Research Article Open Access
Prevalence of Human Herpes Virus 6 (HHV-6) in Patients with Hemodialysis
| Tarhan G1, Yilmaz N2, Ilhan F3, Çeliker H4, Cesur S5* and Çiftçi A6 | |
| 1Department of Medical Microbiology, Faculty of Medicine, Adiyaman University, Ad├?┬▒yaman,Turkey | |
| 2Department of Infectious Diseases and Clinical Microbiology, Faculty of Medicine, Bozok University, Yozgat, Turkey | |
| 3Department of Immunology, Faculty of Medicine, Firat University, Elaz├?┬▒├?┬?, Turkey | |
| 4Department of Internal Medicine, Faculty of Medicine, Firat University, Elaz├?┬▒├?┬?, Turkey | |
| 5Infectious Diseases and Clinical Microbiology Clinic, Ankara Education and Research Hospital, Ankara, Turkey | |
| 6Department of Internal Medicine, Faculty of Medicine, K├?┬▒r├?┬▒kkale University, K├?┬▒r├?┬▒kkale, Turkey | |
| Corresponding Author : | Sarah Cesur Infectious Diseases and Clinical Microbiology Clinic Ankara Education and Research Hospital, Ankara, Turkey Tel: +90-312-3103333 E-mail: scesur89@yahoo.com |
| Received: February 26, 2015 Accepted: July 13, 2015 Published: July 20, 2015 | |
| Citation: Tarhan G, Yilmaz N, Ilhan F, Çeliker H, Cesur S, et al. (2015) Prevalence of Human Herpes Virus 6 (HHV-6) in Patients with Hemodialysis. J Infect Dis Ther 3:224. doi:10.4172/2332-0877.1000224 | |
| Copyright: © 2015 Tarhan G et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
| Related article at Pubmed, Scholar Google | |
Visit for more related articles at Journal of Infectious Diseases & Therapy
Abstract
HHV-6 is the causative agent of exanthema subitum in children. The main modes of transmission of HHV-6 arebody secretions such as infected saliva but it can be transmitted with blood and blood products that are infected with\the virus. Therefore, it is thought that the immunocompromised hemodialysis patients who had multiple bloodtransfusions due to various reasons, and who collectively use devices such as hemodialysis machines are at risk forHHV-6 infection. The present study aimed to determine the incidence of HHV-6 infection in hemodialysis patients.
Twenty-five healthy individuals that were matched with 25 hemodialysis patients in terms of gender and age wereincluded in the study. The IgM and IgG sero-prevalence in the patient and control groups wereinvestigated with theindirect fluorescence antibody method and HHV-6 DNA prevalence and the determination of variant types (variant Aand variant B) were investigated with the PCR-RFLP molecular method. HHV-6 IgG and HHV-6 IgM antibodypositivity ratios in the patient and control groups were 76% and 20% respectively. Out of 50 serum samples of hemodialysis patients and healthy individuals, HHV-6 DNA was positive in seven of 25 samples of hemodialysispatients (28%) and it was positive in eight of 25 samples of the control group (32%). Variant analysis was performedwith the PCR-RFLP method in HHV-6 DNA positive hemodialysis patients and control patients. At the end of theanalysis, while variant A was not detected in the patient and control groups, variant B was detected in a total of 15individuals, including seven patients in the hemodialysis patient group and eight patients in the control group. Inconclusion, the researchers believe that there is a need for controlled studies including more samples to determinethe clinical importance of HHV-6 DNA and HHV-6 variants in hemodialysis patients.
The present study aimed to determine the HHV-6 IgM and IgG sero-prevalence with the indirect fluorescence antibody (IFA) method and HHV-6 DNA prevalence and determination of variant species (variant A and variant B) with the PCR-RFLP molecular method in hemodialysis patients.
Patients: As part of the study, 25 adult (25-60 years) patients undergoing hemodialysis in the Hemodialysis Unit of Fırat University Medical Faculty due to chronic renal failure and 25 healthy individuals that were matched in terms of age and gender as the control group were included to the study.
Preparation of blood samples: Five ml of blood without anticoagulant was taken from peripheral veins of the individuals in the patient and the control groups. After holding the blood at room temperature for 1-2 hours, they were separated into serums by centrifugation for 10 minutes at 3000 × g. The serum samples that were to be used for the PCR test were extracted and purified using DNA extraction and purification kits (Epicentre Technologies, Madison, Wisconsin). After the purification process, the purity control was performed on each sample by the spectrophotometric method. Each sample was kept at -20°C until the study day. The serum samples that were to be used for serological examination by the IFA method were stored at -20°C.
Reproduction of the target gene region with polymerase chain reaction (PCR): For amplification of HHV-6 DNA, a sequence of 830 base pair of LTP (large tegument protein) region was chosen as the target region on viral genome [4]. The region is protected among the HHV-6 species that demonstrate genetic variability, and does not react with other herpes viruses. For this purpose, the A: 5' - GAT CCG ACG CCT ACA AAC AC - 3' and C: 5' - CGG TGT CAC ACA GCA TGA ACT CTC - 3' primer sequences were used. The PCR mixture was prepared by combining apyrogenic water, PCR buffer (1X), PCR enhancer (1X), Mg Cl2 (2.5 mM), dNTP mixture (200 μμM), each primer (20 p mol/μμl), Taq DNA polymerase (5 U/μμl), 5 μμl of sample DNA that will be tested, to produce a final reaction mixture to be 50μμl for each sample. For amplification, the tubes were stored at 94°C for 3 minutes for initial denaturation, producing 40 cycles (denaturation at 94°C for 45 sec., annealing at 62°C for 45 sec., extension at 72°C for 1.15 sec.), at final extension 72°C for 4 minutes (Eppendorf Thermal Cycler, USA Scientific, Inc.) [6].
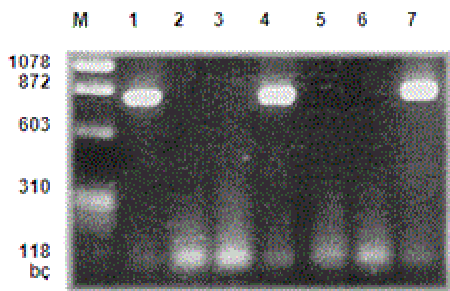
Examination of PCR reproduction products with electrophoresis: PCR products were placed on agarose gel of 1.5%. After this phase, the gel was kept in ethidium bromide solution (0.5 mg/ml), which was previously prepared, for 20 minutes and stained; the bands that were seen under an ultraviolent light source were evaluated. In all studies, negative controls were used. The samples demonstrating reproduction products under 830 bp magnification were taken to restriction enzyme analysis for typing (Figure I).
M- Marker μφ X174 Hae III MV
1-Positive control
2-Negative control
3, 5, 6- HHV-6 DNA (-) samples
4, 7- HHV-6 DNA (+) samples
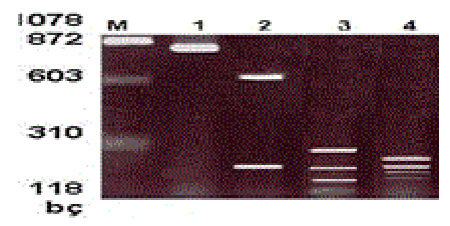
Restriction fragment length polymorphism (RFLP): Restriction endonuclease enzyme analysis was performed to type the visible bands of the samples with positive PCR reaction results on agarose gel. The amplification product of each patient was cut with Hind III, Hinf I and Taq I enzymes. The prepared reaction mixture was incubated at 37°C for one night for cutting with Hind III and HinfI enzymes and at 65°C for cutting with the Taq I enzyme. Two percent agarose gel was prepared for the cutting products. Ten μμl of cut samples and 5 μμl of uncut samples of the same patient were loaded together with the loading tamponade to the wells on the gel. It was run at 110 volts for 40 minutes. After staining with ethidium bromide, the gel was examined with a UV-transilluminator [4-7] (Figure II).
M- Marker μφX174 Hae III MV
1- HHV-6 DNA positive control
2-Amplified product that is cut with Hind III enzyme
3- Amplified product that is cut with Hinf I enzyme
4-Amplified product that is cut with Taq I enzyme
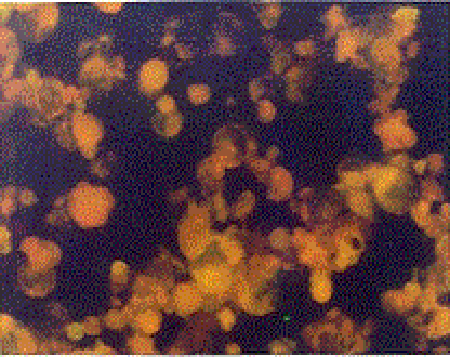
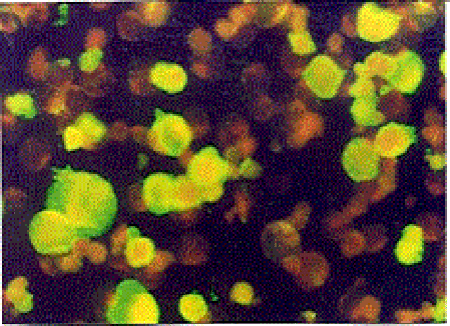
Indirect Immunofluorescence Antibody Test (IFAT): The HHV-6 specific Ig G and Ig M antibodies were investigated by using HHV-6 IgG ( V3 HHV6) and HHV-6 IgM ( V17 HHV6 ) IFA kits obtained from the Biotrin company. In all study phases of the test, the principles and the standards defined in the kit procedure were obeyed. Upon evaluation of the images with a fluorescence microscope under 200-500× magnification in a dark room, the degree of density of the fluorescence reaction was used as a base. The cells with green-yellow fluorescence on a black ground were accepted as (+), other cells were accepted as negative. According to the degree of the fluorescence reaction (+), cells were graded as very bright ++++, bright +++, intermediately bright ++, and weak + (Figure III, IV).
Statistical analysis: All statistical analyses were performed with the SPSS, version 14.0. Upon analysis of the data, Fisher’s exact chi-square tests were used. A P value of <0.05 was considered significant.
When the patient and the control groups were statistically compared, no significant difference was found between the groups in terms of HHV-6 IgG antibody positivity. Furthermore, no statistically significant difference was found between the groups in terms of HHV-6 IgM antibody positivity (p=0.29). HHV-6 IgM sero-positivity ratio in hemodialysis patients did not differ from the healthy control group.
PCR - RFLP results: In the study, HHV-6 DNA was examined with PCR in 50 serum samples of hemodialysis patients and healthy individuals. HHV-6 DNA was positive in 15 samples and negative in 35 out of a total of 50 serum samples. When the HHV-6 DNA positivity ratio was evaluated according to the study groups, it was positive in seven of 25 samples of hemodialysis patients (28%) and it was positive in eight of 25 samples of the control group (32%).The results are presented in Table II.
The positive (+) or negative (-) evaluation of the samples that were amplified with the PCR method in terms of HHV-6 DNA was performed with agarose gel electrophoresis. In agarose gel electrophoresis, the presence of a band in a region of 830 bp was evaluated as PCR (+) and absence of a band in 830 bp was evaluated as PCR (-) (Figure III). For the Hind III enzyme, the isolates that did not show any cut region (830 bp) were accepted as variant A, the isolates that were separated into fragments of 610 and 220 bp were accepted as variant B. For the Hinf I enzyme, the isolates that were separated into fragments of 530, 110, 100, and 90 bp were accepted as variant A and the isolates that were separated into fragments of 300, 200, 150, 100, and 90 bp were accepted as variant B. For the Taq I enzyme, the isolates that were separated into fragments of 630 and 200bp were accepted as variant A, the isolates that were separated into fragments of 270, 200,180 and 160 bp were accepted as variant B (28) ( Figure IV). The variant distribution in healthy individuals and hemodialysis patients after cut of positive PCR samples with the Hind III enzyme is demonstrated in Table III.
While variant B is detected at a rate of 100% in all study groups, no variant a type was observed as a result of RFLP analysis.
In the present study, a correlation was found between HHV-IgG sero-positivity and duration of hemodialysis in hemodialysis patients. Upon the examination of presented study groups by the PCR-RFLP method, HHV-6 DNA positivity in both hemodialysis patients and the control group were at a lower rate than the other studies. This might result from the fact that the nested PCR method is more sensitive than the method that is used in the current study [12]. In the present study, PCR and the IgM positivity as a marker of acute infection at a rate (28%) confirming each other in the hemodialysis patients could be interpreted as acute infection and higher rates of PCR positivity (32%) and lower rates of IgM positivity (12%) in the control group when compared with the hemodialysis patients could be interpreted as a marker of latent infection. There are two different variants of HHV-6. These variants are HHV variant A and variant B [13]. The primary infection is seen mostly due to variant B, however it may develop due to variant A. HHV-6 can be seen in transplantation patients as contamination of the donor, reactivation of the latent infection or reinfection. The most common HHV-6 variant isolated in blood samples of renal transplant patients is variant B, however variant A is more virulent [13-15]. Upon molecular typing of HHV-6 DNA positive samples by RFLP test, the variant B type is found at a rate of 100% in all samples and variant B type was not observed; whereas Yalçın et al. reported HHV-6 DNA positivity at a rate of 63% in renal transplant recipients [12]. Upon type differentiation conducted by the RFLP method they detected that 70% were variant B and 30% were variant A. Similarly to other studies, the current findings demonstrate that variant B is more frequent than variant A. Although HHV-6 variant B reactivation is frequently reported in renal transplant patients, in a study conducted by Csoma et al. in 200 patients who had renal transplantation, they found that HHV-6 variant A viremia is dominant, contrary to previously reported results [13]. In the current study, the HHV-6 variant A in patients with active infection was significantly higher than the patients with latent infection. HHV-6 reactivation is common in the immunocompromised patient group, especially in renal transplant patients. Lempinen et al. investigated the presence of HHV-6 and CMV with immunohistochemical methods in gastroduodenal and colon biopsy samples of 81 renal transplantation patients and 46 chronic dialysis patients [16]. In this study, the HHV-6 variant B positive cell ratio in gastroduodenal biopsy samples was reported as 34% and 28% in renal transplant patients and dialysis patients, respectively. CMV positivity ratio in the same tissue samples were 53% and 28% in renal transplant patients and hemodialysis patients, respectively. In the same study, while HHV-6B positive cell ratio in colon mucosa samples was 36% in renal transplant patients, it was 22% in hemodialysis patients. CMV positivity ratios were 36% and 17% in renal transplant patients and hemodialysis patients, respectively.In this study, HHV-6 variant A positivity was not reported. Both HHV-6 variant B and CMV positive cells were found at the same time in 50 patients who had renal transplantation. In the current study, detected HHV-6B positivity in serum samples at a rate of 28% (7/25) in hemodialysis patients is similar to the study of Lempinen et al. [16]. The HHV-6 variant A was not detected in either the patient group or the control group. As a result, the researchers believe that there is a need for controlled studies including more samples to determine the clinical importance of HHV-6 infection and HHV-6 variants in hemodialysis patients who are immunocompromised hosts, require frequent blood transfusions, and are candidates for renal transplantation.
References
- Levy JA, Ferro F, Greenspan D, LennetteET (1990)Frequent isolation of HHV-6 from saliva and high seroprevalence of the virus in the population. Lancet 335: 1047-1050.
- Okuno T, Takahashi K, Balachandra N, Shiraki K, Yamanashi, K, Takashi M, Baba K (1989) Seroepidemiology of Human Herpesvirus - 6 infection in normal children and adults. J ClinMicrobiol 27: 651-655.
- Luppi M, Marasca R, Barozzi P, Ferrari S, Ceccherini NL, et al. (1993) Three cases of Human Herpesvirus-6 latent infection : Integration of viral genome in peripheral blood mononuclear cell DNA. J Med Virol 40: 44-52.
- Joseps SF, Ablashi DV, Salahuddin SZ, Kramarsky B, Franza BR, et al. (1988) Molecular studies of HHV-6 .J. Virol Methods 21: 179-190.
- Martin MED, Thomson BJ, Honess RW, Craxton MA, Gompels UA, et al. (1991)The genome of Human Herpesvirus 6 : Maps of unit-length and concatemeric genomes for nine restriction endonucleases. J Gen Virol 72: 157-168.
- Luca DD, Mirandola P, De Re VR, Secchiero P, Carbone A, et al. (1994) Human Herpesvirus 6: A survey of presence and variant distribution in normal peripheral lymphocytes and lymphoproliferative disorders. J Infect Dis 170: 211-215.
- Aubin JT, Collandre H, Candotti D, Ingrand C, Rouzioux M, et al. (1991) Several groups among Human Herpesvirus 6 strains can be distinguished by southernblotting and polymerase chain reaction. J ClinMicrobiol 29: 367-372.
- Grose C (1992) Human Herpesvirus type 6 in " Textbook of Pediatric Infectious Diseases", 3rd ed.,(Cherry, F., ed.) Boston, p: 1583-1586.
- Okuno T, Higashi K, Shiraki K, Yamanishi M ,Takahashi Y, et al. (1990) Human Herpes virus 6infection in renal transplantation.Transplantation 49: 519-522.
- Yoshikawa T, Suga S, Asano Y, Nakashima T, Yazaki T, et al. (1992)Aprospective study of human herpesvirus 6infection in renal transplantation.Transplantation 54 :879-883.
- Altay M, Akay H, Ünverdi S, Altay F, Ceri M, et al. (2011) Human herpesvirus 6 infection in hemodialysis and peritoneal dialysis patients. Perit Dial Int 31:320-324.
- YalçinS, Karpuzoglu G, Süleymanlar G, Mutlu G, Mukai T, et al. (1994) Human herpes virus 6 and human herpesvirus7 infections in renal transplant recipients and healthy adults in Turkey. Arch Virol 136:183-190.
- Csoma E, Mészáros B, Gáll T, Asztalos L, Kónya J,et al. (2011) Dominance of variant A in human herpesvirus 6 viraemia after renal transplantation. Virol J8: 403.
- Singh N (2000) Human herpesviruses-6, -7 and -8 in organ transplant recipients. Clin Microbiol Infect 6: 453-459.
- Ljungman P, Singh N (2006) Human herpesvirus-6 infection in solid organ and stem cell transplant recipients. J Clin Virol 37 Suppl 1:S87-91.
- Lempinen M, Halme L, Arola J, Honkanen E, Salmela K, et al. (2012) HHV-6B is frequently found in the gastrointestinal tract in kidney transplantation patients. Transpl Int 25: 776-782.
--
Tables and Figures at a glance
| Table 1 | Table 2 | Table 3 |
Figures at a glance
 |
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 |
Relevant Topics
- Advanced Therapies
- Chicken Pox
- Ciprofloxacin
- Colon Infection
- Conjunctivitis
- Herpes Virus
- HIV and AIDS Research
- Human Papilloma Virus
- Infection
- Infection in Blood
- Infections Prevention
- Infectious Diseases in Children
- Influenza
- Liver Diseases
- Respiratory Tract Infections
- T Cell Lymphomatic Virus
- Treatment for Infectious Diseases
- Viral Encephalitis
- Yeast Infection
Recommended Journals
Article Tools
Article Usage
- Total views: 16715
- [From(publication date):
August-2015 - Nov 21, 2024] - Breakdown by view type
- HTML page views : 12239
- PDF downloads : 4476
