Preoperative Predictors of Successful Weight Loss Outcomes Following Laparoscopic Sleeve Gastrectomy: A Retrospective Single-center Study
Received: 29-Jun-2019 / Accepted Date: 20-Sep-2019 / Published Date: 27-Sep-2019 DOI: 10.4172/2165-7904.1000389
Abstract
Objective: Sleeve gastrectomy (SG) is an established bariatric surgical procedure in the United States yet patient attributes that influence outcomes following SG remain inconclusive. Therefore, identifying baseline variables that predict weight loss (WL) success at one and two-year follow-up was investigated.
Methods: Retrospective analysis of the medical records of 533 patients who underwent SG between January 1, 2014 and June 30, 2015 was completed. Follow-up included 282 subjects at one-year and 136 subjects at two-years. Chi-square and independent samples t tests were used to compare successful and suboptimal WL groups. Multivariate logistic regression analysis was performed to determine which variables independently predicted a successful WL outcome, defined as >50% excess WL (EWL) at one and two-years.
Results: The overall sample was 23% male and 77% female with a mean age of 44.73 ± 11.9, and mean BMI of 46.9 ± 8.1. The follow-up group (n=304) had a significantly higher baseline BMI than those lost to follow-up (n=229) (47.7 ± 8.2 vs. 45.9 ± 7.8, p=0.01), otherwise the groups were similar. At one-year, BMI (p<0.001) was the only independent predictor of successful WL outcome following SG. At two-years, dyslipidemia (p<0.01) and smoking status (p=0.047) were the only independent predictors of successful WL outcome.
Conclusion: BMI appears to be a significant predictor variable for WL following SG. Whether other important baseline patient attributes influence successful WL following SG in the short-, mid-, and long-term warrant further investigation.
Keywords: Obesity; Bariatric surgery; Sleeve gastrectomy; Outcomes; Predictors; Weight loss
Introduction
Obesity has become a worldwide epidemic, with prevalence almost tripling since 1975 [1]. Estimates from the Centers for Disease Control and Prevention (CDC) suggest more than one-third of the United States adult population is obese [2]. The increased obesity rate is attributable to environmental factors, such as large food portions, increase in sedentary activities, pervasive use of medications that induce weightgain, and inadequate sleep [3,4,5]. Despite intervening with diet and exercise to mitigate these environmental factors, persons who initially experience weight loss (WL) often relapse and regain all, if not more, of the weight [3]. This phenomenon is secondary to compensatory mechanisms employed by the body to outweigh the negative energy balance that is experienced during periods of decreased caloric intake and increased energy expenditure [3,6,7].
The obesity epidemic has changed how the disease is treated, with bariatric surgery now widely accepted as one of, if not the most successful obesity treatment available [8]. Given that the safety and efficacy of bariatric surgery has been well established, the number of bariatric procedures performed in the United States has steadily increased over the past five years to over 200,000 annually [9]. Sleeve gastrectomies (SG) have more than quadrupled during the same time span, rendering it the most commonly performed bariatric procedure in the United States [9]. Reportedly, overall benefits of SG over other bariatric surgical procedures include technical ease, shorter operating time, and lower associated morbidity and mortality rates, making it an appealing procedure for surgeons and patients alike [10,11]. SG has proven to demonstrate WL outcomes and comorbidity improvements similar to those following its popular predecessor, the Roux-en-Y gastric bypass (RYGB), escalating its attractiveness [12-14].
Numerous factors may affect a patient’s outcome following bariatric surgery. While previous studies have examined the association between baseline patient characteristics and bariatric surgery outcomes, of which few focused specifically on outcomes following SG, the results remain inconsistent. Accordingly, the need persists to identify predictive indicators of successful SG outcomes. The purpose of this study, therefore, was to identify baseline patient demographics and clinical characteristics that predict greater likelihood of achieving successful WL following SG. The findings may better explain the importance of prior knowledge of these baseline determinants in predicting who is at risk for poor weight loss, weight regain, and possible return of or lack of improvement in associated comorbidities following this procedure. Bariatric programs can then apply these findings to develop enhanced clinical pathways, ultimately maximizing the benefits derived from SG.
Materials and Methods
Setting
The research was conducted at an academic medical center, which is accredited as a Comprehensive Center by the American College of Surgeons’ Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program. The multidisciplinary bariatric team includes three surgeons, three physician assistants, a registered dietitian/ nutritionist, an eating behaviour specialist, and a clinical pharmacist. Perioperative clinical pathways and surgical technique performed by all surgeons at the center were standardized for all included cases.
Population and sample
This study was a retrospective analysis of a convenience sample of 561 medical records of patients who underwent laparoscopic SG between January 1, 2014 and June 30, 2015. All patients were at least 18 years of age and complied with the National Institute of Health’s (NIH) inclusion criteria for bariatric surgery [15]. Exclusion criteria were as follows: (a) conversion to open procedure; (b) conversion to another procedure within the follow-up period; (c) staple line leak within the follow-up period; (d) pregnancy within the follow-up period; (e) reoperation related to the primary procedure within the follow-up period; (f) death within the follow-up period.
Data collection methods
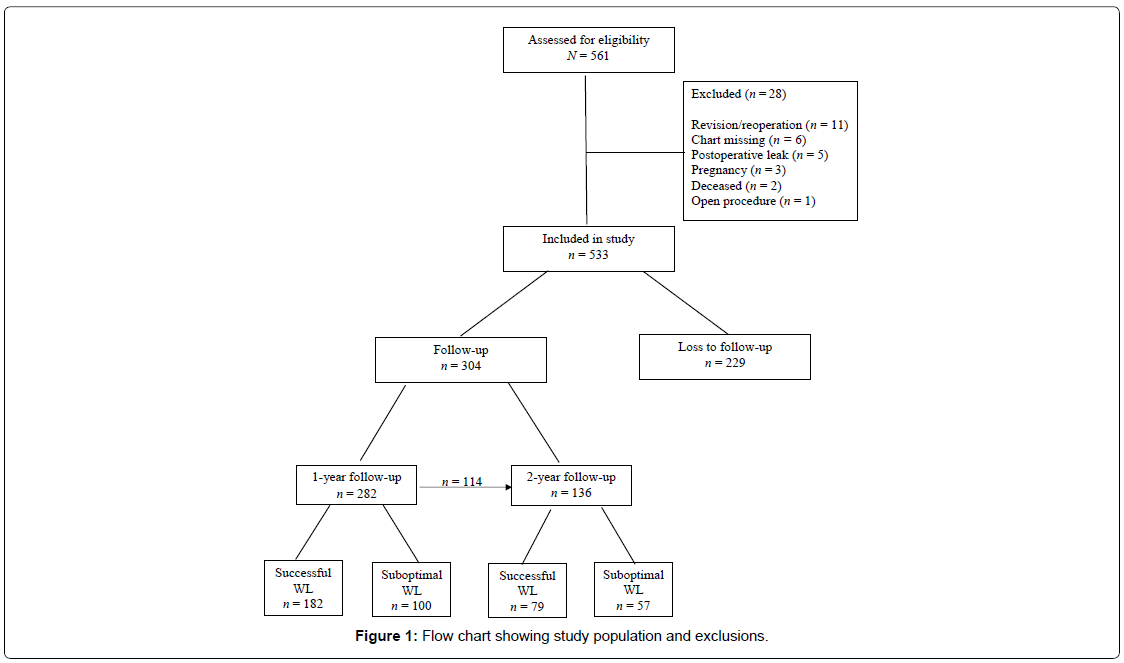
A report, run from the center’s electronic medical record, listing all patients who underwent laparoscopic SG from January 1, 2014 through June 30, 2015, identified 561 potential cases for inclusion. After applying exclusion criteria, 28 subjects were excluded from the cohort. Figure 1 displays reasons for exclusion and the number of subjects in each subgroup. Baseline, one-year, and two-year data were retrospectively collected via review of each participant’s paper and/or electronic medical record. One-year follow-up was defined as 365 ± 90 days and the two-year follow-up period as 730 ± 90 days from each patient’s surgery date. Inclusion in the two year follow-up period was not contingent upon the subject having one-year followup data and vice-versa. Full approval was obtained in accordance from the Institutional Review Board (IRB) at D’Youville College and the Center.
Treatment of data
Data were analyzed using IBM® SPSS® Statistics, version 25. Descriptive statistics were run to provide simple summaries about the overall sample. Subjects were categorized as having suboptimal or successful WL outcomes at one-year and two-year follow-up. Comparisons were made between the suboptimal and successful WL groups at each time point using independent samples t tests or Chi square tests. All tests were two-sided and a p-value<0.05 was considered statistically significant. In areas where significant differences were found between the suboptimal and successful groups further analysis was completed.
The main outcome of interest was WL, with successful WL defined as >50% excess WL (EWL). Predictor variables included: age, sex, race, ethnicity, marital status, employment status, insurance type, length of obesity, smoking status, history of outpatient or inpatient psychiatric treatment, history of eating disorder, weight, Body Mass Index (BMI), Glycated Hemoglobin level (A1C), Fasting Plasma Glucose (FPG), total cholesterol level, High-density Lipoprotein Level (HDL), Low-density Lipoprotein Level (LDL), triglyceride level, presence or absence of Type 2 Diabetes mellitus (T2D), Hypertension (HTN), Gastroesophageal Reflux Disease (GERD), dyslipidemia, and anxiety/depression measured at baseline. Comorbidities were considered present if patient self-reported, was diagnosed by a primary care provider, or was on drug therapy for the disease. In reviewing the two-year data, presence of oneyear follow-up was also represented as a dichotomous independent variable. Logistic regression analyses, utilizing the enter method, were performed to quantify the association between the outcome of interest and explanatory variables. Crude associations were examined and those that were statistically significant were entered into the full model. An adjusted multivariate model was then run to control for age, sex, race, BMI, and insurance type. A multicollinearity assessment was run on all of the variables included in the multivariate model and redundant variables were excluded if the variance inflation factor exceeded 10. Odds ratios and 95% confidence intervals were reported for the model. A p-value<0.05 was considered statistically significant.
Results
The overall sample of 533 subjects was 22.5% males and 77.5% females, of which 82% were Caucasian, 13% Black, and 5% Hispanic/ Latino, with a mean age of 44.73 ± 11.9, and mean BMI of 46.9 ± 8.1. Follow-up data was available on 304 (57%) of the 533 patients, whereby 282 records were available at one-year, 136 at year two, and 114 subjects inclusive in both the one- and two-year periods. Descriptive statistics comparing baseline data for the follow-up (n=304) and no follow-up groups (n=229) revealed the follow-up group had a significantly higher baseline BMI than the no follow-up group (47.7 ± 8.2 vs. 45.9 ± 7.8, p=0.01), otherwise the groups were similar.
Tests of hypotheses
Descriptive statistics of baseline demographic and clinical characteristics grouped for suboptimal and successful weight loss outcomes at one- and two-years follow-up are displayed in Table 1.
| Characteristics | 1-year follow-up (n=282) | 2-year follow-up (n=136) | ||||
|---|---|---|---|---|---|---|
| Successful WL (n=182) | Suboptimal WL (n=100) | p value | Successful WL (n=79) | Suboptimal WL (n=57) | p value | |
| Age in years, mean ± SD | 44.6 ± 12.5 | 48.2 ± 11.7 | 0.02* | 46.6 ± 12.5 | 46.3 ± 13.2 | 0.89 |
| Sex, n (%) | 0.01* | 0.14 | ||||
| Male | 35 (19.2) | 33 (33.0) | 15 (19.0) | 17 (29.8) | ||
| Female | 147 (80.8) | 67 (67.0) | 64 (81.0) | 40 (70.1) | ||
| Race, n (%) | 0.63 | 0.32 | ||||
| White | 161 (88.5) | 81 (81.0) | 65 (82.3) | 46 (80.7) | ||
| Black | 15 (8.2) | 12 (12.0) | 11 (13.9) | 8 (14.0) | ||
| Other | 6 (3.3) | 7 (7.0) | 3 (3.8) | 3 (5.3) | ||
| Ethnicity, n (%) | 0.73 | 0.45 | ||||
| Non-Hispanic/Non-Latino | 132 (73) | 73 (73.0) | 51 (64.6) | 40 (70.2) | ||
| Hispanic/Latino | 9 (4.9) | 3 (3.0) | 7 (8.9) | 2 (3.5) | ||
| Declined | 41 (22.5) | 24 (24.0) | 21 (26.6) | 15 (26.3) | ||
| Marital status, n (%) | 0.47 | 0.27 | ||||
| Single | 54 (29.7) | 27 (27.0) | 20 (25.3) | 21 (36.8) | ||
| Married | 100 (54.9) | 52 (52.0) | 46 (58.2) | 25 (43.9) | ||
| Divorced/annulled | 16 (8.8) | 15 (15.0) | 7 (8.9) | 6 (10.5) | ||
| Widowed | 3 (1.6) | 3 (3.0) | 1 (1.3) | 3 (5.3) | ||
| Declined | 9 (4.9) | 3 (3.0) | 5 (6.3) | 2 (3.5) | ||
| Employment status, n (%) | 0.54 | 0.9 | ||||
| Full time | 93 (51.1) | 46 (46.0) | 39 (49.4) | 25 (43.9) | ||
| Part time | 23 (12.6) | 13 (13.0) | 9 (11.4) | 6 (10.5) | ||
| Unemployed | 61 (33.5) | 40 (40.0) | 28 (35.4) | 24 (42.1) | ||
| Declined | 5 (2.7) | 1 (1.0) | 3 (3.8) | 2 (3.5) | ||
| Insurance type, n (%) | 0.97 | 0.49 | ||||
| Private | 127 (69.8) | 70 (70.0) | 53 (67.1) | 35 (61.4) | ||
| Public | 55 (30.2) | 30 (30.0) | 26 (32.9) | 22 (38.6) | ||
| Length of obesity, n (%) | 0.4 | 0.09 | ||||
| Since birth | 11 (6.0) | 11 (11.0) | 2 (2.5) | 7 (12.3) | ||
| Since adolescence | 54 (29.7) | 31 (31.0) | 25 (31.6) | 16 (28.1) | ||
| 5-9 years | 21 (11.5) | 8 (8.0) | 7 (8.9) | 2 (3.5) | ||
| 10+ years | 96 (52.7) | 50 (50.0) | 45 (57.0) | 32 (56.1) | ||
| History of psych treatment, n (%) | 0.64 | 0.56 | ||||
| No | 128 (70.3) | 73 (73.0) | 56 (70.1) | 43 (75.4) | ||
| Yes | 54 (29.7) | 27 (27.0) | 23 (29.1) | 14 (24.6) | ||
| Smoking status, n (%) | <0.01* | 0.04* | ||||
| Never | 75 (41.2) | 60 (60.0) | 25 (31.6) | 29 (50.9) | ||
| Former | 89 (48.9) | 38 (38.0) | 45 (57.0) | 26 (45.6) | ||
| Current | 18 (9.9) | 2 (2.0) | 9 (11.4) | 2 (3.5) | ||
| History of inpatient psych treatment, n (%) | 0.12 | 0.11 | ||||
| No | 174 (95.6) | 91 (91.0) | 77 (97.5) | 52 (91.2) | ||
| Yes | 8 (4.4) | 9 (9.0) | 2 (2.5) | 5 (8.8) | ||
| History of eating disorder, n (%) | 0.54 | 0.51 | ||||
| No | 180 (98.9) | 100 (100.0) | 77 (97.5) | 57 (100) | ||
| Yes | 2 (1.1) | 0 (0.0) | 2 (2.5) | 0 (0) | ||
| Weight in kg, mean ± SD | 129.2 ± 25.1 | 144.7 ± 30.7 | <0.001* | 129.2 ± 25.8 | 135.9 ± 26.7 | 0.14 |
| BMI in kg/m2, mean ± SD | 45.9 ± 6.7 | 51.3 ± 9.5 | <0.001* | 46.1 ± 7.5 | 48.1 ± 8.4 | 0.17 |
| Excess weight in lbs, mean ± SD | 146.5 ± 48.1 | 179.4 ± 61.4 | <0.001* | 147.3 ± 51.2 | 160.5 ± 54.4 | 0.15 |
| Type 2 diabetes, n (%) | <0.01* | 0.02* | ||||
| No | 138 (75.8) | 58 (58.0) | 61 (77.2) | 33 (57.9) | ||
| Yes | 44 (24.2) | 42 (42.0) | 18 (22.8) | 24 (42.1) | ||
| Hemoglobin A1C in %, mean ± SD | 6.5 ± 1.7 | 6.9 ± 1.7 | 0.42 | 6.5 ± 2.3 | 6.7 ± 0.9 | 0.83 |
| Fasting plasma glucose in mg/dL, mean ± SD | 108.4 ± 30.5 | 127.9 ± 63.3 | <0.01 | 105.9 ± 28.2 | 118.9 ± 52.1 | 0.09 |
| Dyslipidemia, n (%) | 0.02* | <0.01* | ||||
| No | 107 (58.8) | 44 (44.0) | 49 (62.0) | 21 (36.8) | ||
| Yes | 75 (41.2) | 56 (56.0) | 30 (38.0) | 36 (63.2) | ||
| Total cholesterol in mg/dL, mean ± SD | 184.7 ± 38.1 | 183.5 ± 38.6 | 0.8 | 182.4 ± 39.6 | 180.0 ± 34.4 | 0.73 |
| HDLC in mg/dL, mean ± SD | 47.2 ± 13.1 | 44.4 ± 11.0 | 0.07 | 48.5 ± 15.7 | 45.5 ± 12.3 | 0.24 |
| LDLC in mg/dL, mean ± SD | 107.8 ± 32.4 | 106.6 ± 33.9 | 0.77 | 102.9 ± 33.7 | 100.4 ± 31.6 | 0.67 |
| Triglycerides in mg/dL, mean ± SD | 152.4 ± 83.6 | 168.7 ± 91.0 | 0.13 | 155.1 ± 78.4 | 173.0 ± 90.0 | 0.23 |
| Hypertension, n (%) | 0.001* | 0.04* | ||||
| No | 92 (50.5) | 30 (30.0) | 42 (53.2) | 20 (35.1) | ||
| Yes | 90 (49.5) | 70 (70.0) | 37 (46.8) | 37 (64.9) | ||
| GERD, n (%) | 0.83 | 0.63 | ||||
| No | 116 (63.7) | 65 (65.0) | 49 (62.0) | 33 (57.9) | ||
| Yes | 66 (36.3) | 35 (35.0) | 30 (38.0) | 24 (42.1) | ||
| Anxiety/depression, n (%) | 0.47 | 0.05* | ||||
| No | 92 (50.5) | 55 (55.0) | 39 (49.4) | 39 (68.4) | ||
| Yes | 90 (49.5) | 45 (45.0) | 40 (50.6) | 19 (33.3) | ||
| One-year follow-up completed, n (%) | -- | 0.713 | ||||
| No | -- | -- | 12 (15.2) | 10 (17.5) | ||
| Yes | -- | -- | 67 (84.8) | 47 (82.5) | ||
Table 1: Baseline demographic and clinical characteristics of suboptimal and successful weight loss groups at 1- and 2-years follow-up.
One-year follow-up results: Univariate logistic regression analyses were completed with each of the significant predictor variables and the dichotomous dependent variable (WL outcome). Baseline age (p=0.018), weight (p<0.001), BMI (p<0.001), excess weight (p<0.001), fasting plasma glucose (p<0.01), sex (p=0.010), smoking status (p=0.01), T2D (p<0.01), dyslipidemia (p=0.02), and hypertension (p=0.001) were statistically significant and eligible for entry into the multivariate logistic regression analysis.
As expected, a multicollinearity assessment demonstrated a high degree of multicollinearity between baseline weight, BMI, and excess weight, therefore only BMI was entered in the multivariate model. Adjusted multivariate logistic regression analysis indicated that only BMI remained an independent predictor of WL outcome one year following SG (OR 0.920, 95% CI [0.887, 0.955], p<0.001). Restated, for every one unit increase in BMI the odds of achieving successful WL following SG at one year are reduced by 8%. Results of the full, adjusted analysis are reported in Table 2.
| Variables | 1-year analyses | 2-year analyses |
|---|---|---|
| OR (95% CI) | OR (95% CI) | |
| Age | 0.984 (0.960, 1.011) | 1.041 (1.000, 1.084) |
| BMI | 0.920 (0.887, 0.955)* | 0.974 (0.922, 1.029) |
| FPG | 0.994 (0.987, 1.002) | --- |
| Sex (male vs. female) | 0.779 (0.405, 1.498) | 0.925 (0.357, 2.395) |
| Smoking status (never vs. current) | 0.234 (0.049, 1.115) | 0.177 (0.032, 0.981)* |
| Smoking status (never vs. former) | 0.545 (0.113, 2.615) | 0.477 (0.086, 2.644) |
| Type 2 diabetes (no vs. yes) | 1.215 (0.575, 2.566) | 2.106 (0.824, 5.384) |
| Dyslipidemia (no vs. yes) | 1.746 (0.900, 3.387) | 4.127 (1.526, 11.159)* |
| Hypertension (no vs. yes) | 0.930 (0.473, 1.829) | 1.444 (0.537, 3.882) |
| Anxiety/Depression (no vs. yes) | --- | 0.542 (0.242, 1.212) |
| Race (black vs. white) | 0.635 (0.249, 1.617) | 0.904 (0.268, 3.052) |
| Race (else vs. white) | 0.728 (0.197, 2.687) | 0.885 (0.140, 5.604) |
| Insurance type (private vs. public) | 0.873 (0.463, 1.647) | 1.303 (0.557, 3.049) |
| One-year follow up (no vs. yes) | --- | 0.746 (0.231, 2.018) |
Table 2: Multivariate binary logistic regression model results for prediction of successful weight loss vs. suboptimal weight loss outcome at 1-year and 2-years follow-up.
Two-year follow-up results: Univariate logistic regression analyses revealed smoking status (p=0.046), T2D (p=0.017), dyslipidemia (p=0.004), hypertension (p=0.038), and anxiety/depression (p=0.046) were all found to be significant and eligible for entry into the multivariate logistic regression analysis. Additionally, follow-up at oneyear was included as predictor variable in the multivariate model.
No multicollinearity was demonstrated among the predictor variables; thus, all were entered into the model. Adjusted multivariate logistic regression analysis found dyslipidemia (OR 4.127, 95% CI [1.526, 11.159], p<0.01) and smoking status (OR 0.177, 95% CI [0.032, 0.981], p=0.047) to be independent predictors of WL outcome. Those without dyslipidemia were four times more likely to experience successful WL at two years following SG. Additionally, those who never smoked at baseline were 82% less likely than current smokers at baseline to achieve successful WL following SG. Results of the full analysis are reported in Table 2.
Discussion
In this retrospective analysis of 533 patients who underwent SG, we found that BMI was the only independent predictor of successful WL at one-year, while dyslipidemia and smoking status were the only independent predictors at two-years. Furthermore, the results demonstrate SG to be effective in terms of comparable percentage EWL at 12 and 24 months post-operative follow-up, exemplified by Percentage EWL of 57.3% ± 18.0% and 55.9% ± 19.7%, respectively in patients 45 years of age with preoperative BMI more than 45 kg/m2. This evidence suggests SG may lead to stable weight loss, but longer follow-up is warranted.
Other groups have examined the association between predictor variables and outcomes following bariatric surgery, however results vary widely across studies and few have examined the relationship solely among SG patients [16-19]. Despite BMI having been previously reported to be the sole and consistently negatively predictor associated with weight loss following SG [20-22], our results found no relationship between BMI and WL outcome beyond one year. Given that the loss to follow-up group had a significantly lower BMI than our follow-group, as well as the small two-year sample size, successful WL may have been underrepresented in our study resulting in failure to identify a significant relationship between BMI and WL at two-years.
The results from the adjusted analysis confirm previous evidence of a positive association between smoking at baseline and WL outcome at two year follow-up [20]. Nicotine is known to suppress appetite and stimulate metabolism, which perhaps may account for the relationship [23]. Regardless, the multiple health hazards associated with smoking render the additional WL that may occur following SG trivial with respect to clinical practice.
Our study identified a negative relationship between dyslipidemia and WL outcome following SG. The presence of dyslipidemia was based upon self-report, the primary care provider’s diagnosis or documentation that the subject was prescribed a medication for the condition. The diagnosis of dyslipidemia involves measuring plasma levels of total cholesterol, triglycerides, and individual lipoproteins, but for the purposes of this research study this data was not available. Perhaps the prevalence may have been underestimated among our subjects and therefore the result should be interpreted with caution.
Limitations
Our study has methodological limitations that affect interpreting the results, the most notable being attrition. As with most bariatric research of comparable sample sizes, the high attrition rate accounted for only 53% of patients returning at one-year and 26% at two-years.
Given the retrospective nature of our study design the issue of loss to follow-up could not be corrected. Moreover, with no more than 24 months follow-up completed, the results should be interpreted as short-term. As healthcare information systems continue to expand, interoperability of electronic medical records and health information exchanges would greatly benefit retrospective analysis of clinical databases.
While perioperative clinical pathways were fairly standardized, some patients may have required additional visits to address maladaptive eating behaviours, possibly leading to greater WL among these patients. Finally, the list of variables included in this study, although more extensive than that of other studies [18,19,21], was not exhaustive. It is possible that other variables, such as level of education, number of people living in the household, parental status, annual income, and number of clinic visits, should be included in the model.
Conclusion
Our finding that baseline BMI is a significant predictor variable for WL following SG is consistent with existing literature. Whether other important baseline patient demographics and/or clinical characteristics influence successful WL following SG in the short-, mid-, and longterm warrant further investigation.
Disclosures
The authors have no commercial associations that might be a conflict of interest in relation to this article.
References
- Heymsfield SB, Wadden TA (2017) Mechanisms, pathophysiology, and management of obesity. New Engl J Med 376: 254-266.
- Davis RA, Plaisance EP, Allison DB (2018) Complementary hypotheses on contributors to the obesity epidemic. Obes 26: 17-21.
- Berkowitz RI, Fabricatore AN (2011) Obesity, psychiatric status, and psychiatric medications. Psychiatr Clin North Am 34: 747-764.
- MacLean PS, Higgins JA, Giles ED, Sherk VD, Jackman MR (2015) The role of adipose tissue in weight regain after weight loss. Obes Rev 16: 45-54.
- Sumithran P, Prendergast LA, Delbridge E, Purcell K, Shulkes A, et al. (2011) Long-term persistence of hormonal adaptations to weight loss. New Engl J Med 365: 1597-1604.
- Colquitt JL, Pickett K, Loveman E, Frampton GK (2014) Surgery for weight loss in adults. Cochrane Database Syst Rev 8: CD003641.
- American Society for Metabolic and Bariatric Surgery (2016) Estimate of bariatric surgery numbers, 2011-2016.
- Corcelles R, Lacy A (2016) Case for sleeve gastrectomy. Surg Obes Relat Dis 12: 1243-1246.
- ASMBS Clinical Issues Committee (2012) Updated position statement on sleeve gastrectomy as a bariatric procedure. Surg Obes Relat Dis 8: e21-e26.
- Schauer PR, Kashyap SR, Wolski K, Brethauer SA, Kirwan JP, et al. (2012) Bariatric surgery versus intensive medical therapy in obese patients with diabetes. New Engl J Med 366: 1567-1576.
- Schauer PR, Bhatt DL, Kirwan JP, Wolski K, Brethauer SA, et al. (2014) Bariatric surgery versus intensive medical therapy for diabetes 3-year outcomes. New Engl J Med 370: 2002-2013.
- Schauer PR, Bhatt DL, Kirwan JP, Wolski K, Aminian A, et al. (2017) Bariatric surgery versus intensive medical therapy for diabetes 5-year outcomes. New Engl J Med 376: 641-651.
- NIH Conference (1991) Gastrointestinal surgery for severe obesity. Consensus Development Conference Panel. Ann Int Med 115: 956-961.
- Benoit SC, Hunter TD, Francis DM, De La Cruz-Munoz N (2014) Use of bariatric outcomes longitudinal database (BOLD) to study variability in patient success after bariatric surgery. Obes Surg 24: 936-943.
- Gullick AA, Graham LA, Richman J, Kakade M, Stahl R, et al. (2015) Association of race and socioeconomic status with outcomes following laparoscopic Roux-en-Y gastric bypass. Obes Surg 25: 705-711.
- Ortega E, MorÃnigo R, Flores L, Moize V, Rios M, et al. (2012) Predictive factors of excess body weight loss 1 year after laparoscopic bariatric surgery. Surg Endosc 26: 1744-1750.
- Parri A, Benaiges D, Schröder H, Izquierdo-Pulido M, Ramón J, et al. (2015) Preoperative predictors of weight loss at 4 years following bariatric surgery. Nutr Clin Pract 30: 420-424.
- Andersen JR, Aadland E, Nilsen RM, VÃ¥ge V (2014) Predictors of weight loss are different in men and women after sleeve gastrectomy. Obes Surg 24: 594-598.
- Martin DJ, Lee CMY, Rigas G, Tam CS (2015) Predictors of weight loss 2 years after laparoscopic sleeve gastrectomy. Asian J Endosc Surg 8: 328-332.
- Livhits M, Mercado C, Yermilov I, Parikh JA, Dutson E, et al. (2012) Preoperative predictors of weight loss following bariatric surgery: systematic review. Obes Surg 22: 70-89.
- Courtemanche C, Tchernis R, Ukert B (2018) The effect of smoking on obesity: Evidence from a randomized trial. J Health Econ 57: 31-44.
Citation: Scovazzo NC, Rafalson L, Cadzow R, Ramsey DK (2019) Preoperative Predictors of Successful Weight Loss Outcomes Following Laparoscopic Sleeve Gastrectomy: A Retrospective Single-center Study. J Obes Weight Loss Ther 9:389. DOI: 10.4172/2165-7904.1000389
Copyright: © 2019 Scovazzo NC, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 2715
- [From(publication date): 0-2019 - Apr 03, 2025]
- Breakdown by view type
- HTML page views: 1948
- PDF downloads: 767