide in 2015 (range 149–303 million). The African region accounted for most global cases of malaria (88%) followed by the South-East Asia region (10%) and the Eastern Mediterranean region (2%) [3]. About 75% of the land mass of Ethiopia is malarious and 68% of the Ethiopian population estimated at about 54 million in 2010-2014 live in malaria risk areas [4]. The distribution and transmission of malaria in Ethiopia varies from place to place. Risk of malaria is highest in the western lowlands of Oromia, Amhara, Tigray and almost the entire regions of Gambella and Benishangul Gumuz regions [5].
The common causes of human malaria are four species: Plasmodium vivax, Plasmodium falciparum, Plasmodium malaria, Plasmodium ovale and sometimes by a fifth species Plasmodium knowlesi [6]. Plasmodium falciparum accounts for vast majority of malarial infection mainly because of worldwide distribution of the parasite [7]. Plasmodium vivax has the widest geographic range of the four parasites responsible for malaria in man. Historically its range has extended as far North as Finland and Northern China and as far South as North Australia and South Africa [8].
Human infection begins when an infected female Anopheles mosquito bites a person and injects infected with sporozoites saliva into the blood circulation that is the first life stage of Plasmodium (stage of infection). The next stage in malaria life cycle is the one of asexual reproduction that is divided into different phases: the preerythrocytic or exo-erthyrocytic and the erythrocytic phase. Within only 30-60 minutes after the parasite inoculation sporozoites find the way through blood circulation to the first target to the liver [9].
The risk of infection is determined by the number and species of mosquitoes present in a given area as well as the climate and geography. In many parts of the world transmission of malaria coincides with the rainy season when mosquitoes thrive to breed and there is increased agricultural activity. Population shifts caused by political unrest, climatic events and environmental changes brought on by urbanization, deforestation and forced irrigation have all contributed to the increased incidence of malaria [10].
Malaria is one of the public health problem worldwide about 3.2 billion people (that is nearly half of the world’s populations) are at risk of malaria. In 2015 there were 214 million malaria cases [11]. An estimated 3.4 billion people are at risk of malaria of which 1.2 billion are at high risk. In high risk areas more than one malaria case occurs per 1,000 population. Two hundred and seven million cases of malaria in 2012 (uncertainly range 135-287 million) and an estimated 627,000 deaths (uncertainty range 473,000-789,000) with a 90% of deaths due to malaria occur in sub-Saharan Africa [12].
Malaria and poverty are closely linked due to poor access and utilization of malaria prevention, diagnosis and treatment. It is concentrated in low-income and lower income countries. Within these countries the most severely affected communities are those that are the poorest and most marginalized. Such communities have the highest risks associated with malaria and the poor access to effective services for prevention, diagnosis and treatment [13].
Malaria is the leading causes of morbidity and mortality in the Ethiopia. It is the first cause of health facility admissions with the rate of 8% clinical malaria without laboratory confirmation [14]. Malaria and poverty are intimately connected. Malaria is most intractable for poorest countries in the world. It is also a significant problem to social and economic development in the country due to the epidemicity of malaria occurring during harvesting seasons which reduces agricultural productivity and hence leads to food insecurity and poverty [15].
In Nekemtereferral Hospital of East Wollega Zone two species of malaria Plasmodium falciparum and Plasmodium vivax were identified with relatively higher prevalence of P. vivax 71(59.2%) and P. falciparum 27(22.5%). Factor such as low educational level, presence of stagnant water, absence of bed net utilization and illiterate were shown to be associated with relatively high prevalence of malaria [16]. There is high magnitude of malaria at Haro Limmu Woreda according wored ahealth report. But there is no published report about the prevalence and associated factors of malaria in Haro Limmu Woreda. Therefore, this study will determine the prevalence and associated factors of malaria among febrile patients visiting Kalala health center in Haro Limmu Woreda, East wollega, Ethiopia.
Malaria is one of the major public health problems in the world especially in sub-Saharan Africa regions. Ethiopia as a part of sub- Saharan region shares high burden of malaria. Malarias is distributed nationwide, Haro Limmu Woreda will never be free from the same consequence. Therefore, the result obtained provide valuable information about the prevalence and associated factors of malaria for the health center, woreda health bureau and other stake holders for the future management of the expansion of malaria in the area.
Methods And Materials
Study area and period
Haro Limmu Woreda is found in East Wollega zone and 498 km far from Addis Ababa and 165 KM far from Zonal capital city Nekemte to the Northern part. This woreda is bordered by Limmu Galilaworeda and Ebantuworeda in the eastern and Sasigaworeda and Beneshangul Gumuz zone in the western part. The climatic condition of the woreda is almost partially warm zone (kola). Majority of the temperate zone (Wayne dega) farmers travels to the temperate zone (kola) to harvest their farm commonly “Salix”. As the result there is high probability to caught malaria in addition to the 'Kola' people even though their constant resident area is in Wayne dega.
Haro Limmu Woreda has tree health centers and four private clinics and 15 health posts. Kalala Health center is 10km far from Haro Limmu Woreda health office and it serves one cluster (catchment area) which has five Kebeles having one health post each. Total population of Kalala cluster is estimated to be 18, 038 according to woreda statically record projected from census of 2007 EC. In Kalala health center there are seven clinical nurses, two midwiferies, one health officer, one pharmacy technician and laboratory technician. The services provided in this health center are emergency services, nursing assessment, immunization, Antenatal care, delivery conduct, postnatal care and laboratory diagnosis services are routinely done for the society. This study was conducted among febrile patients visiting Kalala Health center from October 15, 2016 to November 15, 2016.
Study design and population
Across-sectional study was conducted at Kalala Health center in Haro Limmu Woreda. The study participants were all febrile patients who were attended Kalala Health center during the study period.
Study population
All febrile patients who were attended Kalala Health center during the study period inHaroLimmu Woreda.
Inclusion and exclusion criteria
Inclusion: Any febrile patients (male and female all age group) attend Kalala health center for fever treatment and interested were participated in the study.
Exclusion: Febrile patients who take anti malaria drugs in the same week and come for follow up.
Sample size and sampling techniques
Sample size: Sample size was calculated using single proportion following formula. In this study area still there is no research data on prevalence of malaria. Since there is no similar study conducted in the selected study area the prevalence of malaria in Assendabo health center 28.8% was taken to determine the sample size [17]. Using sample size formula, the sample size was calculated as follows:
n=(Z2 P(1-P))/d2
n=(1.962*0.288(1-0.288)/0.052=n=315.097252=316
Where: -
n=the total sample size
P=prevalence of malaria in Assendabo health center (28.8%)
Z=Za/2 at 95% CI (1.96)
d=the desired precision of the estimate/ margin of error (5%)
Sampling techniques and study variables
A convenient sampling technique was used to select all febrile patients coming to health center for blood film examination. Sample was collected till the final sample size was reached.
Dependent variable: Malaria Prevalence and Plasmodium species
Independent variable: Socio-economic characteristics: Age, Sex, Religion, Ethnicity, Marital status, Educational status, Occupational status, Income,
Living style: Living near Stagnant water, Residence, Climate condition, Seasons, Housing condition, Toilet condition, visiting lowland, ITNs utilization
Access to information and communication: Health education, Radio access, Access to health facility
Method Of Data Collection
The following data collection method was used:-
Face to face interview
The data was collected using face to face interview by trained laboratory technicians using pretested structured questionnaires. The questionnaire used to collect information like socio-demographic factors like age, sex and other environmental risk factors like living areas with stagnant water.
Blood sample collection and examination
Capillary blood Specimen was collected using strictly disposable sterile blood lancet from finger under aseptic technique. A small drop of blood was added to the center of slide for thin blood film and larger drop of blood at the top end for thick blood film. After smearing, air drying, staining with 10% Giemsa for 10-15 minutes washes with water and dried [18]. Then a drop of oil immersion was applied to both thin and thick blood film and examined systematically under 100X magnification searching for at least 100 fields for the presence of parasite by two laboratory professional.
Data quality control/assurance
The questioner was prepared in English language then translated to Afan Oromo and re-translated back to English language by translator who knows both languages fluently. The questionnaire was pretested on 5% of the sample population 2 weeks prior to data collection out of the study area. The collected data was checked for completeness, accuracy, clarity and consistency by researcher on daily bases.
For laboratory examination the quality of blood film was kept by using standard operating procedure (SOP) starting from sample collection to the end of the procedure. The specimen was fresh whole blood collected by finger puncture and the combination thick-thin blood film provides both options on one frosted glass slide which is labeled by pencil and the slide can be stained as either a thick or thin blood film. The thin blood film was fixed prior staining, and then the smear was read as a thin blood film and unfixed one as thick blood film. One should be able to barely read newsprint through the wet or dry film. The film itself should not have any clear areas or smudges, indicating that grease or fingerprints were on the glass.
The microscope examination of full smear was done systematically to examine the whole slide and the identification of Plasmodium species by laboratory professional and rechecked by investigator, to say positive or negative 100 fields should be checked and then proper registration of the patient with full address and result. The results were recorded and interpreted using standard reporting system by investigator. All patients with positive result were sent to clinician for treatment. SOP was followed throughout the study period [19].
Data analysis and interpretation
Data was collected from each patient and checked for completeness and entered SPSS version 20. After data was cleared and sorted analysis was done. The result was presented using tables, graphs and figures. The prevalence of malaria was determined as the proportion of those study participants who become positive for blood film to total number of participants’ blood film examined. Associated risk factors of malaria were assessed by calculating crude and adjusted odds ratio with 95% confidence interval.
Ethical consideration
Ethical clearance was obtained from respective body and Official letter of permission was obtained from Haro Limmu Woreda Health office and Kalala health center in the woreda. The purpose of the study was clearly explained for the study participants and those willing to participate and greater and equal to 18 years were signed the consent. For those
Results
According to the study design and sample size proposed the study incorporated 316 study participants which make the response rate of the study 100%. The finding of the study is categorized in to: sociodemographic and economic characteristics of respondents, Health seeking behavior of respondents, knowledge and practice of respondent, blood film result of respondents and factors associated with malaria infection among respondents. It is presented by tables, charts, figures and statements for the purpose of clarity and simplicity.
Socio-demographic and economic characteristics of study participants
From all study participants involved in the study 184(58.2%) were male and 132(41.8%) were female with mean age of 29.5(SD ± 16.1) years varying from 1 year to 65 years. Among study participants majority, 265(83.9%) were rural resident, half 159(50.3%) were protestant, 283(89.6%) were Oromo, more than half 176(55.7%) were currently married, nearly half 150(47.50%) were farmer and one fourth 84(26.6%) attended primary and read and write only (Table 1).
| Variables | Response | Number | Percentage |
|---|---|---|---|
| Sex | Male | 184 | 58.2 |
| Female | 132 | 41.8 | |
| Age | 0 to 4 years | 36 | 11.4 |
| 5 to 14 years | 28 | 8.9 | |
| 15 to 24 years | 47 | 14.9 | |
| 25 to 34 years | 74 | 23.4 | |
| 35 to 44 years | 78 | 24.7 | |
| 45 to 54 years | 53 | 16.8 | |
| Residence | Urban | 51 | 16.1 |
| Rural | 265 | 83.9 | |
| Ethnicity | Oromo | 283 | 89.6 |
| Amhara | 33 | 10.4 | |
| Marital status | Single | 125 | 39.6 |
| Married | 176 | 55.7 | |
| Divorced | 15 | 4.7 | |
| Occupation | Farmer | 150 | 47.5 |
| Merchant | 28 | 8.9 | |
| Government employee | 19 | 6.0 | |
| student | 42 | 13.3 | |
| Daily laborer | 23 | 7.3 | |
| Employee(Private sector) | 9 | 2.8 | |
| House wife | 45 | 14.2 | |
| Educational status | Cannot read and write | 66 | 20.9 |
| Read and write only | 84 | 26.6 | |
| Primary (1-8) | 85 | 26.9 | |
| Secondary (9-12) | 55 | 17.4 | |
| College and above | 26 | 8.2 | |
| Family size | 1 to 3 Family members | 110 | 34.8 |
| 4 to 6 Family members | 108 | 34.2 | |
| ≥ Family members | 98 | 31.0 | |
| Average monthly income | ≤ 500ETB | 72 | 22.8 |
| 501 to 1000ETB | 66 | 20.9 | |
| 1001 to 1500 ETB | 106 | 33.5 | |
| 1501 to 2000 ETB | 17 | 5.4 | |
| ≥ 2001 ETB | 55 | 17.4 |
Table 1: Socio-economic and demographic characteristics of respondents Kalala Health center, Haro Limmu Woreda, East Wollega, October to November, 2016.
Average monthly earning history of the study participants were assessed and the mean monthly income was 1328.5 (SD ± 1099.4) Ethiopian Birr (ETB) with a minimum and a maximum of 100 ETB and 5000 ETB respectively. Regarding family size, the mean was 6.70 (SD ± 2.0) family members per household with a minimum and maximum 3 and 13 family members per household respectively (Table 1).
Knowledge of respondents on malaria and other diseases
All 316(100.0%) of the study participants reported that they know the disease malaria and 284(89.9%) of them replied malaria is caused by mosquito bite while the remaining 32(10.1%) of the respondents reported as malaria is caused by parasite. Majority, 276(87.30%) of the respondents ranked malaria as a leading cause of morbidity in their village 240(75.9%) pneumonia and the remaining ranked diarrhea, cough/TB and skin diseases with overlapping response.
Main malaria transmission season in the village was assessed and almost half 156(49.4%) of the respondents reported during rainy time (September to December) with similar period with 58(18.4%) those who reported during maize harvesting. 94(29.7%) and 8(2.5%) reported malaria transmission period during rainy season (June to August) and all year round respectively. Common malaria signs and symptom was assessed and almost all, 302(95.6%) of the respondents reported fever, sweat and headache, 274(86.7%) chills with fever and 215(69.0%) weakness and tiredness as common sign and symptoms.
On how people get malaria, 265(83.9%) of the respondents said that people get malaria by mosquito bites while 35(11.1%) of respondents said by drinking dirty water, 6(1.9%) said by not getting enough food and living near collected water and only 4(1.3%) reported people get malaria by working in the Sun. The study participants were asked which group of people is affected by malaria and two third, 211(66.8%) reported all are equally affected, 56(17.7%) of them replied young children (5 to 15 years), 30(9.5%) all adult (15 to 60 years), 13(4.1%) children
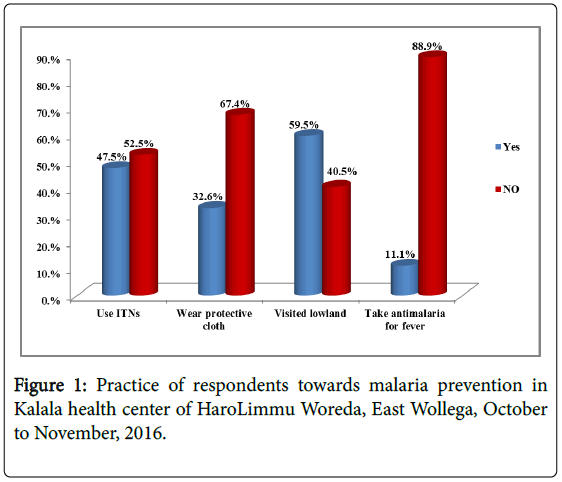
On the knowledge of malaria prevention among the study participants, there is overlapping response on using bed nets, spray of house with chemicals and eliminating stagnant water. Among respondents more than half, 177(56.0%) know preventive method of malaria as using bed nets, 74(23.4%) spraying house with chemicals and 65(20.6%) omitting stagnant water around living house. 251(79.4%) of the respondents knew three of the malaria preventive method; using bed nets, spraying of house with chemicals and eliminating mosquito breeding site (stagnant water).
Practice of respondents towards malaria
In the households of the respondents’ practice of preventing malaria was assessed and practice of not to get malaria according to its decreasing order was 165(52.2%) use mosquito nets, 66(20.9%) spray house with insecticide, 47(14.9%) clean compound (house), 34(10.8%) close door and window early night and 4(1.3%) smoke in house as preventive method of malaria in their households.
In this study only one third, 103(32.6%) of the study participants wear protective cloth which is long sleeve shirts and trousers when they are working around stagnant water to protect themselves from mosquito bites and nearly half, 150(47.5%) of the respondents use ITNs. Among the study participants, 188(59.50%) visited warm zone (lowland) and 98(51.9%) of them used ITNs while they visited lowland (Figures 1-3).
Health seeking behavior, information access of the respondents
Among study participants involved in the study more than three fourth, 248(78.5%) of respondent’s fever started less than 1 week before they visit health center while in 68(21.5%) fever started 2 to 3 weeks before they visited health center. Reason for delay after fever started study participants reported that I go if get sever 119(37.7%) followed by distance of health center (too far) 103(32.6%) and other 94(29.7%) reasoned can’t afford cost of transportation, health facility was closed, shortage of drug, they do not give injection at health facility and they do not give syrup for children. Action taken by respondents when fever started was 185(58.5%) of them went to health facility, 69(21.8%) went to drug store to get drugs, 56(17.7%) done nothing and other 6(1.9%) went to religious leader for prayer or used herbs/natural remedies at home (Table 3).
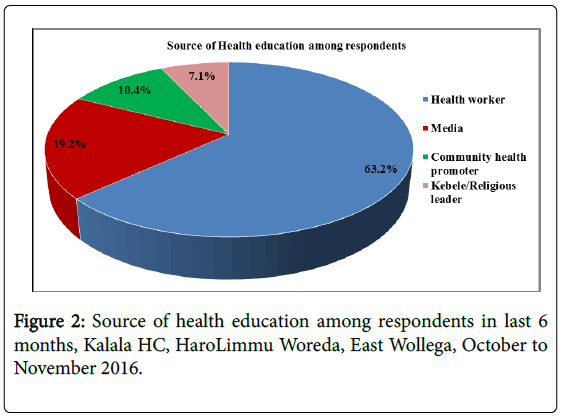
From all study participants 181(57.3%) of them have got health education on malaria in the last 6 months while the remaining 135(42.7%) did not get health education on malaria. Major source of health education among those who got was from health care worker followed by media.
Finding of current study showed that 144(45.6%) of the respondents had radio access (listen to radio) while 172(54.4%) did not have and access to radio or listen to radio. Time at which they attend radio for those who have an access according to its decreasing order was 59(41.0%) night time, 32(22.2%) early evening, 31(21.5%) after noon, 17(11.8%) early in the morning and 5(3.5%) late morning. The radio station commonly listened by the respondents was Fana broad casting, Oromia radio, Ethiopia radio station and Nekemte FM which accounts for 59(41.0%), 51(35.4%), 22(15.3%) and 12(8.3%) respectively.
Malaria prevalence among study participants
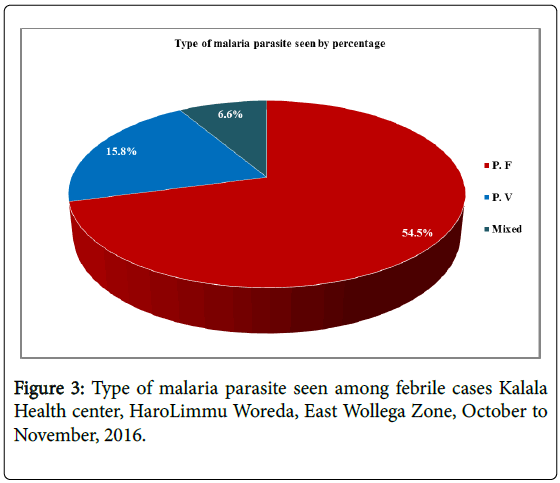
All febrile cases visited the health center was checked microscopically and found that 156 (49.4%) of the febrile cases was positive for malaria using thick blood film. More than half, 85 (54.5%) was Plasmodium falciparum.
Factors associated with malaria among study participants
To identify factors associated with malaria among the study participants regression analysis was done. As shown in the Table 2 below, on bivariate regression analysis factors significantly associated was age, sex, residence, occupation, educational status, family size, average monthly income of the HH, working while wearing protective cloth near stagnant water, taking anti-malarial, ITNs utilization, visiting lowland, access/listen to radio and getting health education.
| Variables | Response | Number | Percentage |
|---|---|---|---|
| Do you know malaria | Yes | 316 | 100.0 |
| No | 0 | 0 | |
| What cause malaria? | Mosquito bites | 284 | 89.9 |
| Parasite | 32 | 10.1 | |
| What are the main malaria transmission seasons? | During rainy season /June –August/ | 94 | 29.7 |
| During rainy time/ Sept – Dec/ | 156 | 49.4 | |
| All year round | 8 | 2.5 | |
| During maize harvesting | 58 | 18.4 | |
| What are the main sign and symptom of malaria? | Fever, Sweat, Headache | 274 | 86.7 |
| Chills/ Shivers | 36 | 11.4 | |
| Weakness / Tiredness | 6 | 1.9 | |
| How do people get malaria? | Mosquitoes bite | 265 | 83.9 |
| Drinking dirty water | 35 | 11.1 | |
| Working in sun | 4 | 1.3 | |
| Didn’t get enough food | 6 | 1.9 | |
| Leave near collected water | 6 | 1.9 | |
| Which group of people is affected by malaria? | All adult / age 15-60 / | 30 | 9.5 |
| Children less than 5 age | 6 | 1.9 | |
| Young children/5-15 age/ | 56 | 17.7 | |
| Children134.1 | |||
| All are equally affected | 211 | 66.8 | |
| What are the prevention methods of malaria? | Using bed net | 177 | 56.0 |
| Omitting stagnant water | 65 | 20.6 | |
| Spraying house with chemicals | 74 | 23.4 | |
| What do you do in your house hold to prevent getting malaria | Using mosquito nets | 165 | 52.2 |
| Smoking in house | 4 | 1.3 | |
| Spray house with DDT | 66 | 20.9 | |
| Clear compound / house | 47 | 14.9 | |
| Close door and window early night | 34 | 10.8 |
Table 2: Knowledge of respondents about transmission, prevention and treatment seeking on malaria Kalala Health center, Haro Limmu Woreda, East Wollega, October to November, 2016.
On multivariate regression analysis factors of malaria were identified using adjusted odds ratio with 95% confidence interval. Among variables significantly associated with malaria on bivariate analysis some of the variables were not associated with malaria during multivariate regression analysis. Those associated were Sex, age, residence, occupation educational status, family size, wearing protective cloth, taking anti malaria drug, using ITNs and access/ listening to radio.
Regarding the sex of the respondents being female is almost twice (AOR=1.89 95% CI 1.25, 8.88) at risk to be attacked with malaria when compared with male participants. Age group between 25 to 34 years (AOR=0.02, 95% CI 0.01, 0.88) and 45 to 54 years (AOR=0.01, 95% CI 0.00, 0.07) are at less risk when compared to other age group of the study participants. Study participants who were rural residents are nearly eight times (AOR=7.86, 95% CI 1.11, 55.71) at risk to be attacked by malaria when compared to their urban counterparts.
Other socio-demographic characteristics significantly associated were occupation and educational status of the respondents. Being Merchants (AOR=0.02, 95% CI 0.00, 0.55) and government employee (AOR=0.00 95% CI 0.00, 0.05) were preventive for malaria attack in the study area as compared with being farmer in occupation. Those study participants whose their level of education were primary (AOR=0.00 95% CI 0.00, 0.12) and secondary (AOR=0.01, 95% CI 0.12) were at less risk to be attacked with malaria in comparison with those study participants with college and above level of education.
Family size of the study participants associated with malaria and those households having 4 to 6 family members are higher risk (AOR=1.58, 95% CI 1.25, 9.98) to develop malaria when we compare them with households having 1 to 3 family members. Other group of family members is not significantly associated with attack of malaria.
As means of preventing mosquito bite, wearing protective cloth during working around stagnant water was inversely associated with malaria attack. Those who wear protective cloth were less likely (AOR=0.17, 95% CI 0.03, 0.90) to develop malaria when compare to their counterpart who did not wear protective cloth while working around stagnant water. In current study, those study participants who did not take anti malaria drugs for prophylaxis were almost ten times (AOR=9.56, 95% CI 4.21, 20.33) to caught malaria in the study area as compare to those who took malaria prophylaxis prior to the study. ITNs utilization is considered as a back bone of malaria prevention in community with less cost or free of charge distribution. Among study participants those who did not use ITNs are fourteen times (AOR=14.24, 95 % CI 2.37, 85.66) at risk to be attacked with malaria than those who use ITNs in the households properly and continuously.
Getting information on malaria prevention and control mechanism is the mainstay in the community. This information can be disseminated through different mechanism among which radio (media) is the commonest. Study participants who have no access to radio or who did not listen to radio were at higher risk (AOR=1.69, 95 % CI 1.08, 4.79) to be attacked by malaria in relation to those respondents who have access to radio in the same community (Table 4).
| Variables | Response | Number | Percentage |
|---|---|---|---|
| When did fever start you? | 24878.5 | ||
| 2-3 weeks | 68 | 21.5 | |
| What did you do when this fever started you? | Get went the health facility | 185 | 58.5 |
| Use herb \fruit or natural remedies | 2 | 0.6 | |
| Went to religious leaders | 4 | 1.3 | |
| Went drug store to get drugs | 69 | 21.8 | |
| We have done nothing | 56 | 17.7 | |
| Reason for delay after fever started | Too far | 103 | 32.6 |
| Cannot afford cost of transport | 24 | 7.6 | |
| I go if get sever | 119 | 37.7 | |
| Health facility was closed | 18 | 5.7 | |
| Shortage of drug | 16 | 5.1 | |
| They do not give injection at HF | 16 | 5.1 | |
| They do not give syrup for children | 20 | 6.3 | |
| Have you get health education on malaria in the last 6 months? | Yes | 181 | 57.3 |
| No | 135 | 42.7 | |
| Do you listen to radio (Have access to radio)? | Yes | 144 | 45.6 |
| No | 172 | 54.4 | |
| If listen to radio, When do you listen? | Early in the morning (5-9am) | 17 | 5.4 |
| Late morning (9-12am) | 5 | 1.6 | |
| afternoon (12-4) | 31 | 9.8 | |
| Early evening (4-8)) | 32 | 10.1 | |
| Night time (8-12) | 59 | 18.7 |
Table 3: Health seeking behavior and access to information of respondents on malaria Kalala Health center, HaroLimmu Woreda, East Wollega, October to November, 2016.
| Variables | Response | Malaria | COR (95% CI) | AOR (95% CI) | |
|---|---|---|---|---|---|
| Positive | Negative | ||||
| Sex | Male | 103 (56.0%) | 81 (44.0%) | 1 | 1 |
| Female | 53 (40.0%) | 79 (60.0%) | 1.89(1.20, 2.98) | 2.16(1.25,8.88) | |
| Age | 0 to 4 years | 12 (33.3%) | 24 (66.7%) | 1 | 1 |
| 5 to 14 years | 10 (35.7%) | 18 (64.3%) | 0.90(0.32, 2.54) | 0.22(0.03, 16.34) | |
| 15 to 24 years | 17 (36.2%) | 30 (63.8%) | 0.88(0.35, 2.19) | 0.08(001, 6.84) | |
| 25 to 34 years | 44 (59.5%) | 30 (40.5%) | 0.34(0.14, 0.78) | 0.02(0.01, 0.88) | |
| 35 to 44 years | 43 (55.1%) | 35 (44.9%) | 0.40(0.17, 0.92) | 0.21(0.04, 12.88) | |
| 45 to 54 years | 30 (56.6%) | 23 (43.4%) | 0.38(0.15, 0.92) | 0.01(0.00, 0.07) | |
| Residence | Urban | 33 (64.7%) | 18 (35.3%) | 1 | 1 |
| Rural | 123 (46.4%) | 142 (53.6%) | 2.11(1.13, 3.95) | 7.86(1.11, 55.71) | |
| Occupation | Farmer | 69 (46.0%) | 81 (54.0%) | 1 | 1 |
| Merchant | 12 (42.8%) | 16 (57.2%) | 1.13(0.50, 2.56) | 0.02(0.00, 0.55) | |
| Government employee | 5 (26.3%) | 14 (73.7%) | 2.38(0.82, 6.95) | 0.00(0.00, 0.05) | |
| student | 18 (42.8%) | 24 (57.2%) | 1.13(0.57, 2.26) | 0.27(0.01, 5.60) | |
| Daily laborer | 18 (78.3%) | 5 (21.7%) | 0.23(0.08, 0.67) | 0.08(0.01, 2.02) | |
| Private Employee | 7 (77.8%) | 2 (22.2%) | 0.24(0.04, 1.21) | 0.00(0.00, 4.76) | |
| House wife | 27 (60.0%) | 18 (40.0%) | 0.56(0.28, 1.12) | 0.06(0.01, 1.18) | |
| Educational status | Cannot read and write | 34 (51.5%) | 32 (48.5%) | 0.41(0.16, 1.09) | 0.01(0.00, 1.19) |
| Read and write only | 26 (31.0%) | 58 (69.0%) | 0.99(0.38, 2.57) | 0.01(0.00, 1.06) | |
| Primary (1-8) | 51 (60.0%) | 34 (40.0%) | 0.29(0.11, 0.75) | 0.00(0.00, 0.12) | |
| Secondary (9-12) | 37 (67.3%) | 18 (32.7%) | 0.21(0.07, 0.59) | 0.01(0.00, 0.12) | |
| College and above | 8 (30.7%) | 18 (69.3%) | 1 | 1 | |
| Family size | 1 to 3 members | 36 (32.7%) | 74 (67.3%) | 1 | 1 |
| 4 to 6 members | 46 (42.6%) | 62 (57.4%) | 0.65(0.37, 1.13) | 1.58(1.25, 9.98) | |
| > or=7 members | 74 (75.5%) | 24 (24.5%) | 0.15(0.08, 0.29) | 0.05(0.01, 1.45) | |
| Average monthly income | <or=500ETB | 70 (97.2%) | 2 (2.8%) | 0.01(0.00, 0.02) | 0.00(0.00, 1.01) |
| 501 to 1000ETB | 58 (87.8%) | 8 (12.2%) | 0.02(0.01, 0.07) | 0.00(0.00, 1.01) | |
| 1001 to 1500 ETB | 12 (11.3%) | 94 (88.7%) | 1.53(0.60, 3.89) | 1.30(0.15, 11.28) | |
| 1501 to 2000 ETB | 7 (41.2%) | 10 (58.8%) | 0.28(0.08, 0.92) | 0.11(0.01, 2.08) | |
| > or=2001 ETB | 9 (16.4%) | 46 (83.6%) | 1 | 1 | |
| Wear protective cloth | Yes | 34 (33.0%) | 69 (67.0%) | 1 | 1 |
| No | 122 (57.3%) | 91 (42.7%) | 0.36(0.22, 0.60) | 0.17(0.03, 0.90) | |
| Take anti malaria | Yes | 28 (80.0%) | 7 (20.0%) | 1 | 1 |
| No | 128 (45.5%) | 153 (54.5%) | 4.78(2.02, 11.31) | 9.56(4.21, 20.33) | |
| Used ITNS | Yes | 94 (62.7%) | 56 (37.3%) | 1 | 1 |
| No | 62 (37.4%) | 104 (62.6%) | 2.81(1.78, 4.44) | 14.24(2.37, 85.66) | |
| Visited lowland | Yes | 79 (42.0%) | 109 (58.0%) | 2.08(1.32, 3.29) | 0.83(0.18, 3.79) |
| No | 77 (60.2%) | 51 (39.8%) | 1 | 1 | |
| Radio access/Listen | Yes | 86 (59.7%) | 58 (40.3%) | 1 | 1 |
| No | 70 (40.7%) | 102 (59.3%) | 2.16(1.37, 3.39) | 1.69(1.08, 4.79) | |
| Health education on malaria | Yes | 73 (40.3%) | 108 (59.7%) | 1 | 1 |
| No | 83 (61.5%) | 52 (38.5%) | 0.42(0.26, 0.66) | 0.48(0.10, 2.29) | |
Table 4: Malaria distribution by socio-demographic characteristics of the respondents’ Kalala Health Center, East Wollega Zone, October to November, 2016.
Discussion
The prevalence of malaria among all febrile cases visited Kalala health center of Haro Limmu Woreda found that 156(49.4%). More than half 85(54.5%) was diagnosed for Plasmodium falciparum 15.8% and 6.6% was diagnosed for Plasmodium vivax and mixed (Plasmodium falciparum and Plasmodium vivax ) respectively. The prevalence was high among the study participants. This could be due to period of data collection which is the time of increase in malaria cases in the community. This study is almost similar with the study conducted in Oromia and Southern Nation Nationalities People Region (SNNPR) in that it shows the dominant species where Plasmodium falciparum [22] and also with the study conducted in Asandabo training center, Jimma Zone of Oromia, among 365 out patients where the dominant species was Plasmodium falciparum [17]. Finding from the current study showed that the common species of malaria identified was P. falciparum (54.5%) and P. Vivax (15.8%) that was less when compared to malaria species identified in Kola Diba Health center, North Gonder, Northwest Ethiopia which accounts for 75% and 25% respectively.23 The altitude of the study area, behavior of the study participants and malaria prevention activities in both study areas can differ that may result in the variation of the finding.
The current study found that prevalence of malaria was very high when compared to cross sectional study conducted on malaria prevalence in Chogoria hospital of Kenya which was 15.9% were positive for Plasmodium falciparum [20]. The dominant species was Plasmodium falciparum for both current study and study conducted in South Eastern Nigeria with a prevalence of 54.5% and 83% respectively. Even though the dominant species for both study was Plasmodium falciparum prevalence of Plasmodium falciparum infection was very high in comparison with current study but Plasmodium vivax (5.0%) was much less as compared to cross sectional study conducted in Nanmdi Azikwe University in Akwa South Eastern Nigeria [21]. The discrepancy with prevalence of malaria could be due to type of study participant’s behavior and practice towards malaria prevention and control. In addition, the time of data collection may vary with current study.
Most of the time malaria infection peaks during September to December in lowlands of Ethiopia which increases number of malaria patients in hospitals and health centers. Different study conducted in various area of regional state of Ethiopia found the prevalence of malaria infection vary from period to period, study method to study method and type of participants involved. Particularly prevalence of malaria among patients attending outpatient department (OPD) during January 2006 to December 2007in SNNPR (4.5%) and Oromia (0.9%) has great discrepancy to each other. The dominant malaria species in SNNPR was Plasmodium falciparum (69.4%) followed by Plasmodium vivax (1.25%).This finding has high prevalence of Plasmodium falciparum and low prevalence when compared to current study 54.5% and 15.8% respectively even though the dominant species of malaria was Plasmodium falciparum [22].
In Oromia regional state the dominant species of malaria parasite was found to be Plasmodium falciparum followed by Plasmodium vivax that goes in line with national malaria infection by species that is estimated to be 60 % and 40% respectively. In Asendabo, Jimma Zone, prevalence of malaria was 28.8% and in Sibu Sire health center, East Wollega the prevalence malaria among febrile cases who visited the health center were 20.1% which was much less than prevalence of malaria in current study area (49.4%) [17,23]. Again in Asendabo training health center like other health facility the dominant species of malaria parasite was P. falciparum (54.3%) followed by P. vivax (45.7%). Regarding P. falciparum, the prevalence is exactly similar with current study but that of P. vivax was much higher than current study which was 15.8%.16 The difference in malaria species is expected to be due to mixed infection not separately reported in case of Asendabo health center but in current study prevalence of mixed infection of malaria parasite was 6.6%.
Prevalence of malaria species in current study was found P. falciparum 54.5% and P. vivax 15.8%. When compared with study conducted in Nekemte hospital of East Wollega zone prevalence of malaria parasite was found that P. falciparum , P. vivax and mixed infection among febrile cases was 59.2%, 22.5% and 18.3% respectively [16] that indicates the dominant species among study participants was P. falciparum which is in line with current study in dominancy but the prevalence was slightly higher than this study. The prevalence of mixed infection with malaria parasite in current study (6.6%) was three fold less when compared with the study conducted in Nekemte hospital. The variation in the finding could be difference in study area, socioeconomic status and awareness on malaria prevention.
Factors associated with of malaria infection in current study was being female, age, rural residence, occupation educational status, family size, wearing protective cloth, taking anti malaria drug, using ITNs and access/listening to radio. These factors were also significantly associated with malaria infection in study conducted in Nekemte hospital [16].
In current study age and sex (female) was significantly associated with malaria infection. Similarly, in Eretria age, time to collect drinking water, geographic location and housing condition of participants positively associated with malaria infection [24,25].
In current study residence and wearing protective clothes while working near stagnant water was significantly associated with malaria prevalence. Similar study conducted in Dilla town of SNNP region, Ethiopia showed that living near stagnant water and having stagnant water in the compound positively associated with malaria infection in the study area [26].
Socio-economic and demographic characteristics of the study participants that increases the risk of malaria infection Kalala health center of Haro Limmu Woreda was being female that could be due to inability to give ITNs in priority for females, age group of 25 to 34 years and 45 to 54 years are less likely to be attacked by malaria, rural residence, not using ITNs in HH, lack of access to radio are at higher risk when compared with their counterparts. This finding goes in line with study conducted in Eretria [25], Dilla town [26], Asendabo health center [17], Sibu Sire woreda [24] and other study conducted in SNNPR and Oromia regional state [22].
Limitation of the Study
Since the main aim of the study is to investigate prevalence of malaria and associated factors among febrile patients in Kelala Health center, Haro Limmu Woreda, East Wollega zone, there may be discrepancy of prevalence between those who seek health care and not seek health care. There may be recall bias and social desirability bias in the study participants.
Conclusion
The finding of the study indicated that the prevalence of Malaria among febrile cases visited Kalala health center was high (49.4%) with a dominance of P. falciparum (54.5%) followed by P. vivax (15.8%). Almost all of the respondents know disease malaria and at list one prevention method.
Factors positively (risk) associated with malaria in febrile cases in the study area was found to be sex, age, residence, working near stagnant water and negatively (preventive) associated factors was ITNs utilization, access/listening to radio.
Recommendation
• Age and sex specific malaria prevention activities should be planned and implemented in the study area specifically and for the general community to tackle the infection during malaria transmission season.
• ITBNs utilization in Households must be strengthened through awareness creation and promotion in the community.
• Priority on using ITBNS should be given for at high risk group of people in the households like pregnant women and children under five.
• Prevention and control measures of malaria like avoiding working near stagnant water, clearing stagnant water near residence should be implemented, strictly followed and monitored in the community as per standard set by national malaria guideline.
• Community health workers and health workers should promote early health seeking behavior of the community when get febrile in the households and community.
Authors’ Contributions
KT and ZK were participated in the design of the study, supervised the data collection process, the quality of data, the statistical analysis and served as the lead authors of the manuscript. All authors read and approved the final manuscript.
Acknowledgments
The authors would like to express their sincere gratitude to Haro Limmu Woreda health office coordinators for giving us an opportunity to conduct this study in Kalala health center. The warmest thanks go to the study participants for their genuine response and Kalala Health Center staff for facilitating data and sample collection during study period. We would also thank our families for their psychological and financial support during this period.
References
- Hennery JB (2001) Clinical diagnosis management by laboratory method 20th end, W.H Saunders Company, Southampton.
- WHO (2014) Fact sheet world malaria report 2014. World Health Organisation.
- WHO (2015) Fact sheet World malaria report, global burden disease. World Health Organisation.
- No authors (2014) Ethiopian Federal Ministry of Health. Epidemiology and distribution of malaria in Ethiopia,2014. The Open University.
- Aschalew A, Tadesse D (2016) Current Status of Malaria in Ethiopia: Evaluation of the Burden, Factors for Transmission and Prevention Methods. Acta Parasitologica Globalis 7: 1-6.
- Warrell DA, Cox TM, Firth JD (2000) Oxford text book of medicine, global patterns of disease and medical practice. Oxford University Press.
- Nicholas JW, Arjen MD, Daniel H (2010) Parasitology plasmodium species, plasmodium falciparum. Antimicrobe.
- Culleton RL, Mita T, Ndounga M, Unger H, Cravo PVL, et al. (2008) Frailer to detect plasmodium vivax in west and central Africa by PCR species typing, Malaria journal 7: 1475-2875.
- Ministry of health (2016) Integrated surveillance and control program for west Nile. MESA.
- Monica C (1980) District laboratory practice in tropical countries. Part 1st end, Cambering university, UK.
- John L, Jeffery DS (2001) The Economic burden of malaria, Am J Trop Med Hyg 64: 85-96.
- FMOH (2011) Health and Health related indicators. Addis Ababa, Ethiopia.
- Amenu D (2014) Prevalence of Malaria among Patients Visiting Nekemte Hospital. J Med Microb Diagn 3: 137
- Fikadu D (2013) The prevalence of malaria in Assendabo training health center. Student research at Jimma University.
- Tekola E, Mulat Z, TesfayeT, Berhan A (2008) Individual house hold and Environmental risk factors for malaria infection in Ahmara, oromia and SNNP regions, Ethiopia. Trans R Soc Trop Med Hyg 10: 4588-4521.
- John LG, Jeffery D (2014) The economic burden of malaria, Center for international development at Harvard.
- Tjitra E, Suprianto S, Dyer M, Currie BJ, Anstey NM (1999) Field evaluation of the ICT malaria P.f/P.vimmunochromatographic test for detection of Plasmodium falciparum and Plasmodium vivax in patients with a presumptive clinical diagnosis of malaria in eastern Indonesia, J Clin Microbiol 37: 2412-2417.
- Ibekwe J, Okonko AC, onunkwo IO (2009) Comparative prevalence of plasmodium in the first Year student,Nnmdiazikwe University in A wka, South East Nigeria,2009. Malaysian Journal of Microbiology 8: 51-54.
- Shargie EB1, Gebre T, Ngondi J, Graves PM, Mosher AW, et al. (2013) Prevalence malaria and Mosquitoes net Coverage in Oromia and SNNPR regions of Ethiopia, BMC Public Health 8: 321.
- Alemu A, Muluye D, Mihret M, Adugna M, Gebeyaw M (2012) Ten-year trend analysis of malaria prevalence in kola Diba, North Gondor, Northwest Ethiopia. Parasit Vectors: 1756-3303.
- Temesgen G, Abdi S, Delensaw Y (2015) Ten-year analysis of malaria Prevalence and its correlation with climatic variables in SibuSire district East Wollega Zone, Wollega University. Star journal 4: 99-105.
- Sintasath DM, Ghebremeskel T, Lynch M, Kleinau E, Bretas G (2005) Malaria prevalence and associated risk factors in Eritrea. Am J Trop Med Hyg 72: 682-687.
- Molla E, Ayele B (2015) Prevalence of Malaria and Associated Factors in Dilla Town and the Surrounding Rural Areas, Gedeo Zone, Southern Ethiopia. J Bacteriol Parasitol 6: 242.
- Ethiopian Federal Ministry of Health (2014) Epidemiology and distribution of malaria. Health Sector Development Programme IV.
Citation: Bidu KT, Babure ZK (2019) Prevalence of Malaria and Associated Factors among Febrile Patients Visiting Kalala Health Center in Haro Limmu Woreda, East Wollega Zone, Western Ethiopia, 2016. Epidemiology (Sunnyvale) 9: 365. DOI: 10.4172/2161-1165.1000365
Copyright: © 2018 Bidu KT, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 3909
- [From(publication date): 0-2019 - Dec 27, 2024]
- Breakdown by view type
- HTML page views: 3101
- PDF downloads: 808