Research Article Open Access
Preparation of Extended-Release Theophylline for Gastric Tube Administration Significantly Impairs Gradual Resorption
Papiež Adriana, Šrámek Vladimír, Pešáková Edita, Matiňáková Libuše and Suk Pavel*Department of Clinical-Pharmacological, International Clinical Research Center, St. Anne's University Hospital Brno, Pekařská 53, 656 91 Brno, Czech Republic
- *Corresponding Author:
- Suk Pavel
Department of Clinical-Pharmacological
International Clinical Research Center
St. Anne's University Hospital Brno, Pekařská 53
656 91 Brno, Czech Republic
Tel: +420 605 436 695/+420 543 183 537
Fax: +420 543 182 555
E-mail: pavel.suk@fnusa.cz
Received date: August 21, 2017; Accepted date: August 29, 2017; Published date: August 31, 2017
Citation: Adriana P, Vladimír S, Edita P, Libuše M, Pavel S (2017) Preparation of Extended-Release Theophylline for Gastric Tube Administration Significantly Impairs Gradual Resorption. Clin Pharmacol Biopharm 6: 175. doi:10.4172/2167-065X.1000175
Copyright: © 2017 Adriana P, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Clinical Pharmacology & Biopharmaceutics
Abstract
Background: Although Extended Release (ER) dosage forms are not suitable for administration via Nasogastric Tube (NGT), they are used in critically ill patients. The aim of this study is to compare pharmacokinetics of intact and crushed ER theophylline capsules and tablets.
Methods: Open-label, randomized controlled trial with two parallel groups was conducted on 10 healthy volunteers. They were randomized into Theo plus® 300 (ER tablets) and Eupyllin CR N® 300 (capsules with ER pellets) group. Each group took the same drug orally twice-first prepared (for the NGT administration) by crushing and secondly as an intact dosage form. Theophylline serum levels were taken at baseline, 30 min, 60 min, 2 h, 4 h, 6 h, 9 h and 12 h after drug administration. maximum serum concentration (Cmax), time to reach Cmax (Tmax) and area under the serum concentration-time curves over 12 h (AUC12h) were calculated. Data are presented as mean ± SD.
Results: Crushing increased Cmax in both Euphyllin (43.8 ± 6.5 vs. 26.5 ± 1.6 μmol/l; p<0.01) and Theoplus (45.2 ± 3.6 vs. 29.4 ± 4.8 μmol/l; p=0.013) groups. Tmax was significantly shorter after administration of crushed dosage forms in Euphyllin (0.9 ± 0.7 vs. 5.6 ± 0.9 h; p<0.001) and Theoplus (1.1 ± 0.5 vs. 9.6 ± 2.5 h; p<0.01) group. Concordantly, drug crushing augmented AUC12 h by 40% in both drugs.
Conclusion: Crushing destroyed ER properties of theophylline tablets and capsules and their pharmacokinetic profiles were comparable with immediate release forms.
Keywords
Theophylline; Pharmacokinetics; Delayed-action preparations; Drug liberation; Volunteers
Introduction
Extended release dosage forms are not suitable for administration via a Nasogastric Tube (NGT) because of the possible pharmacokinetic changes due to modification of the dosage form by crushing [1,2]. Inappropriate dosage forms are often administered through enteral feeding tubes in ICUs. Theophylline is indicated in the treatment of bronchospasms associated with diseases such as asthma or chronic obstructive pulmonary disease and it is administered in intensive and chronic care settings [3,4]. In acute cases, theophylline can be administered intravenously (this formulation contains aminophylline, which is a molecular complex of theophylline with ethylendiamine). However, intravenous administration is sometimes impossible due to the absence of the intravenous line and it is also generally undesirable for long-term hospitalisation. In such cases, the best solution would be to administer theophylline via a nasogastric tube. Unfortunately, no immediate release dosage forms of theophylline are available in the Czech Republic. All the available dosage forms are extended-release formulations: capsules with extended-release pellets (Euphyllin CR N®, Afonilum®) or prolonged release tablets (Theoplus®).
There are almost no data about pharmacokinetic changes of extended-release dosage forms that were modified prior to administration. Primrose et al. studied the influence of halving extended-release theophylline tablets; the results showed that the absorption was faster and plasma levels were higher when the 400 mg tablet was taken as 2 halves [5].
To gain data about crushed extended-release dosage forms, we designed an in-vivo study investigating the impact of dosage form modification prior to administration via NGT on pharmacokinetic properties. The aim of the study was to compare the pharmacokinetic profiles of crushed Theoplus® 300 and Euphyllin CR N® 300 administered per os with intact dosage forms of the same drugs administered per os.
Materials and Methods
This monocentric, open-label, randomised controlled trial with two parallel groups was conducted on 10 healthy volunteers. Two different extended-release formulations of theophylline available on the Czech market were used in the experiment: Theoplus® 300 (Pierre Fabre, France) and Euphyllin CR N® 300 (Takeda, Germany). This clinical trial (protocol number: CPU002, EudraCT number: 2016-002534-72) was approved by the hospital’s Ethics Committee and Czech State Institute for Drug Control. Informed consent was obtained from all individual participants included in the study. All procedures performed in the study were in accordance with the 1964 Helsinki declaration and its later amendments.
The inclusion criteria were: Healthy volunteers between 18 and 45 years of age, BMI 18.5-30 kg/m2, no chronic medication (except for hormonal contraception), and physical examination, ECG and laboratory examination without clinically significant deviations. The exclusion criteria were milk intolerance, pregnancy or lactation, smoking within the last year and alcohol or psychotropic drugs abuse.
The volunteers were randomised into two groups (5 in each group) by using a random code generator. The tested drug was Euphyllin CR N® 300 in Group I and Theoplus® 300 in Group II. The volunteers took the same drug per os twice-firstly, in a crushed form which is suitable for NGT administration and for the second time, as an intact dosage form.
The volunteers were not allowed to consume drinks or meals containing xanthine’s (coffee, tea, and coke, chocolate...) 2 days before and during the duration of the clinical trial. All volunteers fasted 10 hours before and 2 h after the administration of the study drug.
On the first day of the study, suspensions of the examined drugs were prepared by mixing the crushed pellets of Euphyllin CR N® 300 and crushed tablets of Theoplus® 300 with 50 ml of water in beakers. After swallowing the prepared suspension, the beaker was twice rinsed with 50 ml of water and drunk by a volunteer to minimise the losses. Blood samples for theophylline were taken at baseline (60 min before drug administration) and 30 min, 60 min, 2 h, 4 h, 6 h, 9 h and 12 h after drug administration. After 7 days of washout period, the volunteers swallowed an intact capsule of Euphyllin CR N® 300 or intact tablet of Theoplus® 300. The medication was drunk down with 100 ml of water. Blood samples were taken at the same time points.
Blood samples were centrifuged after 60 min and serum was stored at -80°C. Theophylline levels were measured by chemiluminescent immunoassay (Architect i2000SR, Abbott) using Architect itheophylline reagent kit (Abbott).
maximum serum concentration (Cmax) and time to reach Cmax (Tmax) were calculated. The area under the serum concentration-time curves (AUC) was determined using the trapezoidal rule. Based on the sampling schedule, AUC for 12 h after the administration (AUC12h) was calculated. The groups were compared using independent or dependent t-test as appropriate. P-values less than 0.05 were considered significant. To suppress weight difference among volunteers, all serum theophylline levels were standardised to a body weight of 70 kg according to the formula: Ccorrected=Cmeasured × 70/ body weight.
Results
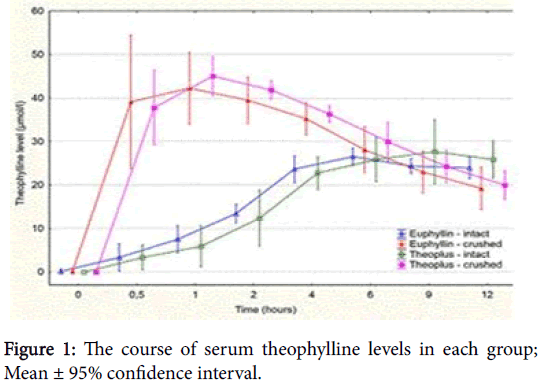
The demographic data of 10 volunteers, who completed the study, are presented in Table 1. The course of serum theophylline levels in each group is depicted in Figure 1. Pharmacokinetic parameters are presented in Table 2. The time to reach peak concentration was significantly faster for the crushed form than for the intact form for both drugs (Table 2). Moreover, Cmax and AUC12h were significantly higher for the crushed forms than for the intact formulas.
| Euphyllin | Theoplus | p | |
|---|---|---|---|
| Age (years) | 33.6 ± 12 | 27.6 ± 7.4 | 0.37 |
| Weight (cm) | 177 ± 9 | 171 ± 7.7 | 0.70 |
| Height (kg) | 77.9 ± 17 | 70.6 ± 13.2 | 0.64 |
| BMI (kg/m2) | 24.6 ± 3.2 | 23.9 ± 2.4 | 0.60 |
| Men/Women | 3/2 | 1/4 | 0.20 |
Table 1: Demographic characteristics. Data are presented as means ± SD; BMI: Body Mass Index.
| Euphyllin | Theoplus | |||||
|---|---|---|---|---|---|---|
| Whole | Crushed | p-value | Whole | Crushed | p-value | |
| Cmax (µmol/l) | 26.5 ± 1.6 | 43.8 ± 6.5 | p<0.01 | 29.4 ± 4.8 | 45.2 ± 3.6 | p=0.013 |
| Tmax (h) | 5.6 ± 0.9 | 0.9 ± 0.7 | p<0.001 | 9.6 ± 2.5 | 1.1 ± 0.5 | p<0.01 |
| AUC12h (µmol/l × h) | 250 ± 17 | 349 ± 48 | p<0.01 | 257 ± 24 | 366 ± 22 | p<0.001 |
Table 2: Pharmacokinetic parameters. Data are presented as means ± SD. Cmax-maximum serum theophylline concentration, Tmax-time from drug administration to reach the maximum concentration, AUC12h-area under the concentration curve over 12 h after administration.
As for comparison between the drugs-the only difference was a significantly shorter Tmax for Euphyllin CR N® 300 than for Theoplus® 300 (5.6 vs 9.6 hours; p<0.01 even if both peaks were flat). The other parameters did not differ.
Side Effects
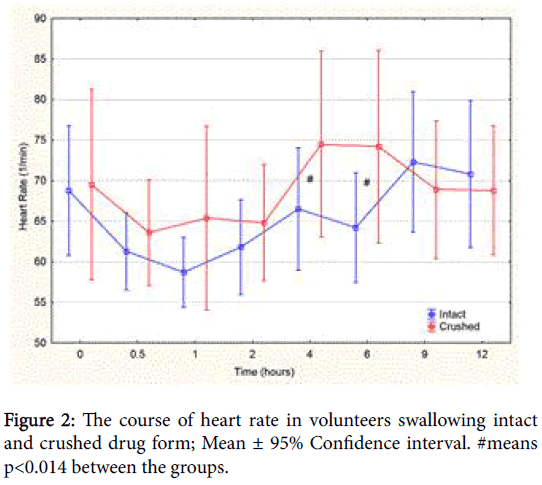
The development of heart rate during the study is shown in Figure 2. There were no changes in blood pressure. Three volunteers reported an episode of hand tremor-in one case accompanied by mild malaisebetween 3 and 4.5 h after the ingestion of the crushed drug forms (2 cases in the Theoplus® group and 1 case in the Euphyllin® group).
Discussion
This study has proven that disruption of extended release drug forms leads to rapid absorption. Tmax of both crushed drugs is approximately 1 hour, which is comparable with immediate release theophylline Tmax of 0.5-3 h [6]. The modified release properties of both theophylline dosage forms were lost by crushing; therefore, the mechanism of formulation of modified-release drugs has likely no influence on the preservation of extended-release properties.
The clinical implication of this study is that the disruption of extended-release drug forms for administration via NGT may cause inappropriately high peak concentrations in combination with shortened duration of action. Therefore, immediate release drug forms should be preferred or, if not available, extended-release drugs should be given in a similar way as immediate release-lower doses with shorter dosing intervals. Another possible solution would be the administration of uncrushed pellets via the NGT. However, this was associated with unexpectedly low blood levels of theophylline in one study [7]. Moreover, the uncrushed pellets administered via NGT in other studies tend to clog tubes sized 14F and smaller [8,9].
We have chosen theophylline as a model drug because it has a narrow therapeutic range [10] and is widely used in critically ill patients with restricted per os intake. The side effects after ingestion of a single dose are mostly mild [11] and therapeutic drug monitoring of theophylline is widely available.
All three volunteers with side effects were women with a relatively low body weight (52-64 kg; average body weight in the study was 74.2 kg). The occurrence of the side effects in our study corresponds with the findings in a study evaluating dosage forms of theophylline with immediate release, where side effects occurred at doses >300 mg [6]. In the case of intact dosage forms, no side effects were noticed during the whole study. It should be mentioned that after a single dose of intact dosage forms the drug levels were in sub therapeutic range. In the case of crushed drugs, Cmax were on the lower border of the therapeutic range. This observation proves that the side effects of theophylline can be present even at therapeutic or nearly sub therapeutic concentrations [3].
Study limitations: The suspension of crushed drugs with water was administered to volunteers per os (and not via NGT) because we wanted to bypass any other factors that might influence the serum concentration of theophylline. Nevertheless, the average losses of drugs associated with administration via NGT are approximately 10% (when an appropriate method is used) [8]. Moreover, this setup would not reflect the reality because drugs in clinical practice are co-administered with enteral nutrition, which may further affect drug absorption [12-14]. This study was designed to detect peak serum theophylline concentrations; therefore, trough levels or the effect of repeated doses were not assessed.
Conclusion
We have proved on the model of theophylline that extended-release dosage forms are not suitable for administration via NGT due to high peak concentrations in combination with shortened duration of action. If it is necessary to administer dosage forms with prolonged release, the dose of the drug should be divided into multiple doses based on the biological half-life of the active substance.
References
- http://www.pharmaceutical-journal.com/learning/learning-article/managing-drug-therapy-in-patients-receiving-enteral-and-parenteral-nutrition/11097109.article
- Gilbar PJ (1999) A guide to enternal drug administration in palliative care. J Pain Symptom Manage 17: 197-207.
- Barnes PJ (2003) Theophylline: new perspectives for an old drug. Am J Respir Crit Care Med 167: 813-818.
- Brenner M, Berkowitz R, Marshall N, Strunk RC (1988) Need for theophylline in severe steroid-requiring asthmatics. Clin Allergy 18: 143-150.
- Primrose WR, Clee MD, Moody JP, Hockings N (1983) Alteration of pharmacokinetics after halving a slow-release theophylline tablet. Pharmatherapeutica 3: 429-432.
- Rovei V, Chanoine F, Strolin Benedetti M (1982) Pharmacokinetics of theophylline: a dose-range study. Br J Clin Pharmacol 14: 769-778.
- Berkovitch M, Dafni O, Leiboviz A, Mayan H, Habut B, et al. (2002) Therapeutic drug monitoring of theophylline in frail elderly patients: oral compared with nasogastric tube administration. Ther Drug Monit 24: 594-597.
- Ruzsíková A, Součková L, Suk P, Opatřilová R, Kejdušová M, et al. (2015) Quantitative analysis of drug losses administered via nasogastric tube-in vitro study. Int J Pharm 478: 368-371
- Ponrouch MP, Sautou-Miranda V, Boyer A, Bourdeaux D, Montagner A, et al. (2010) Proton pump inhibitor administration via nasogastric tube in pediatric practice: Comparative analysis with protocol optimization. Int J Pharm 390: 160-164.
- Raebel MA, Carroll NM, Andrade SE, Chester EA, Feldstein A, et al. (2006) Monitoring of drugs with a narrow therapeutic range in ambulatory care. Am J Manag Care 12: 268-274.
- Ogilvie RI (1978) Clinical Pharmacokinetics of Theophylline. Clin Pharmacokinet 3: 267-293.
- Burns PE, McCall L, Wirsching R (1988) Physical compatibility of enteral formulas with various common medications. J Am Diet Assoc 88: 1094-1096.
- Cutie AJ, Altman E, Lenkel L (1983) Compatibility of enteral products with commonly employed drug additives. JPEN J Parenter Enteral Nutr 7: 186-191.
- Gal P, Layson R (1986) Interference with oral theophylline absorption by continuous nasogastric feedings. Ther Drug Monit 8: 421-423.
Relevant Topics
- Applied Biopharmaceutics
- Biomarker Discovery
- Biopharmaceuticals Manufacturing and Industry
- Biopharmaceuticals Process Validation
- Biopharmaceutics and Drug Disposition
- Clinical Drug Trials
- Clinical Pharmacists
- Clinical Pharmacology
- Clinical Research Studies
- Clinical Trials Databases
- DMPK (Drug Metabolism and Pharmacokinetics)
- Medical Trails/ Drug Medical Trails
- Methods in Clinical Pharmacology
- Pharmacoeconomics
- Pharmacogenomics
- Pharmacokinetic-Pharmacodynamic (PK-PD) Modeling
- Precision Medicine
- Preclinical safety evaluation of biopharmaceuticals
- Psychopharmacology
Recommended Journals
Article Tools
Article Usage
- Total views: 5494
- [From(publication date):
September-2017 - Jul 15, 2025] - Breakdown by view type
- HTML page views : 4639
- PDF downloads : 855