Prevalence and Factors Associated with Anthelmintic Resistance in Gastrointestinal Nematodes of Cattle: A Systematic Review and Meta-analysis
Received: 21-Nov-2018 / Accepted Date: 02-Dec-2018 / Published Date: 17-Dec-2018
Abstract
A systematic review and meta-analysis was conducted with the aim to measure the prevalence of anthelmintic resistance (AR) in cattle gastrointestinal nematodes (GIN) and potential management factors associated with development of such resistance. A search algorithm was constructed and a comprehensive search of the primary literature was conducted in: CAB abstracts (1990-2016), Medline (1860-2016), Agricola (1924-2016) and Lilacs (1985-2016). Prevalence estimates were combined through meta-analysis (MA) using the logit prevalence and between-study heterogeneity was quantified. Twenty-nine publications (5 cross-sectional studies; 14 prevalence surveys and 10 field trials) were included in this review. Random effects MA resulted in an overall AR prevalence of 72.0% (95% CI=58.4% to 80.0%). However, a high heterogeneity was observed (I2=55.9%). From studies reporting the nematode genera involved in the AR, Cooperia spp were present in 91.7% of the studies (n=24); Ostertagia sp. in 44.5% (n=22); Haemonchus sp. in 47.8% (n=23); Trichostrongylus sp. in 36.4% (n=22) and Oesophagostomum spp. in 23.8% (n=21). The included cross sectional studies suggested that frequency of treatments, age of cattle and type of management were potential management factors associated with AR in bovine GINs. However, more detailed studies are necessary to fully evaluate management guidelines for implementation of sustainable GIN control strategies.
Keywords: Ruminants; Parasites; Resistance establishment; Risk factors; Meta-analysis
Introduction
Gastrointestinal nematodes (GIN) are an important cause of production losses in young grazing cattle, particularly in intensive production systems. The prevention of GIN infections and parasitic gastroenteritis relies on broad spectrum anthelmintic drugs. At present, the major classes of anthelmintics available for cattle belong to the families of the imidazothiazoles (levamisole), benzimidazoles (albendazole, febendazole, and oxfendazole) and macrocyclic lactones (avermectins and milbemycins).
For testing drug efficacy, the two most widely accepted tests are the fecal egg count reduction test (FECRT) and the controlled efficacy test [1,2]. The “International harmonization of anthelmintic efficacy guidelines”, indicate that an acceptable product should be at least 90% effective [3]. Even when anthelminitc resistance (AR) occurs at the parasite level, it is diagnosed through the parasitized animals and the outcome expressed at the farm level.
Anthelmintic resistance has been recognized in small ruminants worldwide, and this phenomenon was initially reported in cattle in New Zealand [4]; Australia [5]; South America [6-8]; North America [2] and Europe [9-11].
A systematic review (SR) follows a structured methodology in which each step is conducted by two independent reviewers to minimize bias. Meta-analysis (MA) refers to the statistical methodology for combining results from similar independent studies, with the aim to produce a more precise overall estimate of effect [12]. This methodology allows identification and quantification of factors that can explain variability between studies of the outcome of interest.
The objective of this study was to conduct a SR and MA of the available literature to assess the prevalence of AR in cattle farms and to identify management factors associated with occurrence of AR.
Materials And Methods
Review question, definitions and protocol
This SR studied the farm prevalence of AR in bovine GINs and potential risk factors associated with its occurrence. The PRISMA guidelines (Preferred Reporting items for Systematic reviews and Meta- Analyses statement; [13] were followed and adapted to a prevalence/ exposure SR-MA.
The review question was structured to simultaneously gather information on AR prevalence among bovine GINs and the factors associated with its occurrence.
The population was defined as the bovine species.
The exposures were:
1. The farm prevalence of AR of the GINs Haemonchus placei, H. contortus or H. similis; Ostertagia ostertagi or O. leptospicularis; Trichostrongylus colubriformis, T. axei or T. longispicularis; Cooperia oncophora, C. macmasteri, C. Surbonada, C. punctata or pectinata; Nematodirus battus, N. helvetianus or N. spathiger; Oesophagostomum radiatum; Trichuris globulusa or discolour or Bunostomum phlebotomum. The methodology to diagnose AR was registered without applying any restriction (e.g. FECRT; egg hatch test or larval inhibition migration assay).
The three major classes of anthelmintics available to control bovine GINs were included in the SR: imidazothiazoles (levamisole), benzimidazoles (albendazole, febendazole, and oxfendazole) and macrocyclic lactones (doramectin, eprinomectin, ivermectin, moxidectin and selamectin).
2. Risk factors (RF) associated with the development of AR in bovine GINs. A list of potential risk factors associated with the development of AR was developed while searching literature reviews [14,15] and recent articles in the subject [16-18]. These risk factors included frequency of treatment, cattle management, refugia, use of macrocyclic lactones in previous years, age of cattle treated and breed.
A farm was defined as positive for AR when lack of efficacy for at least one of the studied anthelmintic class was reported by the author. For RFs, the association was measured between the exposure and outcome (e.g. odd ratio or risk ratio).
Data collection
Studies were identified by searching electronic databases (date first search May 06, 2011; updated February 03, 2012 and November 2016). A list of search terms was developed taking into consideration the population (bovine), outcome (AR of GINs) - prevalence and risk factors. The following combination of search terms was used to search the databases CAB abstracts (1990-2016), Medline (1860-2016) and Lilacs (1985-2016), Medline, Cab Direct and LILACS: (bovine OR cattle OR steer OR heifer OR calves NOT (sheep OR ovine OR goat)) AND (((gastrointestinal OR internal) and (parasite* OR nematode*)) OR helmint* or haemonchus OR ostertagia OR cooperia OR trichostrongylus) AND (((anthelmintic OR drench or “macrocyclic lactone*” OR benzimidazol* OR levamisol* OR ivermectin) AND (resistance OR resistan*)) AND prevalence). Adding the RF search terms did not retrieve a new citation beyond those already captured by the anthelmintic resistance terms, therefore; the risk factor search terms were removed. These search terms were adapted to search the database Agricola (1924-2016) from the National Agricultural Library. Additionally, we manually searched the proceedings of the International Conference of the World Association for Advancement in Veterinary Parasitology (WAAVP) and the Veterinary Parasitology journal.
Citations retrieved from databases and manual searches were imported into a reference management software (“RefWorks-COS”). Duplicated references were manually removed. Search verification was performed by hand-searching of 4 literature reviews [14,15,19,20]. All relevant citations identified through manual searching, which were missing from electronic searches, were added into the review process. No language or other restrictions were imposed at this stage of the search.
Relevance screening
Abstract-based relevance screening was conducted using a standardized and pre-tested form (Supplementary material S1). The reviewer agreement was evaluated using 30 abstracts using the variable “pass” (yes or no) to perform the kappa test (kappa>0.8 was considered good reviewer agreement). Conflicts were resolved by consensus between respective reviewers. At this stage, we included primary research investigating AR and/or risk factors on GIN of economical importance in bovine species.
Methodological assessment and data extraction
A protocol form was developed and adapted from a previously form used by the first author (AM) which is included as supplementary online material (S2). This process included three reviewers and three full-text primary research articles for the risk of bias assessment and data extraction step of the pre-test.
Before methodological assessment (BA) and data extraction (DE) were performed, the relevance of articles selected through abstract screening was confirmed using the full-text papers to determine whether:
1. The article was published in English, Spanish, Portuguese, Italian or French.
2. The study designs used cross-sectional, prevalence surveys, longitudinal prevalence surveys, cohort, case-control or field trial.
3. The study reported that the methodology employed to detect AR at the farm level had an appropriate control group when using “in vivo” tests (e.g. FECRT or worm count reduction test).
4. The results reported sufficient detail to provide quantitative data for use in the MA.
The information extracted from each study included variables grouped in:
1. Characteristics of the cattle population and study settings
2. Type of anthelmintic drugs evaluated
3. Type of outcome measured
4. Risk factors evaluated
5. Laboratory method
6. Study results.
Management factors reported in cross-sectional studies associated with AR development were grouped according to the main factors reported in the searched literature as surrogates of potential causes of AR: treatment frequency, grazing management and refugia, age and breed.
The overall methodological quality was assessed using the following criteria:
Method of selection of participants
1. Sampling strategy
2. Follow-up
3. Assessment of confounders
4. Clustering adjustment
5. Sufficiently reported (referenced) laboratory protocols.
Several publication tools or guidelines to conduct observational or experimental trials were followed to build the quality assessment form [21-23].
Further, risk of systematic bias was assessed using guidelines for observational studies or experimental trial studies. The domains for observational studies were selection of participants, confounding variables, measurement of exposure, blinding of outcome assessment, incomplete outcome data and selective outcome reporting according with the RoBANS tool [24]. For experimental studies, the domains were sequence generation, allocation concealment, blinding of outcome assessment, incomplete outcome data and selective reporting [25].
Systematic review management
An electronic SRS nexus review format (Möbius Analytics, Ottawa, Ontario, Canada) was used for all steps of the SR. Each abstract or full paper was assessed by two independent reviewers against each eligibility criteria (Relevance Screening, Bias Assessment and Data Extraction) and any conflicts were resolved through consultation.
Summary measures
Prevalence: The % FECR or percentage total worm count reduction (%WCR) and, when available, a 95% confidence interval (CI), were extracted for each anthelmintic drug for each farm studied. A farm was classified as AR positive for at least one anthelmintic drug with a % FECR or %WCR less than 95 or 90 according to the author cut off. The mean anthelmintic resistance proportion for each study and 95% CI, when not provided by the authors, were estimated using the number of positive farms for AR and the total number of sampled farms reported in the study.
Risk factors: Adjusted odd ratios (OR) were extracted when presented either from a Mantel-Haenszel or logistic regression analysis and 95% CI. When raw data was available (e.g. total sample size, number of farms with anthelmintic resistance with the risk factor present and absent), ORs and 95% CI were estimated. The variables extracted, specified by our a priori categories were:
1. Frequency of treatments (number of annual treatments, number of summer treatments and using only more than 75% of avermectins in the past)
2. Management (grazing management, refugia index and type of control plan)
3. Breed
4. Age.
Meta-analysis
The mean proportion of AR in cattle GINs was analyzed using the logit prevalence to fulfill the assumption of normal distribution to perform a MA of continuous data. Logit prevalence and the standard error were computed using the formula [26]:
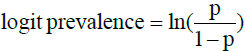 and
and 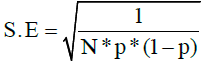
where p is prevalence, S.E is standard error and n is the sample size.
When there was no evidence of AR (e.g. AR=0) a correction of 0.01 was added before logit prevalence estimation [27].
The random effects MA was carried out given the a priori assumption that between study heterogeneity was present. A pooled logit prevalence and 95%CI was generated (forest plots) by the Dersimonian-Lair method stratified by study type, and pooled estimates were back-transformed to prevalence using the formula:
Pr evalence = 1/ (1+ exp(−coeffficient ) )
Between studies heterogeneity was estimated using the I2 statistic, which describes the percentage of variation between studies that is due to heterogeneity rather than chance [12].
Heterogeneity was evaluated using sub-group analysis and univariable meta-regression, a weighted regression of the study results based on study characteristics thought to be a source of variation that may influence the response of subjects to treatment [12].
The study level variables used in the meta-regressions were:
1. Study design (cross-sectional, prevalence survey, field trial)
2. Sampling design (random, convenience or purposive, not applicable)
3. Sample size
4. Continent (Europe, Americas, Oceania, Asia, Africa
5. Clustering (yes, no)
6. Language (English, Spanish or Portuguese)
7. Drug type (benzimidazole, imidazothiazole, macrocyclic lactone).
All the analyses were conducted in STATA V 12.
Results
Study selection and characteristics
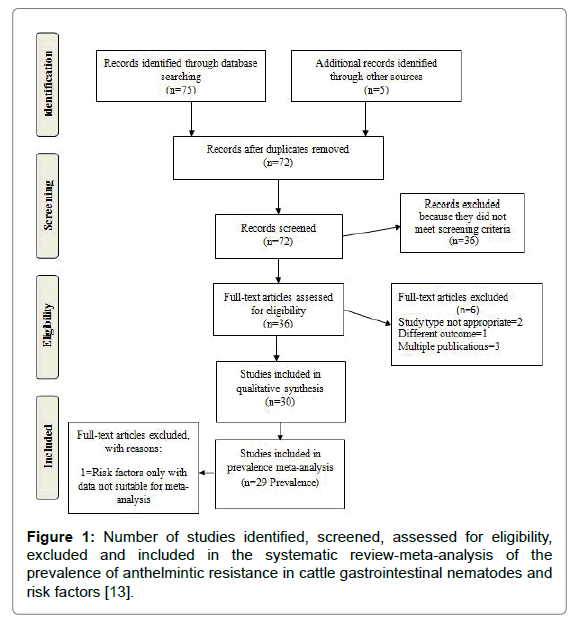
Using the format suggested by [13] the numbers of studies included at each stage of the review with reasons for exclusion are reported in Figure 1. In total, 29 studies were included in the MA, and from these five were qualitatively assessed for risk factors associated with bovine GINs anthelmintic resistance occurrence (Table 1). A table with included articles is presented in Appendix A.
Figure 1: Number of studies identified, screened, assessed for eligibility, excluded and included in the systematic review-meta-analysis of the prevalence of anthelmintic resistance in cattle gastrointestinal nematodes and risk factors [13].
| Reference/year | Country | Risk factor compared with anthelmintic resistance presence (n) | Odd Ratios (95% CI) | p-value | |
|---|---|---|---|---|---|
| Multivariable Logistic | Mantel-Haenzel | ||||
| Number of Annual Treatments | |||||
| El-Abdellati et al. [30] | Belgium | 1st treatment vs. 2nd or>treatments/ML resistance (88) | 0.97(0.84-2.86)a | 0.97 | |
| Soutello et al. [8] | Brazil | 1 to 4 treatments per year/Multiple resistance (25) | High prevalence of AR to perform any analysis | ||
| Suárez and Cristel [28] | Argentina | Number of annual treatments/multiple resistance (25) | 7.68 (2.12-27.9)b | 0.02 | |
| Canul-Ku | Mexico | Treatments per year/Ivermectin resistance (14) | NA | ||
| Number of Summer Treatments | |||||
| Suárez and Cristel [28] | Argentina | Treatments during November-January/multiple resistance (25) | 2.53 (0.26-25.03) | 0.42 | |
| Use only more than 75% Avermectins in the past | |||||
| Suárez and Cristel [28] | Argentina | Yes-No/ML resistance (25) | 18.62 (1.3-254.7) | 0.03 | |
| Use of macrocyclic Lactones in Previous Year | |||||
| El-Abdellati et al. [30] | Belgium | Yes-No/ML resistance (102) | 1.41(0.49-4.12)a | 0.47 | |
| Grazing Management | |||||
| Soutello et al. [8] | Brazil | Rotational-continuous/Multiple resistance (25) | High prevalence of AR to perform any analysis | ||
| Jackson et al. [16] | New Zealand | Land grazed by cattle/multiple resistance (59) | |||
| 25-50% | 0.17 (0.02-1.16) | 0.07 | |||
| 50-75% | 0.34 (0.03-3.35) | 0.35 | |||
| >75% | 0.16 (0.02-1.31) | 0.09 | |||
| Refugia Index | |||||
| Suárez and Cristel [28] | Argentina | Details not provided (25) | 2.15 (0.01-20.69) | 0.69 | |
| Breed | |||||
| El-Abdellati et al. [30] | Belgium | Dairy vs beef vs mixed (83) | NR | <0.01 | |
| Soutello et al. [8] | Brazil | NA | |||
| Age | |||||
| Jackson et al. [16] | New Zealand | -Number of breeding cows and heifers >2 years old (54) | 0.25 (0.08-0.75) | 0.01 | |
| -Number of 1 year old (54) | 4.08 (1.1-15.12) | 0.04 | |||
| -Purchasing more than 2 years old cow and heifers (54) | NR | >0.05 | |||
| Type of Control Plan | |||||
| Suárez and Cristel [28] | Argentina | Strategic programmed- | 1.32 (0.20-8.76) | ||
| Programmed and as required (based on body weight, clinical signs, etc)/multiple resistance (25) | Referent | 0.77 | |||
aOdd ratio estimated from raw data provided by the author(s); NA=Not applicable, statistical analysis was not performed; NR=Measure of association not reported and raw data not suitable for estimation
Table 1: Summary of the main risk factors evaluated for the association with anthelmintic resistance in cattle nematodes, reported on five cross-sectional studies where results were extracted.
Most studies evaluated the efficacy of the three major anthelmintic drug families benzimidazole (n=25), imidazothiazole (n=18) and macrocyclic lactones (n=25). Therefore, we extracted data and analyzed the efficacy of the major drug families and not combinations or narrow-spectrum drugs such as closantel.
A total of 518 farms were investigated in the 29 publications included in this systematic review-meta-analysis (SR-MA). The countries where the studies were conducted were Argentina (n=5), the United States of America (n=3), the United Kingdom (n=3), Brazil (n=4), Belgium and Germany (n=2), New Zealand (n=2), Australia (n=3), Mexico (n=2), Greece (n=1), Nicaragua (n=1), Venezuela (n=1), Bangladesh (n=1) and Cameroon (n=1). The results of the main characteristics and the methodological assessment of the included studies are presented in Tables 2 and 3 respectively.
| Variable | Description | Categories | Number of studies (n=29) |
|---|---|---|---|
| Study design | Type of study design | Cross sectional | 5 |
| Prevalence survey | 14 | ||
| Field Trial | 10 | ||
| Drug | Anthelmintic group that efficacy has been evaluated |
Macrocylic lactones | 25 |
| Bencimidazole | 18 | ||
| Imidazothiazole | 13 | ||
| Laboratory test | Test employed to assess anthelmintic efficacy | FECRT or ECT | 28 |
| In vitro test | 1 | ||
| Cut off | Cut off to define lack of efficacy | ≤ 90% | 2 |
| ≤ 95% | 24 | ||
| Not reported | 3 | ||
| Gastrointestinal Nematodes (GIN) |
Genera of GIN reported to be resistant to the studied drug | Cooperia spp | 22(n=24) |
| Ostertagia ostertagi | 10(n=22) | ||
| Haemonchus spp | 11(n=23) | ||
| Trichostrongylus spp. | 8(n=22) | ||
| Oesophagostomum spp | 5(n=21) | ||
| Date published | Year of study publication | Before 2000 | 1 |
| After 2000 | 28 | ||
| Type of cattle | Type of cattle studied | Dairy | 4 |
| Beef | 14 | ||
| Mixed | 2 | ||
| Not reported | 9 | ||
| Continent | Europe | 6 | |
| Americas | 16 | ||
| Oceania | 5 | ||
| Asia | 1 | ||
| Africa | 1 | ||
| Language | Language of study publication | English | 22 |
| Spanish | 5 | ||
| Portuguese | 2 |
Table 2: Descriptive characteristics of 29 publications which were included in the systematic review-meta-analysis.
| Criteria | Assessment | Number of studies (n=29) |
|---|---|---|
| Was the simple size justified at the farm level | Yes | 5 |
| No | 15 | |
| Not applicable | 9 | |
| Was the simple size justified at the animal level | Yes | 7 |
| No | 22 | |
| How were operations selected for the study? | Not reported | 4 |
| Random | 2 | |
| Convenience or purposively | 23 | |
| Were the laboratory methods described in sufficient detail to be replicated? | Yes | 29 |
| Reference paper | 0 | |
| No | 0 | |
| Did the author report that blinding was used? | Yes | 1 |
| No | 28 | |
| Based on the study design, was clustering accounted for appropriately in the analysis? | Yes | 0 |
| No | 4 | |
| Not applicable | 25 | |
| In cross-sectional studies, was the statistical analysis described adequately that it can be reproduced? | Yes | 2(n=5) |
| No | 0(n=5) | |
| Statistical analysis not done | 3(n=5) | |
| Measurement of exposure | Yes | 4(4) |
| No | 0 | |
| Not applicable | 25 | |
| Incomplete outcome data | Yes | 3 |
| No | 26 | |
| Selective outcome reporting | Yes | 1 |
| No | 28 |
Table 3: Summary for methodological soundness and/or reporting of 29 publications included in the systematic review-meta-analysis.
Summary measures
The overall farm proportion of AR was 85.4% (95%CI=76.2% to 94.6%). When stratified by drug class, 83.3% (95% CI=73.5% to 93.1%) of the studied farms presented resistance to macrocyclic lactones; 47.0% (95% CI= 27.6% to 66.4%) to the benzimidazole and 45.1% (95% CI=19.1% to 71.2%) to imidazothiazole.
From studies reporting AR nematode genera, Cooperia spp. was reported in 91.7% (n=24), Ostertagia sp. in 45.4% (n=22), Haemonchus sp. in 47.8% (n=23), Trichostrongylus sp. in 36.4% (n=22) and Oesophagostomum spp. in 23.8% (n=21).
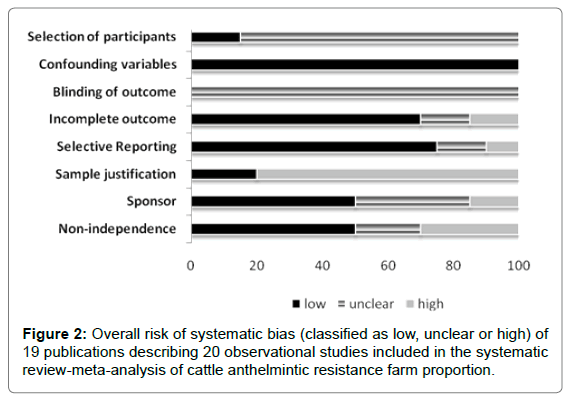
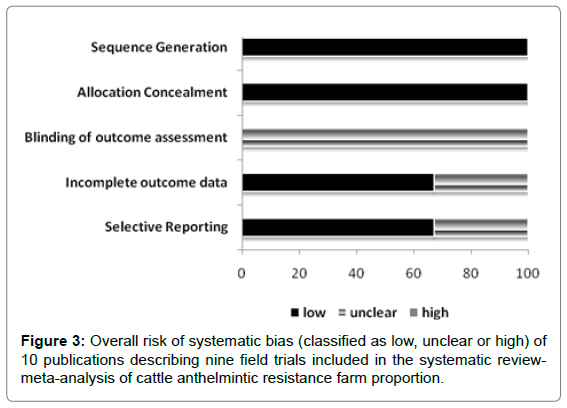
The overall mean risk of bias for observational studies included in the SR-MA is illustrated in Figure 2. Unclear (not reported or unable to assess) was found in selection of farms and blinding of outcome (80% and 100%, respectively) and high in farm sample justification (80%). Figure 3 summarizes the overall mean risk of bias of 10 experimental field trials included in the SR-MA. Except for blinding of outcome assessment (100% unclear), the included studies presented low risk of systematic bias.
Meta-analysis
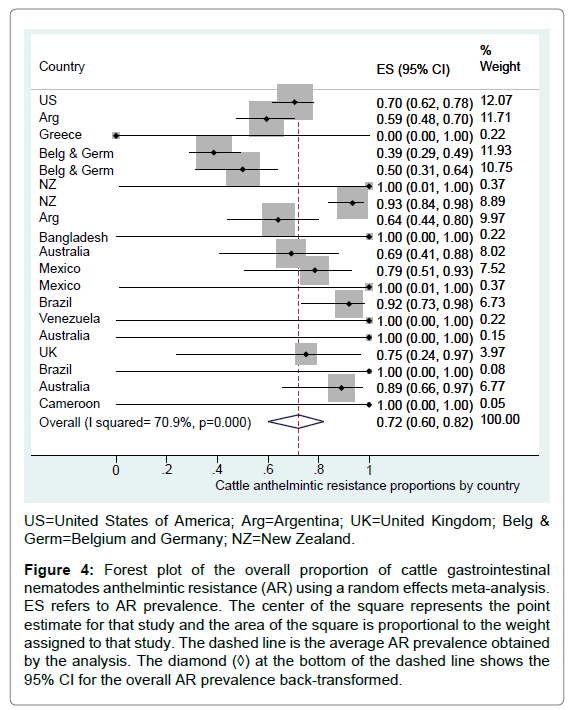
The overall logit AR proportion back-transformed was 72.0% (95% CI=60.8% to 80.8%) for the group of observational studies (n=19), with high between study heterogeneity (I2=70.9%, p<0.001) (Figure 4). The AR proportion for 10 studies following a field trial design was 99.9% (95% CI=16.3% to 99.9%).
US=United States of America; Arg=Argentina; UK=United Kingdom; Belg & Germ=Belgium and Germany; NZ=New Zealand.
Figure 4: Forest plot of the overall proportion of cattle gastrointestinal nematodes anthelmintic resistance (AR) using a random effects meta-analysis. ES refers to AR prevalence. The center of the square represents the point estimate for that study and the area of the square is proportional to the weight assigned to that study. The dashed line is the average AR prevalence obtained by the analysis. The diamond (◊) at the bottom of the dashed line shows the 95% CI for the overall AR prevalence back-transformed.
When exploring potential sources of between study-heterogeneity, results from the univariable meta-regressions, suggested that study location (categorized as “continent”) was associated with AR proportion (p<0.05). This contributed to explain 100% of the between study variation (I2=0.0%) and part of the total variation (Adjusted R2=68.6%). Study type, language, cattle type, study sponsorship and type of nematode developing resistance, were not significantly associated with AR logit prevalence.
Risk factors
A qualitative summary of the main RFs reported in five cross-sectional studies is presented in Table 1. Only two studies had enough data to perform a multivariable logistic model [16,28] while the remaining presented univariable ORs or p-values [29,30]. The results presented from these studies suggest that frequency of treatments and cattle age are associated with the presence of AR.
Conclusions
According to the results of this SR-MA, the phenomenon of anthelmintic resistance in nematodes of cattle has been studied in many parts of the world. Cattle are particularly susceptible to parasitic gastroenteritis at a young age, and then are able to develop immune protection when reaching adult age. Therefore, the number of anthelmintic treatments administered to adult cattle is expected to be low. Nonetheless, the results from this study indicated a high number of farms with bovine GINs resistant to one or more anthelmintic drug worldwide.
However, the high number of farms presenting resistance to the macrocyclic lactones (82%) suggests that this modern and broad spectrum drug has been employed frequently to control not only internal but external parasites such as ticks or screw worms. According to [31] the use of this kind of anthelmintic drugs has been the structural basis of worm management for nearly 40 years and reaffirms that their continual use has led to the global selection of drug-resistant worms populations. From all the studies included in this SR-MA, only one reported that the studied farms were randomly selected while most of them were conveniently selected. Only five studies reported sample size justification. For the observational studies, we identified unclear risk of bias when selecting the farms (85%) and high risk of bias for sample size justification (80%). None of these studies reported blinding, either of the administration of the drugs assigned to each group or of laboratory personnel performing the tests. Because of this, the studies included in this SR-MA are likely to represent a bias selection of farms, because farmers who were aware that anthelmintic treatments were not effective tended to be more likely to participate in the studies, or because researchers selected farms with a previous knowledge of anthelmintic efficacy failure. Thus, AR values are likely to overestimate the true AR in cattle farms. High between-study heterogeneity was expected a priori, in part due to regional characteristics influencing the production systems, epidemiological conditions and GIN control measures applied. This was supported by the fact that “region” was the only variable associated with the outcome and contributed to explain the between-study heterogeneity. This is concordant with Higgins [32] and Ioannidis [33] who are in favor of conducting MA even when the statistics demonstrates that the true effect size varies among studies.
Unfortunately, there were not enough studies to perform a MA on potential risk factors associated with the development of AR. Although some evidence suggests that frequency of treatments, population in refugia and host age at treatment are positive associations [28,30] further research is necessary to establish the main risk factors for GIN AR development [34]. Although some of the included studies were not representative of source populations of cattle ranches, results from this SR-MA suggested that practitioners and producers should be cautious in the frequency of treatment with anthelmintic drugs [35], in order to avoid development of AR which could exacerbate parasitic gastroenteritis and production losses [36].
Acknowledgements
The authors would like to acknowledge Carolina Pereira and Laura Falzon for procuring of some published work. This work was fully funded by the National Research Institute for Agriculture.
Authors Contribution
A. Mederos was responsible for managing the systematic review, execution of the meta-analysis and preparation of the manuscript. A. Minho contributed as second reviewer; B. Carracelas contributed as second reviewer; S. Fernández contributed as second reviewer and J. Sánchez contributed with the meta-analysis.
Conflict of Interest
The authors have not conflicts of interest to declare.
References
- Coles GC, Bauer C, Borgsteede FHM, Geerst TR, Klei TR, et al. (1992) Methods for detection of anthelmintic resistance in nematodes of veterinary importance. World Association for the Advancement of Veterinary Parasitology. Vet Parasitol 44: 35-44.
- Edmonds MD, Johnson EG, Edmonds JD (2010) Anthelmintic resistance of Ostertagia ostertagi and Cooperia oncophora to macrocyclic lactones in cattle from the Western United States. Vet Parasitol 170: 224-229.
- Vercruysse J, Holdsworth P, Letonja T, Barth D, Conder G, et al. (2001) World Organization for Animal Health, International harmonisation of anthelmintic efficacy guidelines. Vet Parasitol 96: 171-193.
- Waghorn TS, Leathwick DM, Rhodes AP, Jackson R, Pomroy WE, et al. (2006) Prevalence of anthelmintic resistance on 62 beef cattle farms in the North Island of New Zealand. N Z Vet J 54: 278-282.
- Rendell DK (2010) Anthelmintic resistance in cattle nematodes on 13 South-West victorian properties. Aust Vet J 88: 504-509.
- Anziani OS, Guglielmone AA, Zimmermann G, Vazquez R, Suarez VR (2001) Avermectin resistance to Cooperia pectinata in cattle in Argentina. Vet Rec 149: 58-59.
- Fiel CA, Saumell CA, Steffan PE, Rodriguez EM (2001) Resistance of Cooperia to ivermectin treatments in grazing cattle of the Humid Pampa, Argentina. Vet Parasitol 97: 211-217.
- Condi GK, Soutello RG, Amarante AF (2009) Moxidectin-resistant nematodes in cattle in Brazil. Vet Parasitol 161: 213-217.
- Papadopoulos E, Hamhougias K, Himona C, Dorchies P (2000) Strongyle anthelmintic resistance in horses and cattle from Greece. Revue de Médecine Vétérinaire 151: 1139-1142.
- Coles GC (2004) Anthelmintic resistance in cattle. Cattle Practice 12: 177-179.
- Demeler J, Zeveren AMJ, Kleinschmidt N, Vercruysse J, Hoglund J, et al. (2009) Monitoring the efficacy of ivermectin and albendazole against gastro intestinal nematodes of cattle in Northern Europe. Vet Parasitol 160: 109-115.
- Borenstein M, Hedges LV, Higgins JPT, Rothstein HR (2009) Introduction to Meta-Analysis. John Wiley & Sons, Ltd, The Atrium, Southern Gate, Chichester, west Sussex, United Kingdom.
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, et al. (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. J Clin Epidemiol 62: e1-e34.
- Prichard RK (1990) Anthelmintic resistance in nematodes: Extent, recent understanding and future directions for control and research. Int J Parasitol 20: 515-523.
- Sutherland IA, Leathwick DM (2011) Anthelmintic resistance in nematode parasites of cattle: A global issue. Trends Parasitol 27: 176-181.
- Jackson R, Rhodes AP, Pomroy WE, Leathwick DM, West DM, et al. (2006) Anthelmintic resistance and management of nematode parasites on beef cattle-rearing farms in the North Island of New Zealand. N Z Vet J 54: 289-296.
- Suarez VH, Cristel SL (2007) Anthelmintic resistance in cattle nematode in the Western Pampeana region of Argentina. Vet Parasitol 144: 111-117.
- Bliss DH, Moore RD, Kvasnicka WG (2008) Parasite resistance in US cattle. The AABP Proceedings 41: 109-114.
- Waller PJ (1993) Control strategies to prevent resistance. Vet Parasitol 46: 133-142.
- Kaplan RM (2004) Drug resistance in nematodes of veterinary importance: A status report. Trends Parasitol 20: 477-481.
- von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, et al. (2008) The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol 61: 344-349
- Sanderson S, Tatt ID, Higgins JPT (2007) Tools for assessing quality and susceptibility to bias in observational studies in epidemiology: a systematic review and annotated bibliography. Int J Epidemiol 36: 666-676.
- Sargeant JM, O’Connor AM, Gardner IA, Dickson JS, Torrence ME, et al. (2010) The REFLECT Statement: Reporting Guidelines for Randomized Controlled Trials in Livestock and Food Safety: Explanation and Elaboration. Zoonoses Public Health 57: 105-136
- Kim YS, Park JE, Lee YJ, Seo HJ, Sheen SS, et al. (2013) Testing a tool for assessing the risk of bias for nonrandomized studies showed moderate reliability and promising validity. J Clin Epidemiol 66: 408-414.
- Higgins JPT, Altman DG, Gotzche PC, Jüni P, Moher D, et al. (2011) The Cochrane Collaboration’s tool for assessing risk of bias in randomized trials. Brit Med J 343: d5928.
- Sanchez J, Dohoo IR, Christensen J, Rajic A (2007) Factors influencing the prevalence of salmonella spp. in swine farms: A meta-analysis approach. Prev Vet Med 81: 148-177.
- Friedrich JO, Adhikari NKJ, Beyene J (2007) Inclusion of zero total event trials in meta-analyses maintains analytic consistency and incorporates all available data. BMC Med Res Methodology pp: 7-5
- Suarez VH, Cristel SL (2011) Risk factors for the development of bovine anthelmintic resistance in Argentina. Proceedings of the 23rd International Conference of the World Association for the Advancement of Veterinary Parasitololgy Argentina Pp: 21-25.
- Soutello RG, Seno MC, Amarante AF (2007) Anthelmintic resistance in cattle nematodes in Northwestern Sao Paulo State, Brazil. Vet Parasitol 148: 360-364.
- El-Abdellati A, Charlier J, Geldhof P, Levecke B, Demeler J, et al. (2010) The use of a simplified faecal egg count reduction test for assessing anthelmintic efficacy on Belgian and German cattle farms. Vet Parasitol 169: 352-357.
- Geurden T, Chartier C, Fanke J, di Regalbono AF, Traversa D, et al. (2015) Anthelmintic resistance to ivermectin and moxidectin in gastrointestinal nematodes of cattle in Europe. Int J Parasitol Drugs Drug Resist 18: 163-171
- Higgins JPT (2008) Commentary: Heterogeneity in meta-analysis should be expected and appropriately quantified. Int J Epidemiol 37: 1158-1160.
- Ioannidis JP, Patsopoulos NA, Rothstein HR (2008) Reasons or excuses for avoiding meta-analysis in forest plots. BMJ 336: 1413-1415.
- Cotter JL, Burgel AV, Besier RB (2015) Anthelmintic resistance in nematodes of beef cattle in south-west Western Australia. Vet Parasitol 207: 276-284.
- das Neves JH, Carvalho N, Rinaldi L, Cringoli G, Amarante AFT (2014) Diagnosis of anthelmintic resistance in cattle in Brazil: A comparison of different methodologies. Vet Parasitol 206: 216-226.
- Ebene NJ, Onyali IO, Mingoas JP, Poungue HB, Mfopit MY, et al. (2016) Efficacy testing of anthelmintics against field strains of Trichostrongyles in cattle farms of the periurban zone of Ngaoundere in Cameroon. AJVS 50: 78-86.
Citation: Mederos AE, Carracelas B, Minho AP, Fernández S, Sánchez J (2018) Prevalence and Factors Associated with Anthelmintic Resistance in Gastrointestinal Nematodes of Cattle: A Systematic Review and Meta-analysis. J Vet Med Health 2: 111.
Copyright: © 2018 Mederos AE, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 3100
- [From(publication date): 0-2018 - Sep 18, 2024]
- Breakdown by view type
- HTML page views: 2377
- PDF downloads: 723