Prevalence and Outcomes of Familial Hypercholesterolemia Patients in a Chinese Myocardial Infarction Cohort
Received: 16-Aug-2017 / Accepted Date: 21-Aug-2017 / Published Date: 24-Aug-2017
Abstract
Background and aims: Familial Hypercholesterolemia (FH) is an inherited metabolic disorder with increased LDL-C levels and coronary heart disease (CHD) risk. The prevalence of heterozygous FH is approximately 1:200 to 1:500 worldwide and higher in CHD populations. We aim to estimate the prevalence of FH and the incidence of recurrent cardiovascular events among FH and non-FH patients in a large myocardial infarction cohort in China.
Methods: We studied the cohort from the China Patient-Centered Evaluative Assessment of Cardiac Events Prospective study (PEACE-Prospective). The eligible cohort included 3367 patients hospitalized for myocardial infarction (MI).
Results: The proportion of potential FH was 0.80% and 4.28% by Dutch Lipid Clinic Network (DLCN) and modified DLCN criteria, respectively. Compared to non-FH, the FH patients were younger, having more personal and family history of premature CHD, current smokers and overweight. The exome sequencing identified 11 cases of pathogenic variants on LDL receptor and ApoB. The risk of recurrent cardiovascular events after MI was greater in FH patients with a hazard ratio (HR) of 1.97 with modified DLCN.
Conclusions: The prevalence of Chinese FH by modified DLCN is comparable to the European estimate by DLCN in the high cardiovascular risk cohort. The patients with FH compared to non-FH have an almost 2-fold adjusted risk of recurrent cardiovascular events. Further consensus on the LDL-C-based threshold may help on the establishment of country specific criteria for FH.
Keywords: Familial hypercholesterolemia; Dutch lipid clinic network criteria; Modified dutch lipid clinic network criteria
5943Introduction
Familial hypercholesterolemia (FH) is an autosomal dominant genetic disorder with elevated LDL cholesterol (LDL-C) and increased risk for premature coronary heart disease [1,2]. FH is primarily attributed to autosomal inheritance of mutations in the low density lipoprotein receptor gene (LDLR), apolipoprotein B (apoB) and proprotein convertase subtilisin/kexin type 9 (PCSK9). These mutations result in an approximately 20-fold increased risk of premature coronary heart disease (CHD) in untreated patients [3,4]. At present, the prevalence of heterozygous FH (HeFH) is estimated to be 1:500 (0.2%) to 1:200 (0.5%) in the general population by clinical diagnosis [1,5]. Several recent studies reported the prevalence rose among patients with CHD ranging from 1.6% to 8.3% depending on the cohort and clinical definition of FH [6,7]. However, systematic evaluation of FH prevalence in China remains unreported.
Referred to the prediction of HeFH prevalence from the European study [1], the number of FH patients in China could be between 2.6 million and 6.5 million. At present, there are no suitable and well established diagnostic criteria for Chinese population. Epidemiology reports suggest LDL-C levels of Chinese population are generally lower than that in Western population [8,9]. Thus, FH in Chinese population may be underdiagnosed by the criteria widely used in the West, such as the Dutch Lipid Clinic Network (DLCN) criteria. Shi et al. used a set of modified DLCN (MDLCN) criteria to identify FH patients in China, and estimated the prevalence of FH as 0.28% in the general population [10]. Sha Li et al. recently reported the prevalence of FH (by DLCN) as 3.9% in a mono-site Chinese MI cohort [11]. However, the FH prevalence from nationwide multi-center cohort has not been investigated. There is limited information about outcome of FH patients after MI, especially on long term major adverse cardiac events (MACE).
This study identified FH patients in a large nationwide cohort (China PEACE-Prospective AMI study including patients enrolled in pilot phase). We aim to 1) estimate the proportion of FH by different diagnostic criteria DLCN vs. MDLCN; 2) compare clinical characteristics of FH patients between different diagnostic criteria; 3) describe FH pathogenic mutations on LDLR, ApoB and PCSK9 genes in Chinese MI patients; and 4) estimate the incidence rate of recurrent cardiovascular (CV) events in FH patients by different diagnostic criteria.
Patients And Methods
Study sample
The study samples were collected from subjects in the China PEACE-prospective AMI [12] as well as the pilot phase cohort. In brief, the China PEACE-prospective AMI study enrolled approximately 4,000 consecutive patients admitted for acute myocardial infarction. These subjects are aged 18 years or older, within 24 h of symptom onset, and from more than 50 diverse hospitals across China from December 30, 2012 to June 1, 2014. The inclusion criteria for pilot phase AMI cohort are the same except that there was no strict record of the symptom onset time. Details of medical history, treatment, and in-hospital outcomes were abstracted from medical records. Comprehensive baseline interviews were conducted to characterize patient demographics, risk factors, presentation, and healthcare status. Post-discharge follow-up interviews were conducted at 1, 6, and 12 months after discharge, to collect information of medication adherence, risk factor control, and report any hospitalization during the follow-up period. Supporting documents for potential outcomes were collected for adjudication by clinicians at the National Center for Cardiovascular Diseases (NCCD). Blood pressure, height, weight, and waist circumference were measured at baseline as well as each follow-up visit. Blood samples were obtained for all patients during the first 24 hours of hospital admission. All laboratory analyses were performed at the NCCD. TC, LDL-C, HDL-C and triglyceride (TG) concentrations were measured by standardized enzymatic methods (Beckman Coulter AU680 analyzers, Beckman AU reagent). Written informed consent was obtained from each patient included in the study. The study protocol of China PEACE-prospective conforms to the ethical guidelines of the 1975 Declaration of Helsinki and the study protocol has been previously approved by the NCCD Institution's ethics committee on research on humans. This study was registered on www. clinicaltrials.gov (Registration No. NCT01624909).
In this study, we finally restricted the samples (n=3367) to patients with a definite discharge diagnosis of AMI, and blood samples taken at baseline, and without missing baseline information. Among these patients, 412 were from pilot phase cohort and 2955 were from PEACE-prospective cohort.
Diagnosis of FH
Diagnosis of FH patients was mainly based on the DLCN criteria and the MDLCN criteria with the LDL-C algorithm adjusted by 1.5-2.5 mmol/L to develop a numerical score [10] (Supplementary Table 1). Information used for definite, probable and possible FH identification included clinical history, family history, LDL-C levels and genetic analysis. By both criteria, patients with a score larger than 8 were classified as definite FH, 6-8 as probable and 3-5 as possible FH. Tendon xanthoma, corneal Arcus and family LDL-C levels were not available in our study and missing information was counted as zero. Family history of premature CHD missing in 141 (4.2%) patients were also counted as zero. For those patients on statin therapy "untreated” LDL-C level was estimated by multiplying correction factor of 1.43 [5]. We combined “definite” and “probable” FH into a single definition of “potential” FH.
Clinical variables and outcome
Patient-level independent variables in this study included demographic information, cardiovascular risk factors, medical and family history of premature CHD which was defined as the onset age of CHD Table 1.
| All (3367) | FH diagnosis by DLCN | FH diagnosis by MDLCN | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Potential (27) | possible | unlikely | p for trend | Potential FH (144) | possible | unlikely | p for trend | ||
| -408 | -2932 | -1369 | -1854 | ||||||
| Demographics | |||||||||
| Age | 61 | 50 | 50 | 62 | <0.000 | 50 | 55 | 65 | <0.000 |
| (52, 70) | (43, 55) | (45, 57) | (55, 71) | (44, 57) | (48, 65) | (59, 72) | |||
| Female | 795 (23.6) | 7 (25.9) | 93 (22.8) | 695 (23.7) | 0.818 | 41 (28.5) | 322 (23.5) | 432 (23.3) | 0.371 |
| Male | 2572 (76.4) | 20 (74.1) | 315 (77.2) | 2237 (76.3) | 0.818 | 103 (71.5) | 1047 (76.5) | 1422 (76.7) | 0.371 |
| Cardiovascular risk factors | |||||||||
| Hyperlipidemia | 1024 (30.4) | 14 (51.9) | 166 (40.7) | 844 (28.8) | <0.000 | 62 (43.1) | 491 (35.9) | 471 (25.4) | <0.000 |
| Hypertension | 1875 (55.7) | 14 (51.9) | 230 (56.4) | 1631 (55.6) | 0.945 | 80 (55.6) | 736 (53.8) | 1059 (57.1) | 0.109 |
| Diabetes | 795 (23.6) | 7 (25.9) | 108 (26.5) | 680 (23.2) | 0.153 | 46 (31.9) | 328 (24.0) | 421 (22.7) | 0.044 |
| Current Smoker | 1933 (57.4) | 16 (59.3) | 264 (64.7) | 1653 (56.4) | 0.004 | 91 (63.2) | 839(61.3) | 1003 (54.1) | <0.000 |
| BMI ≥ 25 | 720 (21.4) | 8 (29.6) | 121 (29.7) | 591 (20.2) | <0.000 | 44 (30.6) | 347 (25.3) | 329 (17.7) | <0.000 |
| Comorbidities | |||||||||
| Pre-exiting CHD | 1445 (42.9) | 14 (51.9) | 182 (44.6) | 1249 (42.6) | 0.266 | 67 (46.5) | 572 (41.8) | 806 (43.5) | 0.758 |
| Premature CHDa | 1062 (31.5) | 21 (77.8) | 322 (78.9) | 719 (24.5) | <0.001 | 116 (80.6) | 737 (53.8) | 209 (11.3) | <0.000 |
| Heart failure | 865 (25.7) | 10 (37.0) | 86 (21.1) | 769 (26.2) | 0.172 | 36 (25.0) | 332 (24.3) | 497 (26.8) | 0.14 |
| Family history | |||||||||
| Premature CHD | 368 (10.9) | 8 (29.6) | 136 (33.3) | 224 (7.6) | <0.000 | 52 (36.1) | 149 (10.9) | 167 (9.0) | <0.000 |
| Lab test at admission | |||||||||
| TC (mmol/L) | 4.7 | 8.1 | 5.9 | 4.5 | <0.000 | 6.5 | 5.4 | 4.1 | <0.000 |
| (3.9, 5.5) | (6.6, 8.7) | (4.9, 6.9) | (3.8, 5.3) | (5.8, 7.8) | (4.8, 6.0) | (3.5, 4.7) | |||
| LDL-Cb | 3 | 6.6 | 4.2 | 2.9 | <0.000 | 5.1 | 3.7 | 2.6 | <0.000 |
| (mmol/L) | (2.4, 3.6) | (5.6, 7.5) | (3.2, 4.9) | (2.4, 3.5) | (4.0, 6.2) | (3.2, 4.1) | (2.1, 3.0) | ||
| HDL-C (mmol/L) | 0.9 | 0. 9 | 0.9 | 0.9 | <0.000 | 0.9 | 0.9 | 0.8 | <0.000 |
| (0.7, 1.0) | (0.8, 1.1) | (0.7, 1.1) | (0.7, 1.0) | (0.8, 1.1) | (0.8, 1.1) | (0.7, 1.0) | |||
| Triglycerides (mmol/L) | 1.4 | 1.9 | 1.8 | 1.3 | <0.000 | 2 | 1.6 | 1.2 | <0.000 |
| (1.0, 2.0) | (1.2, 2.9) | (1.3, 2.4) | (0.9, 1.9) | (1.4, 2.8) | (1.1, 2.2) | (0.8, 1.7) | |||
| Statins use at admission | 246 (7.3) | 13 (48.1) | 50 (12.3) | 183 (6.2) | <0.000 | 36 (25.0) | 105 (7.7) | 105 (5.7) | <0.000 |
Table 1: Patient baseline characteristics stratified according to DLCN and MDLCN diagnosis criteria. BMI: Body Mass Index; CHD: Coronary Heart Disease; TC: Total Cholesterol; LDL-C: Low-Density Lipoprotein Cholesterol; HDL-C: Low-Density Lipoprotein Cholesterol; Data expressed as median (IQR) or percentage. DLCN, Dutch. a Premature CHD was defined as age of onsetb In those on a statin at admission, LDL-cholesterol concentrations were multiplied by 1.43.
The clinical outcome was a composite of cardiovascular events, including cardiac death, nonfatal AMI, coronary revascularization, and ischemic stroke, occurred any time within 1 year after the index AMI hospitalization. The first outcome event of each patient after discharge was analysed after adjudication. In order to assess the association between FH and recurrence of cardiovascular events, we excluded patients who died during the index hospitalization (n=18). Therefore, a total of 3349 patients were included for the association analysis.
Genetic testing and variant calling
In sampling for genetic testing, we referred to the recommendation of the European Atherosclerosis Society [1] and selected 27 DCLN>5 patients (definite and probable FH). Among them 24 available DNA samples were sequenced. Genomic DNAs were isolated using QIAamp DNA Mini kits (Qiagen). DNA libraries were constructed according to the protocol provided by the manufacturer with the Illumina Trusight One Sequencing Panel (Illumina). Exome sequencing data were generated using Illumina Next sequence 500 platform in 2 × 150-bp paired-end reads. All identified variants were annotated against public database, including HGMD [13] and 1000 genomes project (https://www.1000genomes.org/) to determine the pathogenic variants on LDLR, ApoB and PCSK9 genes.
Statistical analysis
The prevalence of potential FH diagnosed by DLCN and MDLCN were calculated as the ratio between the number of cases and the total number of subjects. The values were expressed as mean (SD) or median (first quartile, third quartile) for continuous variables and numbers (percentage) for categorical variables. Patients were categorized according to the DLCN and MDLCN and clinical characteristics were reported in each group. Cochran-Armitage trend test [14] was used for binary variables and Mann-Kendall trend test [15] was used for continuous variables to determine if differences within the DLCN or MDLCN groups were statistically significant. We fitted a Cox proportional hazard model to assess the risk of recurrent events after AMI, with and without adjustment for age, sex, CV risk factors and reperfusion therapy, for DLCN and MDLCN groups, respectively. In the first model, we adjusted only for age and sex. In the second model, we adjusted for known CV risk factors, including age, sex, body mass index, current smoking, hypertension, and diabetes mellitus. In the final model, we further adjusted for reperfusion therapy during the index hospitalization, including thrombolytic therapy, primary percutaneous coronary intervention (PCI) and coronary artery bypass grafting (CABG). Patients who were lost to follow-up within 12 months were censored in the Cox model. All hypothesis tests were 2 sided, and the significance level was set at 0.05. The statistical analysis was performed by SAS version 9.4 software (SAS Institute Inc, Cary, NC, USA).
Results
Prevalence of FH by DLCN and MDLCN
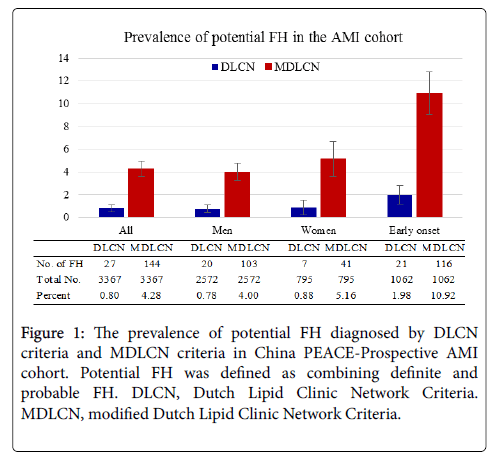
FH patients were diagnosed by DLCN and MDLCN according to published criteria summarized in Supplementary Table 1 [10]. By DLCN criteria, we identified 27 potential FH, the prevalence was 0.80% (95% CI, 0.50%-1.10%) in all 3367 AMI patients. It differentiated in 2572 men 0.78% (95% CI, 0.44%-1.12%) and 795 women 0.88% (95% CI, 0.23%-1.53%). Using the MDLCN criteria, we identified 144 potential FH, the prevalence increased to 4.28% (95% CI, 3.59%-4.96%). It was significantly lower in men 4.00% (95% CI, 3.25%-4.76%) than in women 5.16 (95% CI, 3.62%-6.69%) (Figure 1). Compared to the whole AMI cohort, the 1062 patients with premature MI have more than 2 times higher FH prevalence at 1.98% (95% CI, 1.14%-2.81%) by DLCN and it dramatically increased to 10.92% (95% CI, 9.05%-12.80%) by MDLCN (Figure 1).
Clinical characteristics of FH Patients
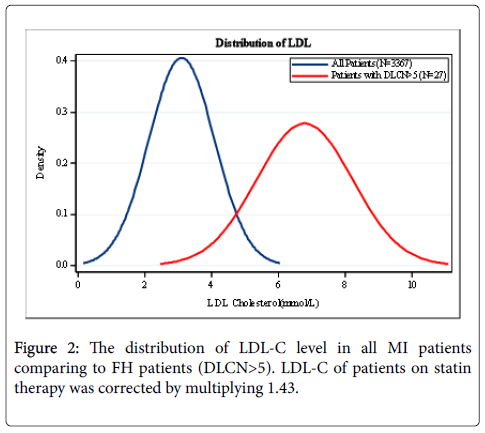
The LDL-C distribution of either 3367 AMI patients or FH patients with DLCN>5 was plotted in Figure 2. The mean values were 3.10 and 6.77 mmol/L, respectively. The baseline characteristics for the whole cohort and FH subgroups were summarized in Table 1. The cohort were divided into three FH groups: potential (definite/probable), possible and unlikely by both DLCN and MDLCN. The median age at admission in the whole population was 61 years. By DLCN, the median ages were 50, 50 and 62 years in potential, possible and unlikely FH groups, respectively. By MDLCN, they were 50, 55, 65 years in the relevant groups. Comparing the three subgroups of potential, possible and unlikely FH, regardless of the diagnostic criteria, the incidence of hyperlipidemia, current smoker, overweight, personal and family history of premature CHD significantly decreased. However, there were no significant trends in the incidence of hypertension and heart failure by either criteria. In the three subgroups stratified by DLCN, the LDL-C levels were 6.6, 4.2 and 2.9 mmol/L, and by MDLCN they were 5.1, 3.7 and 2.6 mmol/L, respectively. Only 7.3% of the patients regularly used statin before the admission on AMI and the rates of statin use among three subgroups significantly decreased regardless of the diagnostic criteria.
Genetic variants in FH patients (DLCN>5)
Genetic analysis identified 11 cases with pathogenic mutations among 24 patients for genetic testing, 10 were on LDLR and 1 on ApoB, no mutations were found on PCSK9 (Supplementary Table 2). Among these LDLR variants, one (c.191-2_196del) was fragment deletion of exon3 and resulted in changes of protein sequence, and one (c.1867dupA) was single nucleotide duplication that led to frame shift, both of which were considered as pathogenic mutations due to their functional impact on LDLR. The rest nine of them were point mutations that resulted in different gene defects. The p.(Trp483*) mutation was previously identified as one of the most common variants in Southern Chinese population [16]. Mutations p. (Arg595Trp), p.(Ala627Thr) and p.(Val797Met) were previously reported in LOVD database [17] as pathogenic, and c.(G1586+5C) was reported as pathogenic in a study on Taiwanese population [18]. The p. (Cys46*) mutation led to an early stop codon and a truncated LDLR protein, thus considered as pathogenic. For p.(Gly218Cys) located on exon4 where most pathogenic mutations were found, a different type of mutation p.(Gly218Valfs) was identified at the same position, which suggested the highly likely pathogenicity of p.(Gly218Cys). Similar case was found for mutation p.(Cys302Phe), where a pathogenic mutation p.(Cys302Tyr) was reported in LOVD. The only mutation of ApoB (p.Arg1388His) was reported before as a likely pathogenic mutation in population from Malaysia [19].
Outcomes among AMI patients with and without FH
During one year after the index hospitalization, among the 3349 patients who were alive at discharge, 82 patients suffered cardiac death, 68 patients had nonfatal AMI, 209 patients underwent coronary revascularization and 24 patients experienced ischemic stroke. Unadjusted rates of cardiovascular events were not significant between patients with and without FH despite the diagnostic criteria. The HR values were then adjusted for age, sex, CV risk factors and further reperfusion therapy in a multivariable model. By DLCN only patients of possible FH had a 1.43 (95% CI, 1.02-2.01) increased risk of recurrent cardiovascular events compared to that in the unlikely patients. Nevertheless, when comparing potential to unlikely FH group by MDLCN, significantly increased risk were observed, the HR was 1.97 (95% CI, 1.21-3.21) (Table 2).
| DLCN criteria | MDLCN criteria | |||||
|---|---|---|---|---|---|---|
| Potential FH† | possible FH | unlikely FH | Potential FH | possible FH | unlikely FH | |
| CV Eventsa/ participants |
2/27 | 43/407 | 287/2915 | 20/144 | 121/1364 | 191/1841 |
| Unadjusted HR (95% CI) |
0.74 (0.19,2.99) |
1.08 (0.78,1.48) |
1.00 (Referent) |
1.36 (0.86,2.15) | 0.85 (0.68,1.07) | 1.00 (Referent) |
| Model1 Adjusted HRb (95% CI) | 1.08 (0.27,4.37) |
1. 48 (1.05,2.08) | 1.00 (Referent) |
2.07 (1.28,3.37) | 1.08 (0.85,1.37) | 1.00 (Referent) |
| Model2 Adjusted HRb (95% CI) | 1.06 (0.26,4.27) | 1.44 (1.02,2.02) | 1.00 (Referent) | 1.98 (1.21,3.22) | 1.07 (0.84,1.35) | 1.00 (Referent) |
| Model3 Adjusted HRb (95% CI) |
1.08 (0.27,4.35) | 1.43 (1.02,2.01) | 1.00 (Referent) | 1.97 (1.21, 3.21) | 1.07 (0.84,1.36) | 1.00 (Referent) |
Table 2: Risks of recurrent events after AMI with respect to the presence of FH (n=3349). HR: Hazard Ratio; CI: Confidence Interval; DLCN: Dutch Lipid Clinic Network Criteria; MDLCN: Modified Dutch Lipid Clinic Network Criteria. a CV Events were defined as the occurrence of cardiac death, nonfatal AMI, coronary revascularization and ischemic stroke. b Model 1, adjusted for age and sex. Model 2, adjusted for age, sex and CV risk factors including BMI, current smoking, hypertension and diabetes mellitus. Model 3, adjusted for age, sex, CV risk factors and reperfusion therapy including thrombolytic therapy, primary percutaneous coronary intervention (PCI) and coronary artery bypass grafting (CABG).
Discussion
This is the first study, to our knowledge, that estimated the FH prevalence by DLCN and MDLCN criteria in a large nationwide AMI cohort in China. The study also included identification and characterization of Chinese FH pathogenic variants by exome sequencing. Furthermore, the incidence of major recurrent cardiac events (1 year follow-up) were also evaluated and compared in FH and non-FH patients. The MDLCN has also been compared to DLCN to explore the appropriate clinical diagnostic criteria for Chinese FH patients.
Prevalence of FH in patients with AMI
FH patients have been reported to be enriched in CHD population although the pravelence of FH varied significantly in different European CHD cohort. A recent study in the Danish population showed that 2.0% of the patients had probable/definite FH and the number increased to 6.9% among patients with premature MI [20]. The SPUM-ACS study enrolled 4778 patients hospitalized with acute coronary syndrome (ACS) in Switzerland, 1.6% of them had FH and the incidence increased to 4.8% in patients with premature ACS [6]. This number was even higher in the EUROASPIRE IV cohort enrolling 7044 patients with coronary events from 24 European countries. The study showed that 8.3% had ‘probable/definite’ FH and 15.4% in patients with premature event [7]. In these cohort studies, the difference in age, sex, ethnical background, and cardiovascular risk factors, and comorbidities, severity of the disease, medications and surgeries may all affect the prevalence estimation.
In our study, the prevalence of FH by DLCN is lower than other studies although FH were diagnosed by both clinical phenotypes and genetic results. Besides aforementioned reasons, one very important factor is that LDL level distribution in Chinese is different from that in Western populations. Data from the World Health Organization identified the age-standardized estimate of mean total cholesterol level at 4.5 mmol/L in Chinese population, comparing to 5.1~5.6 mmol/L in European in 2008 [21]. According to China Nationwide Nutrition and Health Survey 2002, the 95th centile of LDL-C among Chinese living in small/medium size city is about 3.5 mmol/L which is equal to 5 mmol/L in MONICA [9,10,22]. Thus, Shi et al. adjusted the DLCN definition by LDL-C to develop a fit-for-Chinese scoring system. According to the MDLCN, the prevalence of FH in our cohort increased significantly to 4.28% which is about 15 times higher than that in the general population (0.28%) [10]. Gencer et al. reported that the prevalence of FH was about 10 times higher among patients hospitalized for ACS compared with the general population [23]. The higher fold change in PEACE-AMI compared to European cohorts could be due to the inclusion of genetic results in our study.
A recent Chinese AMI cohort study reported the prevalence of FH reached 3.9% and 7.1% in MI and premature MI by DLCN [11], which is higher than our findings (0.80% and 1.98%). One of the reasons may be due to the cohort selection. In our study, the participants were enrolled from 53 hospitals across the country, including 18 secondary hospitals located in 21 provinces in China. While the mono-center cohort is from a specialized cardiovascular tertiary hospital admitting more severe patients, and had much higher rate of statin treatment than PEACE cohort (80.0% vs. 7.3%) The higher prevalence of FH observed could also be, at least partly, explained by the high correction factors used for the adjustment of LDL-C in a large proportion of the cohort [11].
Early onset of CHD is an important indicator of potential FH. The prevalence of FH among premature CHD patients was reported 1.5-20%, which is about 3 times higher than that in the general CHD population [6,7,11,21]. Our study also showed more than 2 times higher incidence of FH in premature AMI patients. Screening such patients may be an effective way to identify the index case followed by cascade screening in family members to facilitate early identification and treatment of FH. This strategy should be promoted especially in areas or countries where universal screening is impracticable.
Clinical characteristics
Our findings on the FH clinical characteristics are mostly comparable to that in Western populations. The age of onset in potential FH is 50 by DLCN and MDLCN which is comparable to the results from SPUM_ACS (50 years) in the Switzerland cohort [6] but is considerably lower than that in EUROASPIRE IV study (58 year) [7]. The AMI patients with FH in our cohort were more than 10 years younger than non-FH patients. The frequency of personal or familial history of premature CHD and level of triglycerides were significantly higher in the potential FH than in the unlikely group, especially when diagnosed by MDLCN. In addition, by both DLCN and MDLCN, the FH prevalence in women was higher than in men, which is consistant with other studies [7].
In the present study only 7.3% patients reported statin treatment. Even in FH patients, only 48.1% (by DLCN) and 25.0% (by MDLCN) patients were on statin treatment, which is lower than that in the Western cohorts [5,7]. It reflects the current status of the early screening and management of AMI patients in China, especially when concomitant with FH.
Clinical outcomes
Our study also for the first time reported an almost 2-fold significantly increased risk of recurrent cardiovascular events in potential FH patients diagnosed by MDLCN in China. Nanchen et al. reported that patients with FH (by DLCN) had a 1.88 increased risk of recurrent cardiovascular event in an acute coronary syndrome (ACS) cohort after adjusted for age and sex. The ratio increased to 2.31 after adjusted for age, sex and traditional risk factors [24]. Our findings on the harzard ratio (1.97) were similar to Nanchen’s report only when using MDLCN. It indicated that early identification and treatment of AMI patients with FH by MDLCN could more effectively prevent the recurrence of cardiovascular events after myocardial infarction.
FH diagnostic criteria in chinese population
The clincial characteristics analysis showed discontinuous trend of some characteristics (e.g. personal and family history of premature CHD and current smoker) in three subgroups of FH patients by DLCN. The risk of recurrent cardiovascular events was not significantly differentiated by DLCN either. The reason could be that some Chinese FH patients were diagnosed “possible” by DLCN criteria with higher LDL-C threshold. Indeed, the mean LDL-C level of 3367 Chinese myocardial infarction patient was 3.10 mmol/L. It is lower than mean value of 3.45 mmol/L derived from a 4778 European cohort with acute coronary syndrome [6]. Nevertheless, the current LDL-C difference between general ethnics populations awaits large scale epidemiology studies. The MDLCN criteria define LDL-C threshold according to actual LDL-C distribution in Chinese population. However, the LDL-C data for the MDLCN were from a 2002 report [9]. With the economic growth and Western-like diet being adopted over the past decade, the population LDL-C level in China is speculated to have increased. An ongoing epidemiology study (led by NCCD) investigating nationwide Chinese resident LDL-C levels will give more accurate estimation and guidance for the fit-for-Chinese diagnostic criteria.
Limitations
The current study have several limitations. Information of physical examination such as tendon xanthomas and corneal arcus were unavailable. But it should have minimum impact because the majority of heterozygote FH patients as this study normally do not have such physical signs. Family LDL-C level was not available in this group of patients. This could potentially lead to a slightly underestimated FH prevalence. In addition, the correction factor 1.43 used to estimate untreated LDL-C levels in patients using statins has not been validated in Chinese population. However, only a few patients (7.3%) on statin were corrected LDL-C level in the cohort. Finally, we used self-reported information on family history of premature CHD which may have recall bias and lead to an overestimation of FH prevalence.
Conclusion
FH is highly prevalent among patients who had CHD/AMI, especially among patients with premature CHD. The 1 year follow-up study demonstrated that AMI patients with FH compared to non-FH had an almost 2-fold adjusted risk of recurrence cardiovascular events. The cascade screening starting from a proband identified in CHD/AMI patients could be a good strategy for early treatment and prevention. The MDLCN has also been compared to DLCN to explore the appropriate clinical diagnostic criteria for Chinese FH patients.
Financial Support
This project was partly supported by the Research Special Fund for Public Welfare Industry of Health (201202025, 201502009) from the National Health and Family Planning Commission of China, the National Key Technology R&D Program (2015BAI12B01, 2015BAI12B02) from the Ministry of Science and Technology of China, CAMS Innovation Fund for Medical SciencesCIFMS 2016- I2M-2-004), and the 111 Project (B16005).
Author Contributions
Drs. Yan Gao and Hong Yin designed and implemented the study, interpreted results and wrote the manuscript. Drs. Lixin jiang and Mingqiang zhang established the study, interpreted the data and reviewed the manuscript. Drs. Ying He and Wenjin Li analyzed the genetic data. Drs. Jihua Wu and Ouhong Wang designed biostatistics method and analyzed clinical data. Dr. Siming Wang completed biochemical analysis of blood samples. Dr. Xi Li contributed to biostatistics analysis design.
Acknowledgement
We appreciate the great contribution by the China PEACE study group at NCCD and cooperative hospitals across China for collection of clinical information and blood samples. We are grateful for the funding support provided by the Chinese government.
References
- Nordestgaard BG, Chapman MJ, Humphries SE, Ginsberg HN, Masana L, et al. (2013) Familial hypercholesterolaemia is underdiagnosed and undertreated in the general population: guidance for clinicians to prevent coronary heart disease: consensus statement of the European Atherosclerosis Society. Eur Heart J 34: 3478-3490.
- Robinson JG (2013) Management of familial hypercholesterolemia: a review of the recommendations from the National Lipid Association Expert Panel on Familial Hypercholesterolemia. J Manag Care Pharm 19: 139-149.
- Scientific Steering Committee on behalf of the Simon Broome Register Group (1991) Risk of fatal coronary heart disease in familial hypercholesterolaemia. BMJ 303: 893-896.
- Hopkins PN, Toth PP, Ballantyne CM, Rader DJ (2011) Familial hypercholesterolemias: prevalence, genetics, diagnosis and screening recommendations from the National Lipid Association Expert Panel on Familial Hypercholesterolemia. J Clin Lipidol 5: S9-17.
- Benn M, Watts GF, Tybjaerg-Hansen A, Nordestgaard BG (2012) Familial hypercholesterolemia in the danish general population: prevalence, coronary artery disease, and cholesterol-lowering medication. J Clin Endocrinol Metab 97: 3956-3964.
- Nanchen D, Gencer B, Auer R, Räber L, Stefanini GG, et al. (2015) Prevalence and management of familial hypercholesterolaemia in patients with acute coronary syndromes. Eur Heart J 36: 2438-2445.
- De Backer G, Besseling J, Chapman J, Hovingh GK, Kastelein JJ, et al. (2015) Prevalence and management of familial hypercholesterolaemia in coronary patients: An analysis of EUROASPIRE IV, a study of the European Society of Cardiology. Atherosclerosi  241: 169-175.
- Li JH, Mi SQ, Li YC, Zhang M, Bi YF, et al. (2012) The levels and distribution of the serum lipids in Chinese adults, 2010. Zhonghua Yu Fang Yi Xue Za Zhi 46: 607-612.
- Wang L (2005) Report of China Nationwide Nutrition and Health Survey 2002: summary report. Beijing: People's Medical Publishing House.
- Shi Z, Yuan B, Zhao D, Taylor AW, Lin J, et al. (2014) Familial hypercholesterolemia in China: prevalence and evidence of underdetection and undertreatment in a community population. Int J Cardiol 174: 834-836.
- Li S, Zhang Y, Zhu CG, Guo YL, Wu NQ, et al. (2016) Identification of familial hypercholesterolemia in patients with myocardial infarction: A Chinese cohort study. J Clin Lipidol 10: 1344-1352.
- Li J, Dreyer RP, Li X, Du X, Downing NS, et al. (2016) China Patient-centered Evaluative Assessment of Cardiac Events Prospective Study of Acute Myocardial Infarction: Study Design. Chin Med J (Engl) 129: 72-80.
- Stenson PD, Mort M, Ball EV, Shaw K, Phillips A, et al. (2014) The Human Gene Mutation Database: building a comprehensive mutation repository for clinical and molecular genetics, diagnostic testing and personalized genomic medicine. Hum Genet 133: 1-9.
- Agresti A (2013) Categorical Data Analysis (3rd edn.), John Wiley and Sons, New York.
- McLeod AI, Hipel KW, Bodo BA (1991) Trend analysis methodology for water quality time series. Environmetrics 2: 169-200.
- Jiang L, Sun Y, Dai YF, Yang SW, Zhang F, et al. (2015) The distribution and characteristics of LDL receptor mutations in China: A systematic review. Sci Rep 26: 17272.
- Fokkema IF, Taschner PE, Schaafsma GC, Celli J, Laros JF, et al. (2011) LOVD v.2.0: the next generation in gene variant databases. Hum Mutation 32: 557-563.
- Yang KC, Su YN, Shew JY, Yang KY, Tseng WK, et al. (2007) LDLR and ApoB are major genetic causes of autosomal dominant hypercholesterolemia in a Taiwanese population. J Formos Med Assoc 106: 799-807.
- Lye SH, Chahil JK, Bagali P, Alex L, Vadivelu J, et al. (2013) Genetic polymorphisms in LDLR, APOB, PCSK9 and other lipid related genes associated with familial hypercholesterolemia in Malaysia. PLoS One 8: e60729.
- Mortensen MB, Kulenovic I, Klausen IC, Falk E (2016) Familial hypercholesterolemia among unselected contemporary patients presenting with first myocardial infarction: Prevalence, risk factor burden, and impact on age at presentation. J Clin Lipidol 10: 1145-1152.
- World Health Organization. Mean blood cholesterol (mmol/L), ages 25+, age standardized, 1980-2008.
- Gostynski M, Gutzwiller F, Kuulasmaa K, Döring A, Ferrario M, et al. (2004) Analysis of the relationship between total cholesterol, age, body mass index among males and females in the WHO MONICA Project. Int J Obes Relat Metab Disord 28: 1082-1090.
- Gencer B, Nanchen D (2016) Identifying familial hypercholesterolemia in acute coronary syndrome. Curr Opin Lipidol 27: 375-381.
- Nanchen D, Gencer B, Muller O, Auer R, Aghlmandi S, et al. (2016) Prognosis of Patients With Familial Hypercholesterolemia After Acute Coronary Syndromes. Circulation 134: 698-709.
Citation: Gao Y, Yin H, He Y, Wu J, Wang S, et al. (2017) Prevalence and Outcomes of Familial Hypercholesterolemia Patients in a Chinese Myocardial Infarction Cohort. Atheroscler Open Access 2: 114.
Copyright: © 2017 Gao Y, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Open Access Journals
Article Usage
- Total views: 5481
- [From(publication date): 0-2017 - Mar 31, 2025]
- Breakdown by view type
- HTML page views: 4554
- PDF downloads: 927