Research Article Open Access
Prenatal Testosterone Exposure Influences Neuronal Sensitivity to Pheromones in Female Mice
| Roger N Thompson, Audrey Napier and Kennedy S Wekesa* | |
| Department of Biological Sciences, Alabama State University, Montgomery, Alabama 36101-0271, USA | |
| Corresponding Author : | Kennedy S Wekesa Department of Biological Sciences Alabama State University, Montgomery, Alabama 36101-0271, USA Tel: 334-229-4196 Fax: 334-229-1007 E-mail: wekesa@alasu.edu |
| Received: June 10, 2015; Accepted: July 08, 2015; Published: July 15, 2015 | |
| Citation: Thompson RN, Napier A, Wekesa KS (2015) Prenatal Testosterone Exposure Influences Neuronal Sensitivity to Pheromones in Female Mice. Biochem Physiol 4:170. doi:10.4172/2168-9652.1000170 | |
| Copyright: © 2015 Thompson RN, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
| Related article at Pubmed, Scholar Google | |
Visit for more related articles at Biochemistry & Physiology: Open Access
Abstract
Exposure to androgens during in utero development in animals which give birth to multiple offspring causes lifelong increased sensitivity in the offspring. Females who develop with a male on each side (designated 2M) are exposed to the testosterone produced by these males due to the steroids freely crossing cell membranes. Female mice exposed to testosterone during development are “masculinized” in their growth and behavior. According to the organizational theory, testosterone is converted to estrogen and it is this estrogen which organizes the brain. Increased sensitivity to testosterone is reflected by the exposed females showing increased aggressive behavior and a reduction in production of IP3 levels in the vomeronasal organ (VNO). These 2M females respond more like males when exposed to the pheromonal compounds 2-Heptanone and 2,5-dimethylpyrazine. In previous studies we showed a distinct difference between male and female IP3 production when male and female VNO microvilli are exposed to these compounds. Production of IP3 by male microvilli exposed to 2-Heptanone and 2,5-dimethylpyrazine were the same as the nonstimulus phosphate buffered saline (PBS). In the present study, we provide evidence showing 2M female IP3 production to be similar to that of male mice. In addition, we provide evidence for increased aggression when 2M females are injected with exogenous testosterone.
| Keywords |
| IP3; Testosterone; Neuronal sensitivity |
| Introduction |
| The brain develops during the early stages of embryogenesis. The organization and pattern formation of the cells during this time is sensitive to steroids. At this critical stage of development, cells proliferate and differentiate as part of the process of forming the brain. Steroids play an important role in this process. The role of androgens in particular is necessary to organize the nervous system and to establish behavior [1,2]. Testicular steroids have been shown to masculinize the genitalia while also masculinizing the developing brain, thus permanently altering behavior. The organizational hypothesis provides a unitary explanation for sexual differentiation: A single steroid signal (androgen) masculinizes the body, the brain and behavior. Following this precept, the nervous system is just another type of tissue for the androgenic signal to organize in a masculine fashion. If the nervous system does not detect the presence of this androgen, it will organize itself in a feminine fashion as the default mechanism. The aromatization hypothesis provides an explanation of the mechanism of action that leads to the brain’s organizational pattern. It suggests that testicular androgens enter the brain and are converted to estrogens and that these estrogens are what masculinize the developing nervous system. Thus, according to the hypothesis, aromatization of testosterone to estrogen is required for this masculinization. In summary animals exposed to either endogenous or exogenous androgens early in life behave like males; whereas those not exposed behave like females. The effects of these hormones on the developing organism are manifest both in structure and behavior in the adult stages. |
| Although the structural difference is subtle, the effect of testicular hormones can be profound. In rats there is a striking sex difference in a group of neurons called the sexually dimorphic nucleus of the pre-optic area [3,4]. The size of this neuronal tissue is about five times smaller in the female than in the male and is due to a decreased number of neurons present. The number of neurons is determined by the perinatal action of gonadal hormones. It has been previously suggested that these hormones affect the rate of apoptosis in the female brain thereby reducing the neurons present. Other areas affected include the medial pre-optic area, ventromedial nucleus, the bed nucleus of the olfactory tract, the accessory olfactory bulb and the vomeronasal organ [3,4]. |
| Male sex hormones play a major role in some forms of aggressive behaviors, especially in social encounters [5]. Circulating levels of androgens relate to different measures of aggressive behaviors. At sexual maturity, inter-male aggression is markedly increased in many species. Reduced levels of circulating androgens following castration is commonly associated with reduced inter-male aggressive behavior. Injection of castrated animals with testosterone increases aggressive behavior [6]. |
| The intrauterine location of an animal to the fetuses of the same and opposite sex has been shown to account for a significant degree of variation between individuals of the same litter [7-10]. In mice, females that develop in utero between two males (2M females) have higher amniotic fluid and fetal blood titers of testosterone than females that do not develop next to a male fetus (0M females) [10]. When compared to 0M females in terms of morphology and behavior, 2M females exhibit longer anogenital spacing at birth (an androgen dependent event) and exhibit higher levels of male-like copulatory behavior as well as inter-female and postpartum aggression [9-11]. Also, 2M females are less sexually attractive, exhibit longer estrus cycles, achieve puberty later, are less proficient in active avoidance responding, and exhibit more locomotors activity [10,12-14]. Despite the longer estrus cycles, 2M females produce fewer offspring and are reproductive for a much shorter time period. |
| Adult 2M female mice injected with testosterone show greater frequencies of chasing and biting, and initiate fights more often than 0M females receiving the same treatment, showing 2M females to be more sensitive to testosterone as adults [15]. Studies in humans have shown that females exposed to higher levels of testosterone display longer anogenital distances [16]. The study concluded that the anogenital distance may predict normal reproductive behaviors in women and may be a new tool of potential clinical interest to evaluate ovarian function. Another study showed an association between perineal length and androgen levels in men, suggesting that serum testosterone levels in adulthood depend on factors involved during the fetal period [17]. |
| In response to pheromones, animals exhibit behavioral repertoires that are innate and require no learning or experience. The perception of pheromones is mainly mediated via the vomeronasal organ (VNO). The VNO is a paired, tubular structure divided by the nasal septum, each side having a crescent shaped lumen lined with receptor neurons on the medial concave side and filled with fluid from the vomeronasal gland [18]. The transduction of pheromones is mediated by G-Protein coupled vomeronasal receptors that show an increase in IP3 production when stimulated. |
| In a previous study, the production of IP3 was correlated with microvillar membrane stimulation by the pheromonal compounds 2-heptanone and 2,5-dimethylpyrazine [19]. Additional studies show correlation between the production of IP3 and aggression when male mice are exposed to urine or glandular extracts [20]. In order to investigate neuronal sensitivity to testosterone, we used IP3 production as a bioassay, and the resident-intruder paradigm to determine the effect of prenatal exposure to testosterone on 0M and 2M female mice. |
| Methods and Materials |
| Animals |
| CD-1 mice were obtained from Charles River Laboratories (Kingston, NY, USA) and maintained in a breeding colony in the Department of Biological Sciences at Alabama State University. Animals were housed in Institutional Animal Care and Use Committee (IACUC) inspected and approved facilities and cared for according to the NIH Guide for Care and Use of Laboratory Animals. Food and water were provided ad libitum. |
| Membrane preparations |
| VNOs from adult female mice were dissected from their crevices in the nasal cavity, removed from the cartilaginous capsule, and frozen on dry ice. The tissues were then minced with a razor blade and subjected to sonication for 2-5 min in ice-cold (PBS). The resulting suspension was layered on a 45% (wt/wt) sucrose cushion and centrifuged at 4°C for 30 min at 3000 g in a Beckman SW55Ti rotor. The membrane fraction on top of the sucrose was collected and centrifuged as before for 15 min to pellet the membranes. The membranes were re-suspended in 100 μl of ice-cold PBS. Protein concentration was determined using bovine serum albumin as standard. The procedure used for the preparation of microvillar membranes is modeled after well-established methods. These preparations have been previously characterized and are sufficiently enriched in chemosensory membranes. |
| Second messenger assays |
| For IP3 assays, reactions were incubated for 1 min at 37°C in 25 mmol 1-1 Tris-acetate buffer pH 7.2, 5 mmol 1-1 Mg-acetate, 1 mmol 1-1 DTT, 0.5 mmol 1-1 ATP, 0.1 mmol 1-1 CaCl2, 0.1 mg ml 1-1 bovine serum albumin, 10 μmol 1-1 GTP, and 20 μg VNO membrane proteins. Reactions were terminated by the addition of 1 M trichloroacetic acid. IP3 was measured with a kit from Perkin Elmer, Inc. (Boston, MA, USA) according to the manufacturer’s instructions and is based on displacement of [3H] IP3 from a specific IP3 binding protein. Differences between experimental and control animals were analyzed by analysis of variance (ANOVA). |
| Determination of ano-genital distance |
| The ano-genital distance index (AGDI) was determined at weaning on postnatal day 21. Female mice were weighed, the ano-genital distance measured, and both sets of data were recorded. Each female was toe-clipped and sorted into groups of five animals per cage. After calculating the AGDI, females were re-sorted into groups based on the index. Those with the lower AGDI were placed into the group labeled 0M and those with the higher AGDI were placed in the 2M group. |
| Behavioral tests |
| In order to evaluate neuronal sensitivity of adult females to the effects of testosterone, we observed aggressive displays of female mice to pheromones using the resident-intruder paradigm. A conditioning or training period is not required as this assay relies on innate and stereotyped behaviors initiated by chemosensory cues. After an isolated resident female mouse has established the cage as its territory, a male mouse is introduced into the cage. The number and length of attacks were recorded during a five minute observation period. Behavioral test were conducted in an isolated, darkened room using a 40-W red bulb for visibility. Differences between experimental and control animals were analyzed by analysis of variance (ANOVA). |
| ELISA assays for testosterone |
| After the completion of all behavioral testing, each group of females (0M and 2M) was euthanized and the vomeronasal organ extracted and blood samples collected. The blood samples were centrifuged and the serum collected and stored at 4°C until assayed. Assays to determine the testosterone levels of the females were done using an assay kit from Assay Designs, Inc. (Ann Arbor, MI) as per the manufacturer’s guidelines. |
| Results |
| AGDI measurements |
| Following weighing the female mice and measuring their anogenital distance, the anogenital distance index was calculated using the formula anogenital distance (mm) at weaning on postnatal day 21 divided by the body weight (grams) multiplied by one hundred. The average anogenital distance and body weight for the 0M females was 2.7 mm ± 0.35 and 14.4 g ± 2.05, respectively. For the 2M females, the average anogenital distance and body weight was 3.9 mm ± 0.85 and 12.7 g ± 0.06, respectively. |
| 0M and 2M female IP3 experiments |
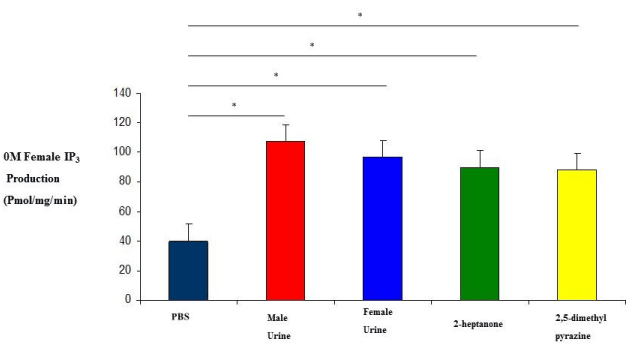
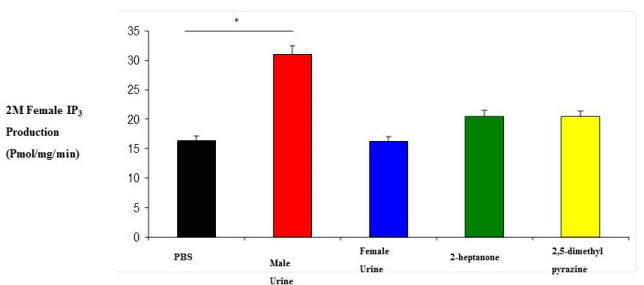
| 0M and 2M adult females were subjected to variety of stimuli known to elicit an IP3 response in membrane preparations. IP3 experiments revealed that 0M females responded to stimulation by male urine, female urine, 2-heptanone and 2,5-dimethylpyrazine with IP3 production levels which are higher than 2M female responses (Figure 1 and 2). Their responses were also higher than our regular membrane preparation responses which are a mixture of 0M, 1M and 2M females (Data not shown). Male urine displayed the strongest response in both 0M and 2M females which is consistent with previous results reported from our membrane preparations. |
| Testosterone levels of 0M and 2M females |
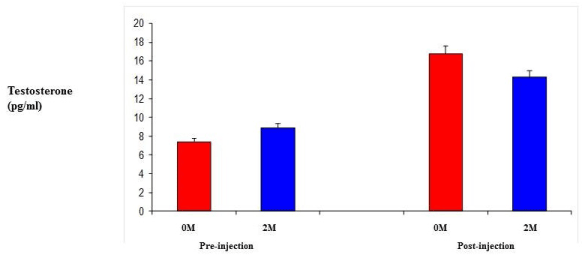
| Testosterone levels were measured using un-treated female 0M and 2M female mice in order to establish circulating levels of testosterone. Following a regime of daily injections of TP at 0.5 mg/kg daily [21] for six days, testosterone levels were measured again for both groups. While the 2M female levels were slightly higher initially, they were not that dissimilar. After the daily injections, the 0M females had higher circulating levels, but these were not significantly different from the 2M female levels (Figure 3). |
| 0M and 2M female aggressive behavior |
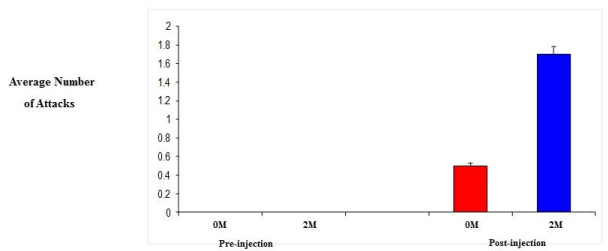
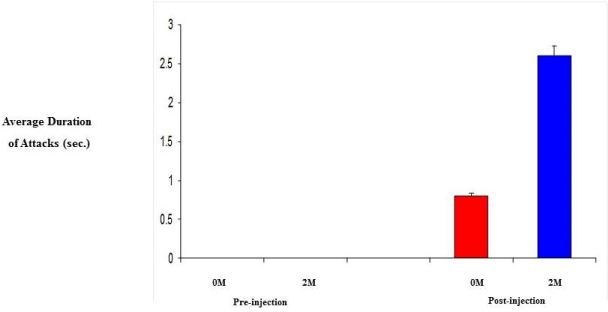
| 0M and 2M adult female mice were observed in behavioral test for aggression (resident-intruder paradigm). Specifically, we looked at the number and duration of attacks since aggression levels are related to the level of testosterone. Initially, both groups showed no aggression towards the intruder, however, following daily injection of testosterone propionate, aggression levels did increase. As a result of increased testosterone levels, the 2M females displayed a 3-fold increase in both the number of attacks and the duration of those attacks compared to that of the 0M females (Figure 4 and 5). |
| Discussion |
| Although it is well established that the vomeronasal organ is important for reproductive and social behavior, little is known about its role in developmental processes (in utero) in the organization of the nervous system. The mechanism of IP3 stimulation and aggression is only recently being elucidated by studies such as the ESP1 protein found in the lacrimal gland, along with unknown compounds of the sub maxillary and parotid glands [22]. Studies of behavior have documented a number of activities and events but none have been correlated to IP3 production except aggression [20]. Here we show that there is a correlation between IP3 production, aggression and testosterone levels. Females who were exposed to testosterone in the uterus display male-like behavioral characteristics later in adulthood as seen in the results from both our IP3 experiments and our behavioral studies (Figures 1, 2, 4 and 5). In addition, when testosterone is supplemented by injections of testosterone, the 2M female’s aggression level increases more than that of female mice which had not been previously exposed (Figures 4 and 5). In our study we also looked at the production of IP3 of 2M females when stimulated with the pheromonal compounds 2-heptanone and 2,5-dimethylpyrazine, chemical compounds found in female mouse urine and responsible for estrus extension and puberty delay, respectively. When male mice are exposed to 2-heptanone and 2,5-dimethylpyrazine they respond with very little increase in IP3 [19]. As expected, the 2M female responses were more like that of the male response to the two compounds (Figures 1 and 2). This higher sensitivity in the 0M mice may be due to a lack of in utero exposure to the masculinizing effects of testosterone. Alternatively, our results can be viewed as a reduced response in the 2M mice which is most likely due to the influence of testosterone being secreted by the adjacent males (in utero) and having a masculinizing effect on the 2M female. These two views can be linked to receptor activation during development. Androgen receptors are critical for establishing masculine characteristics in mammals. Activation of these receptors is necessary and occurs through the exposure of testosterone in the developing embryo. Since the 0M mice lack environmental testosterone, the less male characteristics are displayed both in brain development and behavior. The indication is that these 0M females are more readily primed for female stimuli than the 2M females. However, the mechanism of action is not yet known [23,24]. |
| Steroids play a critical role in the development of sex differences in neuroendocrine functioning when present at critical developmental periods such as organizing the brain. Differing levels of prenatal testosterone will alter the organizational function and exert effects that persist through adulthood. Past studies have indicated that 2M females are more aggressive, have longer and more irregular estrus cycles, are less sexually receptive to males, are less preferable to males when males have a choice, have a greater anogenital distance, reach puberty later, show lower levels of learning when in an active avoidance situation, exhibit higher levels of locomotors activity, lactating 2M females are more aggressive towards a male intruder, and experience increased body weight and fat storage [12,13]. The 2M females require a shorter duration of testosterone treatment to induce aggression. Adult 2M female mice injected with testosterone show greater frequencies of chasing and biting and initiate fights more than do 0M females receiving the same treatment (Figure 4). 2M mothers fight for longer duration than 0M females (Figure 5) and 2M pregnant mice show more intense aggression than do 0M female mice [13,25]. |
| In our studies we have been able to show that 2M females exposed to testosterone in utero do not respond to female pheromones as the 0M females as measured by production of IP3. This suggests that the pheromone receptors of females exposed to testosterone during utero have reduced sensitivity to female hormones. These females also tend to have higher aggression levels towards other females and fought for longer durations when injected with testosterone. On the other hand the females with no exposure to testosterone (0M) females did not lose their sensitivity to pheromone stimulation and were not aggressive to other females when injected with testosterone. Our data shows that masculinization due to intra-uterine position not only affects behavior but may also affect receptor sensitivity. The present study illustrates a correlation between the developing embryonic brain of mice and adult behavior. The role of testosterone in the pattern formation of the brain has been previously documented [1,2]. However, the role of testosterone in IP3 production had not recently been explored. We speculate that the lack of exposure of the developing mouse brain to testosterone will establish the organizational structure of the brain to be female oriented. This orientation primes the brain to be more receptive to stimuli such as 2-heptanone and 2,5-dimethylpyrazine. Previous studies conducted in our lab have shown that these compounds act through G-protein coupled vomeronasal receptors and stimulate the production of IP3. This structural orientation that is established early in development leads to adult behavior patterns such as aggression in males. The results of these studies provide a foundation for exploring the molecular mechanisms and physiological pathways that lead to adult sexual orientation and behavior. |
References
|
Figures at a glance
 |
 |
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 | Figure 5 |
Relevant Topics
- Analytical Biochemistry
- Applied Biochemistry
- Carbohydrate Biochemistry
- Cellular Biochemistry
- Clinical_Biochemistry
- Comparative Biochemistry
- Environmental Biochemistry
- Forensic Biochemistry
- Lipid Biochemistry
- Medical_Biochemistry
- Metabolomics
- Nutritional Biochemistry
- Pesticide Biochemistry
- Process Biochemistry
- Protein_Biochemistry
- Single-Cell Biochemistry
- Soil_Biochemistry
Recommended Journals
- Biosensor Journals
- Cellular Biology Journal
- Journal of Biochemistry and Microbial Toxicology
- Journal of Biochemistry and Cell Biology
- Journal of Biological and Medical Sciences
- Journal of Cell Biology & Immunology
- Journal of Cellular and Molecular Pharmacology
- Journal of Chemical Biology & Therapeutics
- Journal of Phytochemicistry And Biochemistry
Article Tools
Article Usage
- Total views: 15220
- [From(publication date):
September-2015 - Apr 03, 2025] - Breakdown by view type
- HTML page views : 10621
- PDF downloads : 4599
