Preoperative Dietary Behaviour as Predictive Factor for Inadequate Weight Loss after Sleeve Gastrectomy
Received: 15-Nov-2017 / Accepted Date: 22-Nov-2017 / Published Date: 12-Dec-2017 DOI: 10.4172/2165-7904.1000358
Abstract
Laparoscopic sleeve gastrectomy was the most common procedure performed worldwide. It was feasible and associated with fewer complications and metabolic deficiencies compared than other procedures. Inadequate weight loss (IWL) was a critical issue of this procedure and it could depend on technical fault or on preoperative patient factors. The aim of this study was to identify predictive factors of inadequate weight loss investigating on preoperative psychological and dietary behaviour.
Method: A retrospective analysis of patients undergoing sleeve gastrectomy was acquired. Patients with inadequate weight loss (excess weight loss (EWL) <50% at 1 years after procedure) and patients with EWL>50% were compared. Bivariate analysis and multivariate model was performed in order to study preoperative factors focused on psychological (binge eating disorder (BED) and grazing) and dietary (compliance to preoperative diet) behaviour.
Result: 20/85 (23.5%) patients were included in IWL group. In bivariate analysis, predictive factors for IWL were diabetes (p=0.034), insulin-treatment (p=0.037) and patient misbehaviour to preoperative diet (p=0.042). In multivariate model including BMI, BED, grazing and compliance to preoperative diet, misbehaviour to preoperative diet was the predictive factor for IWL (p=0.046).
Conclusion: Patient misbehaviour to preoperative diet was a predictive factor for IWL. Preoperative patients could be based on compliance to preoperative diet.
Introduction
Laparoscopic sleeve gastrectomy (LSG) is the most common bariatric procedure performed worldwide [1]. Indeed, LSG is gaining popularity because it is technically less demanding, requires less operative time, and may be associated with fewer complications and metabolic deficiencies compared than other procedures [2]. Increasing experience with LSG shows that some patients will have a procedure failure because complications (i.e. reflux symptoms), weight regain or inadequate weight loss (IWL) [3]. Despite failure for weight regain must be evaluated at least after 5 years, IWL could be assessed in the first year after procedure [4].
There is no consensus on the definition of IWL. Several authors considered excess weight loss percentage (EWL%) the most practice parameter to evaluate IWL [3,5,6]. Although patients presented a great EWL heterogeneity, Reynaud criteria defined unsatisfactory results as EWL lower than 50% [7], and literature defined IWL as EWL<50% [8]. IWL (EWL<50%) could be depending on by preoperative [9] and/or postoperative [10] patient behaviour whether if LSG procedure was well performed and standardized. An attentive postoperative follow-up could prevent a negative eating behaviour and a preoperative assessment could be facilitating patient selection.
Anthropometric data (i.e. BMI), comorbidities (i.e type 2 diabetes) and psychological and dietary behaviours (i.e. eating disorders and diet compliance) are the most important factor in order to predict postoperative LSG success [8,9,11,12].
Despite the well-defined importance of preoperative diet was well defined in terms of preoperative weight loss and its benefit on perioperative bariatric surgical outcomes, its efficacy on postoperative EWL was is not clear [13-16].
The goal of this study was to identify predictive factors for IWL after LSG, with respect to preoperative anthropometric parameters and patient’s psychological and dietetic behaviour.
Materials and Methods
1) Patient selection
A retrospective review of a prospectively maintained database was performed. We included all consecutive laparoscopic sleeve gastrectomy (LSG) procedures performed between November 2014 and February 2017 at Bariatric Unit of San Michele Hospital.
All patients included in this study met the National Institutes of Health consensus criteria for bariatric surgery and the Italian guidelines for morbid obesity surgery and fulfilled the institutional guidelines of medically supervised weight loss and psychological clearance [17].
One board-certified surgeon (RM) considered to be experienced in laparoscopic bariatric surgery and one junior surgeon (GF) considered to be in learning process, performed all procedures. Signed informed consent was obtained from all patients.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
2) Technical considerations
All procedures were performed laparoscopically. Primary SG procedure and revisional cases were included in this study. SG as first step to another bariatric procedure and all definitive procedures were included. All procedures were performed in French position and all patients met perioperative recommendations as indicated by Italian guideline for morbid obesity [18]. Drainage was left three-days and nasogastric tube was removed one-day after the operation. Liquid alimentation was allowed two-days after operation if no-fistulas and no-stenosis was documented from gastric swallow and/or clinically. A complete clinical and laboratory follow-up 1,3,6,12 months after operation was performed as indicated by Italian guideline for morbidity obesity [18].
3) Data collection
Data were recorded prospectively on specific excel sheet forms. All patients were consecutive and ranked according to the date of operation (case rank).
Preoperative data - Several preoperative criteria were collected: sex, age, weight, height, BMI, comorbidities (type 2 diabetes, insulinrequirement, dyslipidaemia, hypertension, obstructive sleep apnea syndrome).
Hypertension was defined as preoperative elevated blood pressure (>140/90 mmHg) or patients taking medication to control blood pressure. Type 2 diabetes was defined as elevated glycemic values with or without the need to take medication according with International guidelines.
Obstructive sleep apnea syndrome (OSAS) was diagnosed by polysomnography and all patients with moderate to severe OSAS were preoperative treated by continuous positive airway pressure (CPAP). We also collected other comorbidities including knee arthrosis requiring medical or surgical treatment and upper gastrointestinal disorders (gastro-oesophageal reflux, gastritis and/or gastric or duodenal ulcer). Previous bariatric surgery was collected.
Preoperative psychological and dietetic behavior
Binge eating disorder (BED) was defined as a compulsive overeating without possibility of excessive consumption of food ingest during a subject state of feeling both out of control and distressed without compensatory, purgatory behaviour [19].
Grazing was defined as an eating behaviour characterized by the repetitive eating of small/modest amounts of food in an unplanned manner and/or not in response to hunger/satiety sensation [20].
All patients were evaluated by at least two clinical evaluations performed by the same psychologist (CS).
All patients received a preoperative Very Low Ketogenic Caloric Diet (VLKCD) (11 patients) or a Very Low Caloric Diet (VLCD) (74 patients) by a medical dietician (SP). VLKCD was 930-995 kcal with carbohydrate intake 40.1-47.6 g, VLCD 975-1057 kcal with carbohydrate intake 109-123.5 g.
Patient compliance to preoperative diet was calculated 4 to 8 weeks after prescription. All patients that experimented stable weight or weight gain were considered misbehaviour to preoperative diet. We also calculated preoperative total weight loss.
Operative data - Operative time frames were defined as skin incision to skin closure. Nasogastric tube was 34 to 40 Fr. Intraoperative methylene blue test at the end of the procedure and upper gastrointestinal series at postoperative day 2 were systematically performed to detect gastric leak. Conversion to open or laparoscopy, transfusions and intraoperative complications were evaluated.
Postoperative data - Mortality rate was evaluated within a postoperative period of 60 days after the initial surgical procedure. Sixty days postoperative morbidity rate was also evaluated using Clavien-Dindo classification [21]. Total hospital and ICU length of stay, rehospitalisation data (with or without reoperation) within 60 days were also collected. Excess weight loss percentage (EWL%) at 1,3, 6 and 12 months after surgery was calculated.
4) Outcomes
The primary endpoint outcome variable was one-year EWL% (group 1: EWL>50% vs. group 2: EWL<50%).
5) Statistical analysis
Descriptive statistics for quantitative variables were expressed as a mean + SD or median (range) and for qualitative variables as percentages. Bivariate analyses - preoperative, operative and postoperative characteristics between groups were compared using the Pearson or Fisher’s exact test for categorical variables and the Student’s t test or Wilcoxon-Mann-Whitney test for continuous variables. Variables were considered significant at <0.05 level.
Multivariate model – BMI, psychological eating disorder and compliance to preoperative diet were control variables considered in fitting logistic regression models. Response variable was EWL<50%. The level of significance for variables retained in the multivariate models was set at 0.05. Statistical analysis was performed using SAS 9.4 statistical software.
Results
1) Preoperative and intraoperative data
A total of 85 consecutive patients that underwent a laparoscopic sleeve gastrectomy (SG) were included. Junior surgeon performed 44 (51.7%) procedures and senior 41 (48.3%) procedures.
Eighty-one patients (95.3%) had a SG as an initial bariatric procedure and 4 patients (4.7%) had a previous gastric banding removed 3 months before SG.
Mean age was 44.09 years (SD 10.78), mean weight was 122.1 kilograms (SD 26.8), mean height was 160.1 centimetres (SD 10.83), and mean BMI was 46.61 kg/m2 (SD 8.58). Demographic and anthropometric data in male and female were showed in (Table 1).
| Age (mean) | Female | Male | Total |
| 45.3 | 44.3 | 45.09 | |
| Age (SD) | 10.69 | 11.53 | 10.78 |
| Weight (mean) | 116.7 | 147.1 | 122.1 |
| Weight (SD) | 21.9 | 33.6 | 26.8 |
| Height (mean) | 157.8 | 171 | 160.1 |
| Height (SD) | 10.16 | 6.45 | 10.83 |
| BMI (mean) | 45.87 | 50.02 | 46.61 |
| BMI (SD) | 7.99 | 10.56 | 8.58 |
Table 1: Demographic and antropometric data
Thirty (35.3%) patients presented binge eating disorder and 26 (30.5%) patients grazing. Total 16 patients (18.8%) were diabetics. Diabetes was revealed in 16 (18.8%) patients and 13 (15.3%) patients required insulin-treatment. 21 (24.7%) patients presented OSAS and 32 (37.6%) hypertension.
Mean operative time was 109.9 (min 45-max 248) minutes. Not intraoperative complications occurred.
VLKCD Low caloric ketogenic (LCKD) diet was performed in 11 (12.9%) patients with a mean preoperative weight loss of 18 kg and there were not misbehaviour patients to LCKD in this arm of the study. VLCD (LCBD) was performed in 74 (87.1%) patients with a mean preoperative weight loss of 10 kg. In total, we revealed 28 (32.9%) patients misbehaviour to preoperative diet (LCBD).
2) Postoperative data
Postoperative mortality rate was nil. Postoperative 60-day morbidity rate was 9.4% (8 patients). Not major postoperative complications (grade 3 and 4) occurred. 1 patient needed postoperative transfusion and 1 patient had portal thrombosis successfully treated by oral anticoagulant.
A total of 10 (11.7%) patients required a hospitalization in intensive care unit with a median stay of 24 hours. Mean overall length of hospital stay was 4.4 days (min 4- max 18 days), 8 (9.4%) patients needs more than 4 days.
3) Outcomes
Mean EWL was 20.5%, 41.2%, 54.17% at 1-3-6 months, respectively. At 1 years mean EWL was 62.67%, mean weight loss was 42.4 kg, mean BMI was 31.8 kg/m2. (Table 2) shows weight outcomes in male and female patients.
| BMI (mean) | Female | Male | Total |
| 31.05 | 33.86 | 31.55 | |
| BMI (SD) | 6.04 | 6.02 | 6.1 |
| Weight loss (mean) | 38.33 | 47.55 | 39.98 |
| Weight loss (SD) | 14.66 | 22.08 | 16.46 |
| EWL% (mean) | 62.76 | 58.24 | 61.96 |
| EWL% (SD) | 17.43 | 14.72 | 16.99 |
Table 2: One-year outcomes.
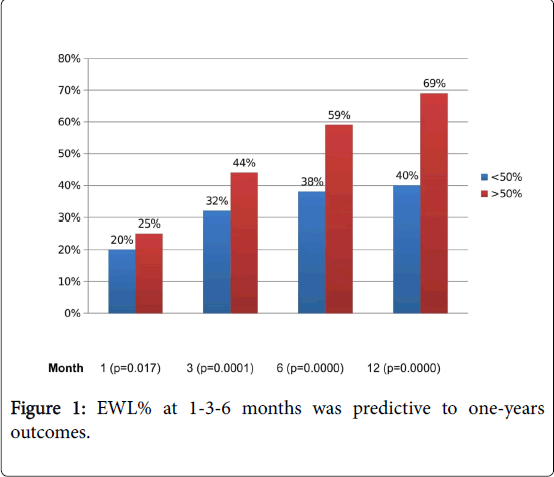
EWL% at 1 (p=0.017), 3 (p=0.0001) and 6 (p=0.0000) months was predictive to one-year outcomes (Figure 1).
4) Univariate analysis and multivariate modelZ
A total of 65 (76.4%) patients were included in group 1 (EWL>50%) and 20 (23.5%) patients group 2 (EWL<50%). Diabetes (p=0.034), needing of insulin treatment (p=0.037) and misbehaviour patients during preoperative diet (p=0.042) were negative predictive factor to obtain good outcomes (EWL>50%) (Tables 3 and 4).
| Variables | EWL<50% (N, mean) | EWL>50% (N, mean) | p |
|---|---|---|---|
| Female | 16 (80%) | 54 (83%) | 0.75 |
| Age (years) | 45 | 45 | 0.98 |
| BMI (kg/m2) | 47.9 | 46.1 | 0.42 |
| BED | 6 (30%) | 24 (36%) | 0.57 |
| Grazing | 7 (35%) | 19 (29%) | 0.62 |
| Diabetes | 7 (35%) | 9 (13%) | 0.034 |
| Insulin | 6 (30%) | 7 (10%) | 0.037 |
| Dyslipidaemia | 8 (40%) | 19 (29%) | 0.36 |
| OSAS | 5 (25%) | 16 (24%) | 0.97 |
| Hypertension | 10 (50%) | 22 (33%) | 0.19 |
| Junior surgeon | 8 (40%) | 36 (55%) | 0.22 |
| Operative time (min) | 103 | 112 | 0.28 |
| Misbeahavior to diet | 10 (50%) | 18 (27%) | 0.042 |
| Preoperative WL (kg) | 9.8 | 12.2 | 0.53 |
Table 3: Univariate analysis.
| EWL > 50% | Estimated Coefficient. | Std Err | p |
|---|---|---|---|
| BMI | -0.03 | 0.0301 | 0.315 |
| Binge | 0.484 | 0.6055 | 0.424 |
| Grazing | -0.253 | 0.5882 | 0.667 |
| Misbehaviour to diet | -1.1 | 0.5501 | 0.046 |
Table 4: Multivariate model.
Compliance to preoperative diet was obtained in 57 patients: 11 (100%) patients undergoing LCKD and 46 (62%) patients LCBD. In analysis of all patients responder to preoperative diet, no difference was detected in diet type in group 1 (EWL>50%) patients: VLCKD 9/11 patients (81%), VLCD 37/42 patients (82%).
BMI, eating disorder and preoperative weight loss had not impact on IWL. Junior surgeon and his learning process seemed to have no impact on IWL.
In multivariate model misbehaviour patients to preoperative diet patients was the only predictive factor for IWL (p=0.046).
Despite the small sample size of this retrospective study some useful information can be obtained also on the effect of the predictors on the probability of EWL >50%. The confidence intervals (CI) for the estimated coefficients of the multivariate logistic regression model fitted (Table 5) provide us clear clues on the direction of the effect (albeit statistically significant only for misbehavior to diet) of the predictors on the probability of an EWL >50%.
| EWL>50% | Estimated Coef. | Std Err | p | Confidence interval |
|---|---|---|---|---|
| BMI | -0.03 | 0.0301 | 0.315 | [-0.089,0.288] |
| BED | 0.484 | 0.6055 | 0.424 | [-0.700,1.670] |
| Grazing | -0.253 | 0.5882 | 0.667 | [-1.400,0.890] |
| Misbehaviour to diet | -1.1 | 0.5501 | 0.046 | [-2.170,-0.020] |
Table 5: Multivariate model.
High values of BMI and BED tend decrease the probability to an EWL> 50%; being classified as a grazer or with a misbehavior to preoperative diet act in the same direction decreasing the probability to an EWL >50%. Note that, (Table 5) misbehaviour during preoperative diet was the only predictive factor statistically significant for IWL (p=0.046).
Discussion
This study shows that BMI and age, eating disorders, and surgeon learning process did not impact on IWL whereas however, it reveals that bariatric patients with misbehaviours during preoperative diet were subject to have IWL one-year after procedure and this fact seemed to be independently by to preoperative weight loss. Moreover, patients with IWL at one-year could be detected at first month after procedure.
Recently Al-Khyatt [8] reported data of 227 patients undergoing gastric bypass. 12 months after surgery 32 (14%) did not achieve 50% of EWL. Multiple regression analysis demonstrated that older age (>60 years), diabetes, BMI >60 kg/m2, preoperative weight gain and waiting-list for surgery longer than 18 months were predictors of IWL one-year after procedure.
Higher BMI and age are frequently correlated to IWL [5,9,11,22] but these data are not confirmed by our study. Diabetes seems to have a positive correlation with IWL (p=0.034 in univariate analysis).
Furthermore, as Al-Khyatt study, our data emphasized the importance of preoperative patients selection and preoperative education for surgery. Indeed, authors demonstrated a significant positive association between preoperative EWL and that at 12 months (p=0.009) and, in multivariate analysis, a negative association between preoperative weight gain and 12-months postoperative EWL.
In our study, a multivariate model has been created in order to analyse preoperative psychological and dietary behaviour factors hypothetically influencing IWL, with respect of main predictive factor (BMI). This model revealed that preoperative diet was important not only for preoperative weight loss but also to select patient potentially subject to IWL. Indeed, preoperative diet was prescribed to all patients but, in multivariate model, IWL was associated to patient with preoperative stable or gained weight related to dietetic misbehaviours (p=0.046). Moreover, preoperative weight loss and dietetic model seemed to be not associated with IWL. Instead In relation to the importance of preoperative diet type in regard at patient’s compliance, indeed ketogenic diet compliance was totally, as justified by its anorexigenic effect [23].
Psychological and socioeconomic behaviour has been already welldiscussed in literature [6,16,24,25]. To our knowledge, no data were available about grazing as predictor of IWL but consistent literature identified this disorder as a predictor of weight regains [20]. Data about binge eating disorder were quite divergent as negative predictor or not of IWL [25,26].
In our data grazing and binge eating disorder seemed to have no impact on IWL but, while grazing seemed to be a negative factor (controlling for binge the estimated coefficient has a negative sign, Table 4), binge eating disorder seemed to have a positive impact (controlling for grazing, the estimated coefficient has positive sign, Table 4).
LSG is a well-standardized procedure and operative technique should not affect the postoperative EWL [27]. Our data confirmed that learning process in high volume bariatric centre did not impact on IWL; indeed junior surgeon performed 51.7% procedures without differences of IWL than senior surgeon.
Trend of EWL in the first year revealed an interesting data. An attentive follow-up could early detect patients with IWL at one-year after procedure. One-month after SG, patients with IWL at 1-year showed significant lower EWL than patients with a 1-year adequate weight loss (p=0.017) and this trend was also observed at 3 (p=0.0001) and 6 (p=0.0000) months after procedure.
Conclusion
Patient compliance to preoperative diet reflected patient’s postoperative dietary behaviour and could predict IWL. In this setting, a good patient behaviour during preoperative diet could be criteria to select patient for SG. Furthermore, an attentive follow-up could early detect IWL in order to increase dietetic and psychological surveillance of dietetic behaviour.
Conflict of interest
There is no conflict of interest between any authors of the manuscript.
References
- Angrisani L, Santonicola A, Iovino P, Formisano G, Buchwald H, et al. (2015) Bariatric surgery worldwide 2013. Obes Surg 25:1822–1832.
- Benaiges D, Mas-Lorenzo A, Goday A, Ramon JM, Chillaron JJ, et al. (2015) Laparoscopic sleeve gastrectomy: More than a restrictive bariatric surgery procedure? World J Gastroenterol 21:11804–11814.
- Yorke E, Sheppard C, Switzer NJ, Kim D, de Gara C, et al. (2017) Revision of sleeve gastrectomy to Roux-en-Y gastric bypass: A canadian experience. Am J Surg 213: 970-974.
- Switzer NJ, Karmali S, Gill RS, Sherman V (2016) Revisional bariatric surgery. Surg Clin North Am 96: 827–842.
- Snyder B, Nguyen A, Scarbourough T, Yu S, Wilson E (2009) Comparison of those who succeed in losing significant excessive weight after bariatric surgery and those who fail. Surg Endosc 23: 2302–2306.
- Dilektasli E, Erol MF, Cayci HM, Ozkaya G, Bayam ME, et al. (2017) Low educational status and childhood obesity associated with insufficient mid-term weight loss after sleeve gastrectomy: A retrospective observational cohort study. Obes Surg 27: 162–168.
- Reinhold RB (1982) Critical analysis of long term weight loss following gastric bypass. Surg Gynecol Obstet 155: 385–394.
- Al-Khyatt W, Ryall R, Leeder P, Ahmed J, Awad S (2017) Predictors of inadequate weight loss after laparoscopic gastric bypass for morbid obesity. Obes Surg 27: 1446–1452.
- Livhits M, Mercado C, Yermilov I, Parikh JA, Dutson E, et al. (2012) Preoperative predictors of weight loss following bariatric surgery: systematic review. Obes Surg 22: 70–89.
- Stanford FC, Alfaris N, Gomez G, Ricks ET, Shukla AP, et al. (2017) The utility of weight loss medications after bariatric surgery for weight regain or inadequate weight loss: A multi-center study. Surg Obes Relat Dis Off 13: 491–500.
- Lutfi R, Torquati A, Sekhar N, Richards WO (2006) Predictors of success after laparoscopic gastric bypass: A multivariate analysis of socioeconomic factors. Surg Endosc 20: 864–867.
- Martin DJ, Lee CMY, Rigas G, Tam CS (2015) Predictors of weight loss 2 years after laparoscopic sleeve gastrectomy. Asian J Endosc Surg 8: 328–332.
- Van Nieuwenhove Y, Dambrauskas Z, Campillo-Soto A, van Dielen F, Wiezer R, et al. (2011) Preoperative very low-calorie diet and operative outcome after laparoscopic gastric bypass: a randomized multicenter study. Arch Surg Chic 146: 1300–1305.
- Ruiz-Tovar J, Zubiaga L, Diez M, Murcia A, Boix E, et al. (2016) Preoperative regular diet of 900 kcal/day vs balanced energy high-protein formula vs immunonutrition formula: Effect on preoperative weight loss and postoperative pain, complications and analytical acute phase reactants after laparoscopic sleeve gastrectomy. Obes Surg 26: 1221–1227.
- Gerber P, Anderin C, Gustafsson UO, Thorell A (2016) Weight loss before gastric bypass and postoperative weight change: Data from the scandinavian obesity registry (SOReg). Surg Obes Relat Dis 12: 556–562.
- Deb S, Voller L, Palisch C, Ceja O, Turner W, et al. (2016) Influence of weight loss attempts on bariatric surgery outcomes. Am Surg 82: 916–920.
- NIH conference (1991) Gastrointestinal surgery for severe obesity. Consensus development conference panel. Ann Intern Med 15: 956–961.
- Badiali M (2005) Technical options in bariatric surgery and their mechanisms of action. Ann Ital Chir. 76: 433–438.
- Woldeyohannes HO, Soczynska JK, Maruschak NA, Syeda K, Lee Y, et al. (2016) Binge eating in adults with mood disorders: Results from the international mood disorders collaborative project. Obes Res Clin Pract 10: 531–543.
- Goodpaster KPS, Marek RJ, Lavery ME, Ashton K, Merrell Rish J, et al. (2016) Graze eating among bariatric surgery candidates: Prevalence and psychosocial correlates. Surg Obes Relat Dis 12: 1091–1097.
- Clavien PA, Barkun J, Vauthey JN, Dindo D, Schulick RD, et al. (2009) The Clavien-Dindo classification of surgical complications: Five-year experience. Ann Surg 250: 187–196.
- Nagao Y, Diana M, Vix M, Mutter D, Marescaux J, et al. (2014) Age impact on weight loss and glycolipid profile after laparoscopic sleeve gastrectomy: Experience with 308 consecutive patients. Surg Endosc 28: 803–810.
- Paoli A (2014) Ketogenic diet for obesity: Friend or foe? Int J Environ Res Public Health 11: 2092–2107.
- Livhits M, Mercado C, Yermilov I, Parikh JA, Dutson E, et al. (2010) Behavioral factors associated with successful weight loss after gastric bypass. Am Surg 76: 1139–1142.
- Brunault P, Jacobi D, Miknius V, Bourbao-Tournois C, Huten N, et al. (2012) High preoperative depression, phobic anxiety, and binge eating scores and low medium-term weight loss in sleeve gastrectomy obese patients: A preliminary cohort study. Psychosomatics 53: 363–370.
- Sioka E, Tzovaras G, Oikonomou K, Katsogridaki G, Zachari E, et al. (2013) Influence of eating profile on the outcome of laparoscopic sleeve gastrectomy. Obes Surg 23: 501–508.
- Major P, Wysocki M, Dworak J, Pedziwiatr M, Malczak P, et al. (2017) Are bariatric operations performed by residents safe and efficient? Surg Obes Relat Dis 13: 614–621.
Citation: Fantola G, Porcu M, Pinuts S, Sollai C, Moroni R (2017) Preoperative Dietary Behaviour as Predictive Factor for Inadequate Weight Loss after Sleeve Gastrectomy. J Obes Weight Loss Ther 7: 358. DOI: 10.4172/2165-7904.1000358
Copyright: © 2017 Fantola G, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 4845
- [From(publication date): 0-2017 - Apr 03, 2025]
- Breakdown by view type
- HTML page views: 4038
- PDF downloads: 807