Prevalence and Factors Associated with Death in Healthcare-Associated Bacteremia in the Fann National University Hospital, Infectious and Tropical Diseases Department
Received: 26-May-2023 / Manuscript No. JIDT-23-100216 / Editor assigned: 29-May-2023 / PreQC No. JIDT-23-100216 (PQ) / Reviewed: 13-Jun-2023 / QC No. JIDT-23-100216 / Revised: 20-Jun-2023 / Manuscript No. JIDT-23-100216 (R) / Published Date: 27-Jun-2023 DOI: 10.4172/2332-0877.1000553
Abstract
Background: Healthcare-associated bacteremia is a real public health problem because of its high morbidity and mortality. The objectives of this study were to describe the characteristics of bacteremia and to identify death – associated factors.
Methods: This was a retrospective, descriptive and analytical study based on the records of patients hospitalized in the department of infectious and tropical diseases, whose diagnosis of healthcare-associated bacteremia was retained during the study period from January 1, 2016 to December 31, 2017.
Results: Fifty-two cases of healthcare-associated bacteremia were collected. The hospital prevalence was 2.6%. Male sex was predominant with a sex ratio=1.2. The average age was 42 ± 16 years. Twenty-two patients were HIV-infected. The majority of patients (32 cases) had been on antibiotics before their current hospitalization. Regarding the reasons for hospitalization, pulmonary signs dominated the series, followed by neurological and gastrointestinal signs with respectively 27, 26 and 18 cases. Invasive devices were dominated by peripheral venous catheters (100%) followed by urinary catheterization (87%). The main germs found were Staphylococci (26.6%), Enterobacter spp (23.5%), Klebsiella pneumonia (18.7%) and Escherichia coli (14.1%). Staphylococci were highly resistant to cefoxitin (88.2%) and methicillin (70%). There was a high level of resistance of gram-negative bacilli to 3rd line cephalosporin. Case fatality was 35%. Acute renal failure (p=0.01) and male gender (p=0.05) were associated with the occurrence of death.
Conclusion: Healthcare-associated bacteremia is a real public health problem. Standard hygiene measures play an important role in the control of these infections.
Keywords: Healthcare; Associated bacteremia death; Omicron variant; Respiratory distress
Introduction
The resurgence of Multi-Resistant Bacilli (MRB) in hospitals is a worldwide phenomenon observed for all bacterial species but to varying degrees depending on the country and the department. These bacteria are more dangerous in certain infections such as bacteremia, in particular due to the delay in introducing effective treatment, which is consequently a factor in excess mortality [1]. In fact, the lethality attributable to this healthcare-associated bacteremia is estimated to be between 10 and 50% [2]. In France, the last National Prevalence Survey (NPS) was conducted in 2017 and the overall prevalence rate of healthcare-associated infections was 5.21%; bacteremia represents the fourth most frequent infectious site with a prevalence of 0.6% [3]. In the United States, the latest prevalence survey conducted in 2015, shows a prevalence of healthcare-associated infections of 3.2% including 0.41% of healthcare-associated bacteremia [4]. In resource-limited countries, the prevalence of healthcare-associated infections is 2 to 20 times higher than in developed countries. In Africa, the prevalence ranges from 2.5 to 14.8% depending on the study [5]. Senegal, like other countries with limited resources, is also faced with the emergence of these healthcare-associated infections. The studies conducted show prevalence of healthcare-associated infections that range from 4 to 13.8% [6]. In the Fann Hospital, it was estimated at 10.9% in 2007 and in 2010, healthcare-associated bacteremia represented 46.7% of HAIs in the infectious and tropical diseases department [7-9]. Also, the emergence and increase of bacterial resistance to antibiotics further complicates the management of healthcare-associated bacteremia. In Senegal, in order to eliminate or at least reduce this scourge, control actions have been taken, precisely with the creation of a Nosocomial Infections Control National Program as well as a control committee within each health care facility. But few data are available on the extent and the problems related to this healthcare associated bacteremia. This is why we conducted this study, in order to:
• Determine the prevalence of healthcare-associated bacteremia in the infectious diseases department of the Fann National University Hospital
• Describe the epidemiological, clinical, bacteriological, therapeutic and evolutionary aspects of healthcare associated bacteremia,
• Identify factors associated with death.
Materials And Methods
Type of study
This was a retrospective descriptive and analytical study conducted from records of patients hospitalized in the department of infectious and tropical diseases from January 1, 2016 to December 31, 2017.
Inclusion criteria: Were included in this study, all patients, hospitalized in the Infectious and Tropical Diseases Department during the study period and for whom the diagnosis of healthcare associated bacteremia was made with bacteriological evidence.
Variable Definitions: To facilitate the retrospective collection of chart items, the following definitions were used.
An infection is said to be associated with care: If it occurs during or after the treatment (diagnostic, therapeutic, palliative, preventive or educational) of a patient, and if it was neither present nor incubating at the beginning of the treatment [8].
Bacteremia:It defined as the presence of at least one positive blood culture (justified by clinical signs), except for the following organisms:
• Coagulase-negative staphylococci
• Bacillus spp. (except B. anthracis)
• Corynebacterium spp.
• Propionibacterium spp.
• Micrococcus spp.
or other saprophytic or commensal microorganisms with comparable pathogenic potential, for which two positive blood cultures for the same microorganism, taken at different punctures, at different times, and within a short interval (a maximum of 48 hours is usually used), are required [10]. This bacteremia must occur 48 hours after admission or contact with the health care facility to be considered as associated with care.
Multi-Resistant Bacilli (MRB)
These are extended-spectrum beta-lactamase-secreting Enterobacteriaceae (ESBL), methicillin-resistant Staphylococci, Pseudomonas and Acinetobacter resistant to ticarcillin and/or ceftazidime, and Streptococci with decreased susceptibility to penicillin G.
Non-inclusion criteria: Patients treated for bacteremia whose records were incomplete and unusable, i.e., without documentation of blood culture data, were not included.
Data collection: Data were collected from a standard questionnaire including:
• Socio-demographic characteristics: age, sex, geographical origin and profession
• Bioclinical characteristics: reasons for hospitalization, site of infection, comorbidities, history of hospitalization, previous antibiotic therapy, blood count, urea, creatinine, C-reactive protein, aspartate aminotransferases, alanine aminotransferases, Prothrombin Level (PT), bacteriological data and resistance profile of isolated bacteria
• Evolutionary and therapeutic data
Data entry and analysis: Data entry was performed with Epi-Info version 7 software and the exploitation was done with R software. The qualitative variables were expressed in proportions and the quantitative variables in mean standard deviation in case of normal distribution, in median with the extremes if necessary. For the bivariate analysis, the factors associated with death were identified by comparing the different variables. The difference was statistically significant if p<0.05.
Results
Epidemiological aspects
During our study period, 52 cases of associated bacteremia were recorded out of a total of 1987 hospitalized patients, i.e., a hospital prevalence of 2.6%. The total number of Healthcare-Associated Infections (HCAI) was 123 cases, i.e. a proportion of bacteremia of approximately 42.3%. The predominant sex was male with an M/F ratio of 1.2. The average age was 42 ± 16 years and the 40-60-year age group represented more than half of the patients (51.9%). The majority of patients lived in suburban (46.2%) and rural (42.3%) areas. Among the 52 cases of healthcare-associated bacteremia, 22 were living with HIV (42%). Other comorbidities such as chronic renal failure (4%), diabetes (2%) and high blood pressure (2%) had also been found (Table 1).
| Variables | Number | Percentage (%) |
|---|---|---|
| Geographic origin | ||
| - Sub-urban | 24 | 46.2 |
| - urban | 22 | 42.3 |
| - rural | 5 | 9.6 |
| Sex | ||
| - Male | 28 | 53.9 |
| - Female | 24 | 46.1 |
| Age range (years) | ||
| - < 20 | 6 | 11.5 |
| - 20-40 | 13 | 25 |
| - 40-60 | 27 | 51.9 |
| - > 60 | 6 | 11.5 |
| Marital status | ||
| - Married | 26 | 50 |
| - Bachelor | 17 | 32.7 |
| - Widower | 5 | 9.6 |
| - Divorced | 4 | 7.7 |
| Occupation | ||
| - Employee | 11 | 21.2 |
| - Unemployed | 11 | 21.2 |
| - Student | 6 | 11.5 |
| - Informal sector | 6 | 11.5 |
| Comorbidities | ||
| - Yes | 38 | 73 |
| - No | 14 | 27 |
Table 1: Distribution of patients according to socio-demographic aspects.
Clinical aspects
A recent previous hospitalization had been noted in 33% of cases and its duration was less than 10 days in 53.9% of cases, with a mean of 13 days ± 10.3 (1;35). Of the patients with a previous hospitalization, 94% were hospitalized in the medical department and the reasons were diverse and varied (headache, fever, gastroenteritis). The majority of patients (61.5%) had been on antibiotics before their current hospitalization. Amoxicillin-clavulanic acid (20%), Ceftriaxone (16%), Ciprofloxacin (9%), Cotrimoxazole and Metronidazole (7% each) were the most used molecules (Table 2).
| Variables | Number | Percentage (%) |
|---|---|---|
| Previous hospitalisation | ||
| - Yes | 17 | 33 |
| - No | 34 | 65 |
| - Not specified | 1 | 2 |
| Hospital ward | ||
| - Medicine | 16 | 94 |
| - Surgery | 1 | 6 |
| Previous antibiotherapy | ||
| - Yes | 32 | 61 |
| - No | 17 | 32.7 |
| - Not specified | 3 | 5.8 |
Table 2: Distribution of patients according to medical history.
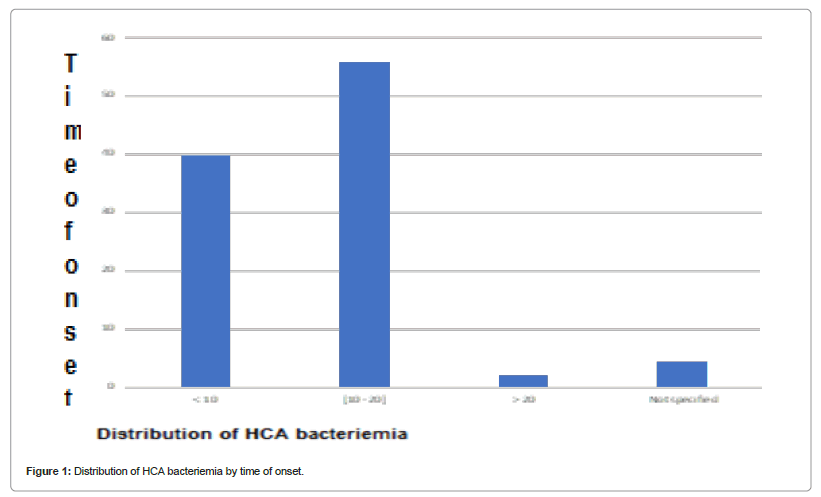
Regarding the reasons for current hospitalization, pulmonary signs dominated the series, followed by neurological and gastrointestinal signs with 27, 26 and 18 cases respectively. Care-associated bacteremia occurred during the first 20 days of hospitalization, mainly between the 10th and 20th day (56%). Another infection associated with care was identified in 19 patients (36.54%). They were urinary (14 cases), genital (2 cases), cutaneous (2 cases) or ENT (1 case). The invasive devices were dominated by peripheral venous catheters (100%) followed by urinary catheterization (Figure 1 and Table 3).
| Reason for current hospitalization | Number | Percentage |
|---|---|---|
| Tuberculosis | 17 | 30.9 |
| Other pulmonary infections | 5 | 9.1 |
| Meningitis and/or encephalitis | 5 | 9.1 |
| Severe malaria | 3 | 5.5 |
| Others | 22 | 45.5 |
| Exposure factors | ||
| Venous catheters | 52 | 100 |
| Urinary catheter | 45 | 87 |
| Nasogastric tube | 26 | 50 |
| Bed sores | 4 | 8 |
Table 3: Distribution of patients according clinical aspects.
Paraclinical aspects
In our series, the mean hemoglobin level was 8.81 ± 2.6 g/dl, with extremes of (4.1-14.6). The mean white blood cell count was 11521.9 ± 10079.6/mm3 with a range of (2200-56280). Hyperleukocytosis was found in 21 cases (40.38%) and leukopenia in 5 cases (9.62%). The C-reactive protein assay was performed in 50 cases and showed a mean level of 119.52 ± 89.42 (mg/L), with extremes of (6-431.17) the mean creatinine level was 16.9 ± 21.8 (4-127). The blood culture was single- microbial in 62% of cases. A total of 11 germs were isolated (Table 3) and the most frequent among them were Staphylococci (26.6%), Enterobacter spp (23.5%) and Klebsiella pneumonia (18.7%) (Table 4).
Among the 17 strains of Staphylococci isolated, ¾ had high resistance to some antibiotics, including Penicillin (82.4%), Cefoxitin (88.2%), Methicillin (70.6%). However, no resistance was found for Vancomycin and Colistin (Table 4). The Streptococcus strains isolated were all sensitive to Imipenem, Vancomycin, Colistin and Chloramphenicol. However, resistance to methicillin, penicillin and cefoxitin was noted with 21%, 18% and 22% respectively.
| Gram | Bacteria | Number | Percentage |
|---|---|---|---|
| Gram positive | Staphylococcus | 17 | 26.6 |
| Streptococcus | 2 | 3.1 | |
| Gram negative | Enterobacter spp | 15 | 23.5 |
| Klebsiella pneumonie | 12 | 18.7 | |
| Echerichia coli | 9 | 14.1 | |
| Acinetobacter | 2 | 3.1 | |
| Enterococcus | 2 | 3.1 | |
| Flavobactérium | 2 | 3.1 | |
| Others (Serratia, Citrobacter, Pseudomonas) | 3 | 4.6 |
Table 4: Distribution of healthcare-associated bacteremia cases according to the germs isolated on blood culture.
The Enterobacter spp strains expressed resistance to Cephalosporins, Amoxicillin and Ciprofloxacin (60%). For the 9 Escherichia strains, 3/4 of them were also resistant to 3rd generation cephalosporins (88.9%), to amoxicillin (88.9%) and to quinolones. The same was true for Klebsiella pneumonia strains, which were all resistant to C3G (100%) and to Quinolones. Concerning the Enterococcus strains isolated, they both presented a total resistance to Ampicillin and Fosfomycin. As for the Acinetobacter strains, they showed high resistance to C3G and they were both sensitive to Colistin and Fosfomycin. For Serratia, they showed total resistance to several families of antibiotics but were 100% sensitive to C3G and Chloramphenicol. The Citrobacter strain isolated showed total resistance to Amoxicillin, Cephalosporins and Ciprofloxacin. It was sensitive to Chloramphenicol, Fosfomycin, Colistin and Imipenem. The same was true for Flavobacterium. The isolated Pseudomonas strain expressed several resistances to almost all families of antibiotics (Table 5).
| Antibiotics | Enterobacter spp | E. coli | K. pneumoniae | Staphylococcus |
|---|---|---|---|---|
| Resistance (%) | ||||
| Amoxicillin | 80 | 88.9 | 91.7 | 11.8 |
| Penicillin G | 6.7 | 0 | 25 | 82.4 |
| Methicillin | 6.7 | 0 | 25 | 70.6 |
| Ceftazidime | 66.7 | 77.8 | 91.7 | 11.8 |
| Cefepime | 66.7 | 77.8 | 83.3 | 11.8 |
| Cefoxitin | 80 | 33.3 | 50 | 88.2 |
| Cephalosporin | 80 | 88.9 | 100 | 29.4 |
| Piperacillin | 66.7 | 55.6 | 33.3 | 17.6 |
| Carbapenem | 0 | 11.1 | 0 | 0 |
| Kanamycin | 33.3 | 22.2 | 0 | 52.9 |
| Gentamicin | 40 | 44.4 | 50 | 52.9 |
| Amikacin | 6.7 | 0 | 0 | 17.6 |
| Fusidic acid | 5.9 | |||
| Tetracyclin | 6.7 | 0 | 0 | 11.8 |
| Vancomycin | 0 | 0 | 0 | 0 |
| Ciprofloxacin | 60 | 77.8 | 83.3 | 52.9 |
| Levofloxacin | 26.7 | 55.6 | 33.3 | 5.9 |
| Colistin | 0 | 0 | 0 | 0 |
| Fosfomycin | 46.7 | 55.6 | 33.3 | 23.5 |
Table 5: Resistance profile of the isolated bacteria.
Therapeutic and evolutionary aspects
The majority of healthcare-associated bacteremia were treated with a single antibiotic, and Imipenem with 19 cases (61%) was the most used molecule. The two most commonly used antibiotics in dual therapy were Vancomycin and Amikacin. The combinations of antibiotics used in poly-antibiotic therapy were diverse and varied. The average duration of antibiotic therapy was 8.7 ± 2.6 days (2-15). The average length of hospitalization was 25.8 ± 14.2 days (6-68). Of the 52 patients, 18 died, with a case fatality rate of approximately 35%. Acute renal failure (p=0.01) and male gender (p=0.05) favored death.
Discussion
Since over a 24-month period, we collected 52 cases of healthcare- associated bacteremia out of a total of 1987 patients and 123 healthcare-associated infections. Thus, the study found a hospital prevalence of healthcare-associated bacteremia of 2.61%. This low percentage, compared to the reality, can be explained on one hand by the high denominator (1987 patients) and on the other hand by the fact that many cases were not eligible because they did not meet the CDC definition of healthcare-associated bacteremia. This under- diagnosis could also be due to the low request for blood cultures and/ or unmet sampling conditions [6]. All this is due to our patients’ low living standards and/or our poor technical facilities. In Morocco, the prevalence of bacteremia is similar with 3% [10]. In Senegal, Lakhe, et al., found a slightly higher rate (4.11%), which could be explained by the fact that his study included both community-acquired and healthcare-associated bacteremia [11,12]. However, the prevalence of healthcare-associated bacteremia in the total number of HCAIs in our study was 42.3%. Bacteremia comes therefore second behind urinary tract infections (82.1%). In 2010, an incidence survey carried out at the Infectious and Tropical Diseases Department by Fortes, et al., in Dakar (Senegal) found a similar result to ours (46.7%).
Males were predominant with a sex ratio of 1.2. Similar ratios were found by Diallo and Wendy [13,14]. However, in the literature, women contracted more healthcare-associated bacteremia, as reported by Wassa (sex ratio of 0.79) or Moumile and Seydi (53%), and by Lefort, et al., [10,13-15]. Several studies have shown that the proportion of women with nosocomial infections in general is higher than that of men [6,16-17]. The mean age was 42 ±16 years comparable to the mean found by Wendy, Fortes (43 years), Badaoui (40 years) and by Dia, et al., (41.4 years) (7,9,12,16). This could be explained by the fact that most of the patients hospitalized in the infectious diseases department are adults. As for the existence of a defect, it was found in 38 patients. It was mainly HIV infection (22 patients or 42%). This can be explained by the fact that the infectious diseases department of Fann Hospital is the reference department in Senegal for patients living with HIV case management. Moreover, in this department, 60% of the beds are occupied by PLWHA [18]. Bacteremia associated with care occurred during the first 20 days of hospitalization, especially between the 10th and 20th day (56% of patients). This delay was similar to that found in a multicenter study in Spain [19-21]. On the other hand, the study carried out by Sagne, et al., at Aristide LeDantec the National University Hospital found a smaller average of 3.88 days [2-9,21]. In 19 of our patients (36.5%), a second infection associated with care was found. It was mainly urinary (14 cases), followed by genital and skin infections (2 cases) or ENT (1 case). In fact, in the literature, among the least frequent infections, we note soft tissue infections, skin, genital and joint infections [21]. Urinary tract infections, on the other hand, are one of the most frequent infections, especially in medical wards [7,20]. They also constitute the entry point for half of the cases of bacteremia [22- 24]. In terms of invasive devices, all patients with healthcare-associated bacteremia had a peripheral venous catheter (100%). Peripheral venous catheters may indeed constitute an entry point for these healthcare- associated bacteremia (secondary bacteremia via catheter-related infection) [7]. The same holds true for the nasogastric tube, which would further favor the occurrence of nosocomial pneumopathy, and by ricochet, nosocomial bacteremia [10]. In our study, the patients who had a nasogastric tube represented half of the population (50%). This high rate could be explained by the recurrent use of gastric tubes for the diagnosis of pulmonary tuberculosis, in addition to its classical use, i.e. parenteral nutrition, in case the patient’s condition required it. The different time period of catheterization which could also be factors favoring bacteremia, were not recorded in our study; the risk of infection increases with a time of period of more than 72 hours [25].
The mean white blood cell count was 11521.92 ± 10079.6/ mm3 (2200-56280); C-reactive protein was measured in 50 patients, with a mean level of 119.5 ± 89.4 mg/l. Creatinine levels were measured in 50 patients, with an average of 17 ± 21.8 mg/l (4-127). The blood culture was mono-microbial in 62% of the samples; similar data have also been found in the literature [13,26].
In terms of gram staining, gram-negative bacilli dominated the series with 70% of cases. The main germs detected in our study were Staphylococcus (26.6%), Enterobacter spp (23.5%), Klebsiella pneumoniae (18.7%) and Escherichia coli (14.1%). It can therefore be noted that Enterobacter were the most frequently isolated germs during the study, with Enterobacter spp. at the top of the list. In fact, these Enterobacter have become an increasing cause of healthcare-associated bacteremia [27]. For his part, Compaore, during his prevalence survey “on a given day” in the Mother and Child Department of Dakar Military Hospital in 2009, recalled that his 3 documented cases of infection were gram negative bacilli [28]. At the same time, this finding had already been made in 2007 and 2008 in Dakar in various level 3 hospitals during prospective studies of bacterial agents after an infection. Nearly 78% of the bacteria isolated were enterobacteria [27]. However, taken separately, Staphylococci remain the germs that cause the most healthcare-associated bacteremia. This result is similar to that obtained in a study carried out at Aristide Le Dantec the National University Hospital, which found a rate of 29% [29]. Staphylococci were responsible for 58% of catheter-related infections, 37% of surgical site infections, and 36% of bacteremia in France in 2000 [30]. Their frequency could be explained by their nosocomial nature, linked to the increase in invasive procedures: implantation of foreign material, insertion of perfusion or chemotherapy catheters, etc. [31]. The staphylococcal strains isolated were found to be resistant to methicillin in (70.6%). In Kenya, the prevalence of Methicillin-Resistant Staphylococcus aureus (MRSA) was smaller, 53.4% [32]. This is also found in Mediterranean countries such as Egypt, Greece and Cyprus with rates of 48, 45 and 52% respectively [20,33]. None of the strains was resistant to imipenem, and vancomycin or colistin offered possibilities of treatment with these molecules. However, the high cost of treatment of these different molecules is an obstacle to their use. The presence of ciprofloxacin in type IB pharmacies exposes it to overuse and therefore to the possibility of resistance to this molecule as noted in our study (59.2%).
Furthermore, we confirm the results of numerous studies that have shown that Klebsiella pneumoniae, Escherichia coli, and Enterobacter spp are the predominant species of ESBL [34,35]. These ESBL enterobacteria, grouped together, remain in the majority in our study (68.8%) with : Klebsiella pneumoniae (14.1%), Escherichia coli (18.7%) and finally Enterobacter (23.5%). Poor hygiene conditions, the often anarchic antibiotic prescription and the non-respect, or even the ignorance of asepsis elementary rules of asepsis such as the drawing blood or other pathological products without wearing gloves and the handling or irrational use of invasive devices often select the most resistant of these microorganisms. Thus, their frequent transmission between patients ends up creating an infection in many of them. In addition, they are resistant to several families of antibiotics: Cephalosporins, Quinolones, Macrolides, among others. The 2007 Dakar prevalence survey found that 50% of the BMR isolated were enterobacteria, with Enterobacter cloacae in the lead: producer of Extended-Spectrum Beta-Lactamase (ESBL), then Escherichia coli: high-level penicillinase secretor; there was also Pseudomonas aeruginosa: resistant to ticarcillin and ceftazidime [6]. At the same time, the Pseudomonas strain isolated in our study expressed resistance to almost all antibiotics; only Imipenem, Amikacin, Colistin and Chloramphenicol were susceptible. Elsewhere, in Brazil for example, a study in 2007 revealed that 77% and 55% of Pseudomonas strains were resistant to aztreonam and Amikacin respectively [36].
Care-associated bacteremia was treated in 78.9% of patients and among them mono-antibiotic therapy was the most prevalent in ¾ (76%), led by Imipenem (61%). Contrary to the majority of studies in which Ceftriaxone was the most used molecule either as monotherapy or in combination with either Gentamicin alone or with Gentamicin, Spiramycin, and Metronidazole [10]. This frequent use of Ceftriaxone was noted in Côte d’Ivoire (53%) in 2015, in Burkina Faso (70%) in 2011, and also, in a France (44.1%) study on the antibiotic therapy of bacteremic patients admitted to the emergency room in 2007 [27,37,38]. In bi or tritherapy, Vancomycin, Fucidic acid and Amikacin are the most used molecules in our study. Moreover, we could not differentiate between probabilistic treatment and adapted treatment after antibiotic susceptibility testing. The reason for this was that most of the patients’ files did not contain details in this respect. Moreover, it should be remembered that our study was carried out in an infectious and tropical diseases department where the majority of patients were already being treated with antibiotics. The readjustment of the treatment was made directly towards molecules that were more sensitive to these bacteria, namely imipenem (61%). The average duration of antibiotic therapy was 8.7 ± 2.6 days. A study of 55 patients with S. aureus bacteremia on a catheter, associated with a 1992 literature review of the literature, allowed the authors to conclude that intravenous treatment should not be less than 10 days and that it was not necessary to continue it beyond 14 days [22]. In Canada, Thosmas found a median time period of 11 days in his study of 100 bacteremic patients, close to that found in our series [39].
Among our 52 patients, 18 died, i.e. a lethality of about 35%. Indeed, bacteremia associated with treatment often worsens patients’ condition and impairs their vital prognosis. Thus, a case fatality rate of 30-50% has been frequently reported in the literature [33]. Acute renal failure (p=0.01) and male gender (p=0.05) would favor death. Indeed, the literature shows that the condition of patients with healthcare-associated bacteremia admitted to a nephrology department or those with renal disorders was associated with death [12,23,40]. Moreover, this was the only parameter that seemed to have an influence on lethality, contrary to several other studies where other factors, whether clinical (reasons for hospitalization such as sepsis, confusion, history of hospitalization; altered general condition and immunosuppression, comorbidities, invasive devices or paraclinical (hypoalbuminemia, moderate fever or hypothermia, elevated CRP) influenced death [12,41-43].
Conclusion
The morbidity and mortality related to bacteremia, particularly to healthcare-associated bacteremia remain a real concern. Hence the significance of a rational prescription of antibiotics and the strict respect of hygiene measures during treatment. This will limit the emergence of multi-resistant bacteria but also the incidence of healthcare-associated infections.
References
- Mortensen VH, Søgaard M, Mygind LH, Wolewitz M, Kristensen B, et al. (2022) Incidence and mortality of hospital-acquired bacteriemia: a population-based cohort study applying a multi-state model approach. Clin Microbiol Infect 28:879e9-879.e15.
- Bearman GM, Wenzel RP (2005) Bacteremia: A leading cause of death. Arch Med Res 36: 646-659.
- Centre for prevention of healthcare associated infections (CPIAS) (2017) Results of National prevalence of diseases list.
- Wilson ME (2019) Antibiotics: What everyone needs to know. Oxford University press, Oxfordshire, UK.
- Nejad BS, Allegranzi B, Syed SB, Ellis B, Pittet D (2011) Health-care-associated infection in Africa: a systematic review. Bull World Health Organ 89: 757-765.
- Dia NM, Ka R, Dieng C, Diagne R, Dia ML, et al. (2008) Prevalence of nosocomial infections in a university hospital (Dakar, Senegal). Med Mal Infect 38:270-274.
- Deguenonvo LF, Traore K, Dia NM, Diouf A, Lakhe A, et al. (2015) Results of a survey incidence of the cases of nosocomial infections with multidrug resistant bacteria in a hospital center in Dakar (Senegal). Rev Mal Inf Micr 6.
[Crossref]
- Garner JS, Jarvis WR, Emori TG, Horan TC, Huges JM (1988) CDC definitions for nosocomial infections, 1988. Am J Infect Control 16: 128-140.
- Badaoui L, Lahsen TO, Daoudi N, Sodqi N, Chakib MI, et al. (2016) Current profile of bacteremia in the infectious diseases department: About 216 cases. Rev Mal Inf Micro 6.
[Crossref]
- Lakhe NA, Sylla K, Mbaye KD, Ndiaye R, Diallo VMPC, et al. (2017) Bacteremia: Profile and antibiotic resistance at the infectious and tropical diseases clinic in fann hospital, dakar, senegal. J Infect Dis Ther 6:348.
[Crossref]
- Seydi M, Sow AI, Soumare M, Diallo HM, Hatim B, et al. (2004) Staphylococcus aureus bacteremia in the Dakar Fann University hospital. Med Mal Inf 34: 210.
- Sligl WI, Dragan T, Smit SW (2015) Nosocomial gram-negative bacteremia in intensive care: Epidemiology, antimicrobial susceptibilities and outcomes. Int J Infect Dis 37: 129-134.
- Moumile K, Carbonne A, Rouquet ML, Gamard MN, Rousselot AB, et al. (2004) Descriptive study of bacteremia in a geriatric hospital. Pathol Biol 2: 557-565.
- Seydi M, Soumare M, Sow AI, Diop BM, Sow PS (2005) Escherichia coli meningitis during bacteremia in the Ibrahima-Diop-Mar infectious diseases clinic, Dakar Fann National Hospital Center (Senegal). Med Mal Infect 35:344-348.
- Lefort A et al. (2009) COL6-02 Predictive factors for the severity of Escherichia coli bacteremia (BEc): COLIBAFI study. Rev Med Intern 30: S57.
[Crossref]
- Kakupa DK, Muenze PK, Byl B, Wilme MD (2016) Study of the prevalence of nosocomial infections and associated factors in the two university hospitals of Lubumbashi, Democratic Republic of Congo. Pan African Med 24: 275.
- Schultz L, Walker SAN, Elligsen M, Walker SE, Simor A, et al. (2003) Identification of predictors of early infection in acute burn patients. Burns 39:1355-1366.
[Crossref]
- Seydi M, Sow PS, Soumare Mr, Ndour CT, Slide NM, et al. (2003) Bacteremia during AIDS in Dakar, Senegal. Med Mal Infect 33: 323-326.
[Crossref]
- Valles J, Leon C, Lerma FA (1997) Nosocomial bacteremia in critically ill patients: A multicenter study evaluating epidemiology and prognosis. Clin Infect Dis 24: 387-395.
- Monnet T (2011) Nosocomial infections: the importance of follow-up epidemiology and rapid identification of causative bacteria: example of some techniques of diagnosis allowing this early identification. Sci Pharma. dumas-00656963.
- Havey TC, Fowler RA, Pinto R, Elligsen M, Daneman N (2013) Duration of antibiotic therapy for critically ill patients with bloodstream infections: A retrospective cohort study. Can J Infect Dis Med Microbiol 24: 129.
- Lee CC, Chang CM, Hong MY, Chin HH, Ko WC (2013) Different impact of the appropriateness of empirical antibiotics for bacteremia among younger adults and the elderly in the ED. Am J Emerg Med 31: 282-290.
- Tehrani MS, Hajage D, Fihman V, Tankovic J, Cau S, et al. (2014) Gram-negative bacteremia: Which empirical antibiotic therapy? Med Mal Infect 44:159-166.
- Girou E (2008) How to reduce nosocomial infections in intensive care in practice? 17 : 275-279
[Crossref]
- Isendahl J, Manjuba C, Rodrigues A, Xu W, Normark BH, et al. Prevalence of community-acquired bacteremia in Guinea-Bissau: an observational study. BMC Infect Dis. 2014; 14:3859.
- Ravahatra ZDR, Randriatsarafara FM, Rakotovao AL, Rasamindrakotroka A (2021) Prevalence and factors associated with extended-spectrum ßlactamase producing Enterobacteriaceae bacteraemia in University Hospital of Befelatanana, Madagascar. Afr J Clin Exper Microbiol 22: 52-59.
[Crossref]
- Compaoré TSA, Gueye-Ba M, Ka-AS, Dionne P, Wade B (2010) Surveillance des infections nosocomiales: bilan de quatre années d’enquête de prévalence « un jour donné » dans le département Mère-Enfant de l’hôpital Principal de Dakar (Sénégal). Rev Med Perinat 2: 213-218.
[Crossref]
- Kliebe C, Nies BA, Meyer JF, Neutzling RMT, Wiedemann B (1928) Evolution of plasmid-coded resistance to broad-spectrum cephalosporins. Antimicrob Agents Chemother 28: 302-307.
- Monitoring group of the regional hospital hygiene relay of the center (2001) First multicenter monitoring of bacteraemia in the Region. Centre Bull Epide´miol Hebd.16.
- Lagier JC (2008) Bactériémies et endocardites a` Staphylococcus aureus. Ann Cardiol Angeiol 71-77.
- Moran GJ, Krishnadasan A, Gorwitz RJ, Fosheim GE, Mcdougal LK, et al. (2006) Methicillin-resistant S. aureus infections among patients in the emergency department. N Engl J Med 355: 666-674.
- Wangai FK, Masika MM, Maritim MC, Seaton RA (2019) Methicillin-resistant Staphylococcus aureus (MRSA) in East Africa: Red alert or red herring? BMC Infect Dis 19: 596.
- Brun-Buisson C (1997) Prevalence of multidrug-resistant bacteria according to hospitals and patient specialties and characteristics. Clean Hospital Forum II. 3-28.
- Monnet DL, Biddle JW, Edwards JR, Culver DH, Tolsen JS, et al. (1997) Evidence on interhospital transmission of extended beta lactam-resistant Klebsiella pneumonia in the USA, 1986 to 1993. Infect Control Hosp Epidemiol 18: 492-498.
- Leao LSNO, Passos XS, Reis C, Valadao LMA, Silva MRR, et al. (2007) Phenotyping of bacteria isolated in blood cultures from critical patients. Rev Soc Bras Med Trop. 40:537-540.
- Akoua-Koffi C, Tia H, Plo JK, Monemo P, Cisse A, et al. (2015) Epidemiology of community-onset bloodstream infections in Bouake´, central Co^te d’Ivoire. New Microbes New Infect 7: 100-104.
- Viallon A, Marjollet O, Leveques Y, Robert F, Berger C, et al. (2007) Antibiotic therapy in bacteremic patients admitted to the emergency department: analysis of its pertinence. J Eur Urg 20: 70-76.
[Crossref]
- Lucet JC, Chevret S, Decré D (1996) Outbreak of multiply- resistant Enterobacteriaceae in an intensive care unit: epidemiology and risk factors for acquisition. Clin Infect Dis 22: 652-4.
- Culver DH, Horan TC, Gaynes RP, Martone WJ, Jarvis WR, et al. (1997) Surgical wound infection rates by wound class, operative procedure, and patient risk. Am J Med 91:152-157.
- Gavazzi G, Escobar P, Olive F, Debray M, Couturier P, et al. (2001) Prognostic factors of nosocomial bacteremia in the elderly.
- Said H, Rejeb MB, Khefacha S, Chebil D, Dhidah L, et al. (2013) Bacteraemia associated with care in the intensive care unit – incidence study at CHU Sahloul, Sousse, Tunisia (2010–2011). J Epidemiol Public Health 61: 467.
- Diekema DJ, Beekmann SE, Chapin KC, Morel KA, Munson E, et al. (2003) Epidemiology and Outcome of Nosocomial and Community-Onset Bloodstream Infection. J Clin Microbiol 41:3655-3660.
- Burlaud A, Mathieu D, Falissard B, Trivalle C (2010) Mortality and bloodstream infections in geriatrics units. Arch Gerontol Geriatr 51: 106-110.
Citation: Daye KA, Fall NM, Massaly A, Sall K, Diop ND, et al. (2023) Prevalence and Factors Associated with Death in Healthcare Associated Bacteremia in the Fann National University Hospital, Infectious and Tropical Diseases Department. J Infect Dis Ther 11:553. DOI: 10.4172/2332-0877.1000553
Copyright: © 2023 Daye KA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 643
- [From(publication date): 0-2023 - Feb 21, 2025]
- Breakdown by view type
- HTML page views: 550
- PDF downloads: 93