Prenatal Sonographic Findings and Prognosis of Craniosynostosis Diagnosed during the Fetal and Neonatal Periods
Received: 19-Mar-2018 / Accepted Date: 17-May-2018 / Published Date: 24-May-2018 DOI: 10.4172/2376-127X.1000377
Abstract
Introduction: Craniosynostosis, defined as the premature closure of one or multiple cranial sutures, has a variable genotype-phenotype association, comprising approximately 180 different syndromes. Reported cases of prenatal diagnosis are relatively rare and detection in the fetal period is difficult, although the incidence is three to five in 10,000 live births. Most cases of craniosynostosis are compatible with survival, although a high mortality rate is observed during the neonatal period in some conditions. Objective: To explore the sonographic findings of fetuses with craniosynostosis and investigate their prognosis. Method: We conducted a 5-year, multicentre retrospective study and collected data on patients with craniosynostosis diagnosed in the perinatal period. Results: Of 41 cases, 30 cases (73%) were syndromic craniosynostosis, eight cases (20%) were non-syndromic craniosynostosis and the other three cases (7%) were secondary craniosynostosis of chromosomal deletion syndromes. The prenatal ultrasound detection rate was 61%. Half of the cases of syndromic craniosynostosis detected during the perinatal period were Pfeiffer syndrome, there were also six cases of Apert syndrome, three cases of Crouzon syndrome and other rare form of syndromic craniosynostosis (Beare-Stevenson syndrome, Saethre- Chotzen syndrome, cranioectodermal dysplasia and thanatophoric dysplasia). Abnormal head biometry was closely correlated with deformation of the cranial shape, which was the most frequently detected finding leading to prenatal diagnosis. Three cases presented with ventriculomegaly and exophthalmos but normal cranial shape and size. The overall survival rate of infants with syndromic craniosynostosis was 79%, while all of the infants with non-syndromic craniosynostosis survived. Conclusion: Prenatal diagnosis of craniosynostosis is difficult, especially when dysmorphic change of the fetal cranium is not evident. Abnormal head biometry and distortion of ventricles in shape could potentially be additional markers of fetal craniosynostosis and consequently increase the prenatal detection rate.
Keywords: Craniosynostosis; Prenatal diagnosis; Ventriculomegaly; Cranial deformation; Screening; Prognosis
Abbreviations
US: Ultrasonographic; CI: Cephalic Index; 3D: Three-Dimensional; CNS: Central Nervous System; BPD: Biparietal Diameter; NT: Nuchal Translucency; VSD: Ventricular Septal Defect; CSF: Cerebrospinal Fluid
Introduction
Craniosynostosis, defined as the premature closure of one or multiple cranial sutures, has a variable genotype-phenotype association, comprising approximately 180 different syndromes. Reported cases of prenatal diagnosis are relatively rare and detection in the fetal period is difficult, although the incidence is three to five in 10,000 live births [1-4]. Most cases of craniosynostosis are compatible with survival, although a high mortality rate is observed during the neonatal period in some conditions [5,6]. Careful planning of the delivery method and perinatal care is crucial because of the potential for complex anomalies of the upper airway due to midfacial hypoplasia [7]. Moreover, multiple reconstructive surgeries requiring the coordination of dental and orthodontic specialists may be necessary after the infancy period [8- 10]. Various types of neurological pathophysiology, including elevated intracranial pressure, hydrocephalus and Chiari malformations in growing children can cause brain damage associated with neurologic and cognitive dysfunction [9]. All of these facts support the rationale for prenatal diagnosis of craniosynostosis.
Some US signs of craniosynostosis are well known. While prenatal detection is straightforward when distinguishing cranial shape abnormalities, such as cloverleaf skull, there are other conditions which present with minimum cranial shape abnormalities, whose accurate identification remains challenging [5]. Several authors have reported characteristic US findings. For instance, an increased CI or the ratio of biparietal and occipto-frontal diameters, was thought to be a reliable marker with 95% diagnostic sensitivity [4,11]. Normal range of CI is defined between 75% and 85% and increased CI indicates the CI value of more than 85% [12]. The direct observation of a fused cranial suture is effective in the third trimester, although cranial deformity may be present four to 16 weeks earlier [13]. Decreased US penetration through the cranial bone, known as the brain-shadowing sign, can be confirmed retrospectively in cases of craniosynostosis [4]. 3D-US can be used to evaluate the patterns of the development of metopic suture in the middle of the second trimester, which correlate with brain malformation as well as the detection of craniosynostosis or to visualize abnormal facial features in a more realistic way [14,15].
Despite these tools, the rate of prenatal diagnosis of craniosynostosis is low and it is typically a secondary finding after the identification of associated anomalies [16]. Possible reasons for this low detection rate are that some cases of craniosynostosis present with none of the previously discussed characteristic features. Moreover, there is no universal marker for prenatal detection of craniosynostosis because craniosynostosis encompasses diverse phenotypes.
The objective of this study was to reveal sonographic findings in fetuses with craniosynostosis and to describe the findings that may facilitate the identification of this condition. Prognosis was also investigated to show the postnatal course, of this rare disease.
Materials and Methods
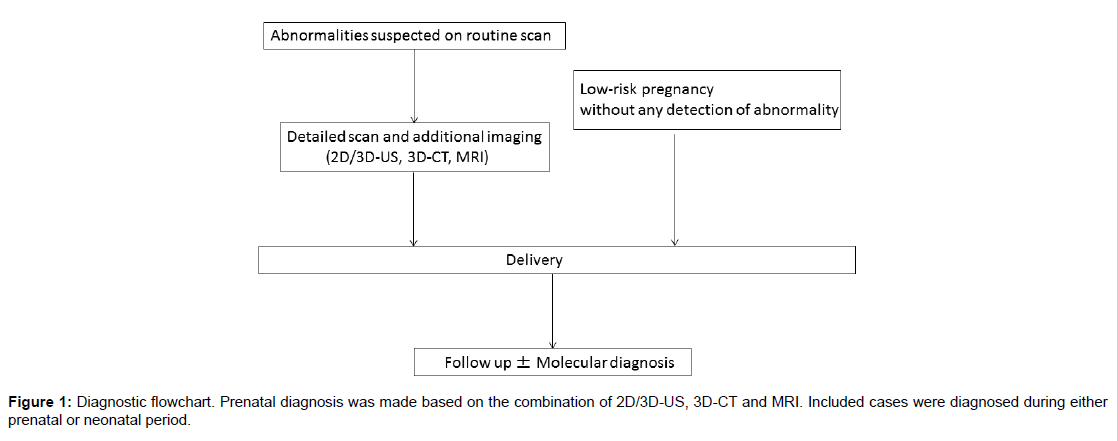
This was a multicenter retrospective study involving facilities participating in the Perinatal Research Network Group in Japan. Through a primary survey, the number of cases detected in each hospital was determined. Patients with craniosynostosis born from January 2011 to December 2015 were enrolled. Both cases of prenatal and neonatal diagnosis were included. The latter were transferred from other hospitals to the participating facilities during neonatal period when craniosynostosis was suspected after birth. Most major tertiary hospitals treating fetal anomalies in Japan participate in this network and the survey responses were from the representative of the facilities (Figure 1).
Through a secondary survey, detailed data of the affected babies were collected. The gestational week of prenatal diagnosis, cranial biometry, maximum width of the posterior horn of the lateral ventricle, any other positive findings on fetal scans and the presence of polyhydramnios were investigated using the prenatal medical records. Birth weight, gestational week of birth, delivery method, postnatal diagnosis, hydrocephalus requiring postnatal interventions and the infants’ current condition were also analysed.
Pregnant women routinely receive multiple US examinations during pregnancy course in Japan, typically every 4 weeks before 26 weeks’ gestation, every 2 weeks from 27 to 36 weeks and every week thereafter, according to the practice guideline of Japan Society of Obstetrics and Gynecology [17]. When fetal abnormalities were detected or suspected on routine scans, women were referred to regional tertiary hospitals for further examinations. Detailed scan including 3D-US at the tertiary hospitals confirmed abnormal findings. When the US investigation was not sufficient to diagnose the fetal conditions, additional prenatal assessment such as MRI and/or 3D-US was added after adequate discussion of the possible risks and benefits. There was no standard protocol commonly shared with all participating institutions for the diagnosis and perinatal management of fetal craniosynostosis. Delivery method was decided by obstetric indications. Fetal karyotype was performed in early second trimester in some cases for other reasons, but microarray or other genetic tests for single gene disorders were not prenatally performed to confirm the specific diagnosis of craniosynostosis. Ventriculomegaly was considered to be present when the maximum width of the posterior horn of the lateral ventricle was more than 10 mm. The shape of the fetal cranium was determined to be deformed if the examiner noted abnormalities at the time of scanning. Key images for prenatal diagnosis were sent to the authors and two examiners (A.H. and J.M.) independently assessed the shape of the cranium and ventricles in BPD view.
The final diagnosis was determined postnatally based on either molecular diagnosis or clinical manifestations examined by experienced plastic surgeons, neurosurgeons, paediatricians or geneticists. The clinical courses of the infants were followed up to July 2016 and their age at time of the survey ranged from eight months to five years. Cases were divided into three groups for further analysis: syndromic, nonsyndromic and craniosynostosis complicated with chromosomal abnormalities. This research was approved by the Ethical Committee at Miyagi Prefectual Children’s Hospital (application No. 290).
Results
One hundred and ten (59%) of 185 institutes replied to the primary survey. After the exclusion of one case, 41 cases from 23 institutions were enrolled in this study. We decided to exclude one case because the final diagnosis was uncertain following intrauterine fetal demise at 27 weeks.
The final diagnosis was Pfeiffer syndrome in 15 patients, Apert syndrome in 6, Crouzon syndrome in 3, Beare-Stevenson syndrome and thanatophoric dysplasia in 2 and Saethre-Chotzen syndrome and cranioectodermal dysplasia (Sensenbrenner syndrome) in 1 patient each. The other eight cases were non-syndromic and three cases were craniosynostosis complicated with chromosomal deletion syndromes. Maternal and neonatal characteristics are shown in Table 1. The rate of caesarean delivery was 79% in the syndromic group and 38% in the non-syndromic group. Molecular confirmation was performed in three cases with the following results: Pfeiffer syndrome type 3 (case 12), Apert syndrome (case 19) and thanatophoric dysplasia type 2 (case 29) (Table 1).
| Syndromic craniosynostosis (n=30) | Non-syndromic craniosynostosis (n=8) | Chromosomal aberration (n=3) | Total (n=41) | |
|---|---|---|---|---|
| Maternal age (y) | 34.0 ± 5.4 | 31.3 ± 2.5 | 36.0 ± 2.0 | 33.8 ± 5.0 |
| Parity | ||||
| primipara | 13 | 3 | 1 | 17 |
| multipara | 16 | 1 | 2 | 19 |
| unknown | 1 | 4 | - | 5 |
| Twin pregnancy | 2 | 1 | - | 3 |
| Gestational age of delivery (wk) | 36.9 ± 2.4 | 35.5 ± 4.1 | 36.7 ± 3.5 | 36.6 ± 2.9 |
| Cesarean section | 22 (73%) | 3 (38%) | 2 (66%) | 27 (66%) |
| Birth weight (g) | 2891 ± 432 | 2362 ± 643 | 1665 ± 890 | 2698 ± 620 |
Table 1: Maternal demographics and neonates at birth.
Prenatal diagnosis
Twenty-five cases (61%) were detected prenatally by either only US or the combination of US and further imaging modalities including MRI and/or 3D-CT. Regarding the timing of the detection of abnormal findings, four cases (9.7%) were detected before 22 weeks of gestation. Two cases were diagnosed as Pfeiffer syndrome at a later stage, one of these fetuses presented with only mild ventriculomegaly at 20 weeks, while a clover-shaped skull became evident in the third trimester. In the other case, increased nuchal fold thickness and facial abnormalities including a hypoplastic nasal bone, low-set ears, hypertelorism and exophthalmos were noted at 21 weeks, but direct observation of the cranial suture by a specialist excluded the possibility of craniosynostosis at that time. This fetus was diagnosed with Pfeiffer syndrome after birth. In the third case, the fetus presented with cystic hygroma and short extremities at 16 weeks. A conventional G-banding chromosome analysis of amniotic fluid cells showed a normal karyotype and the women decided to continue the pregnancy, resulting in the postnatal diagnosis of cranioectodermal dysplasia. A correct prenatal diagnosis was made before 22 weeks only in one case of thanatophoric dysplasia and the patient elected to terminate the pregnancy based on the US findings and the diagnosis was confirmed by both autopsy imaging and molecular testing. The other 21 cases were detected after 25 weeks of festation and no abnormalities were found at the second trimester anomaly scan.
The sonographic findings leading to prenatal diagnosis are described in table 2. There were four cases whose prenatal diagnoses were difficult or missed. Exophthalmos and ventriculomegaly were noted, but cranial deformity was not evident in three cases of syndromic craniosynostosis. Establishing definitive diagnoses at the time of the scans was difficult due to the lack of conclusive findings in these cases. There was one case in which abnormal head biometry was the only finding. Increased BPD was noted at 30 weeks, but a detailed scan suggested a normal fetus. The diagnosis of craniosynostosis was made postnatally in this case (Table 2).
| Sonographic findings | Syndromic craniosynostosis (n=30) | Non-syndromic craniosynostosis (n=8) | Chromosomal aberration (n=3) | Total (N=41) |
|---|---|---|---|---|
| Prenatal diagnosis | 22 (73%) | 3(38%) | 0 | 25 (61%) |
| Abnormal shape of skull | 18 (60%) | 3 | 2 | 23 (56%) |
| Abnormal biometry (head) | 17 (57%) | 1 | 3 | 21 (51%) |
| Ventriculomegaly | 15 (50%) | 1 | 2 | 18 (41%) |
| (Ventriculomegaly without skull deformation) | 3 | - | 1 | 4 |
| Exophthalmos | 9 (30%) | 1 | - | 10 (24%) |
| Related anomalies in extremities or spine | 11 (37%) | - | - | 11 (27%) |
Table 2: Sonographic findings detected prenatally.
Pfeiffer syndrome
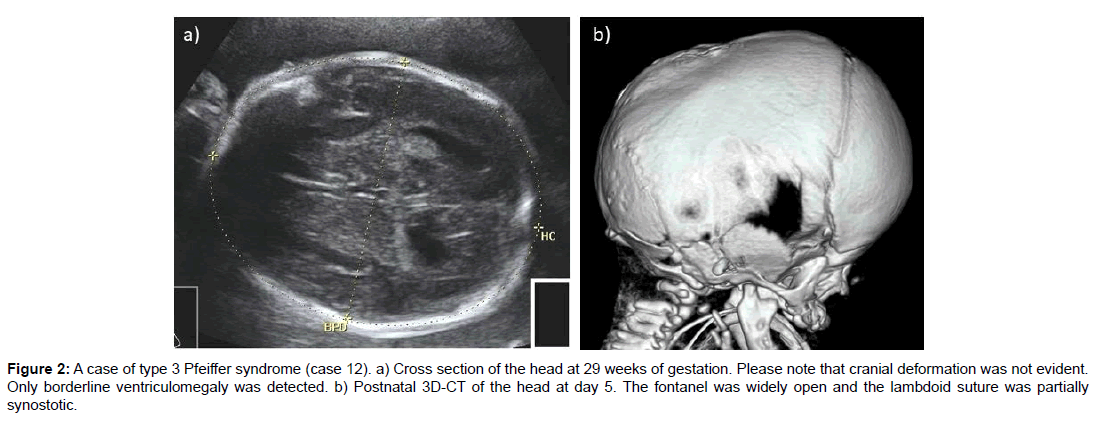
Twelve cases were prenatally diagnosed and three cases were diagnosed after birth. Several common characteristics were noted in the fetal period. Detection of a cloverleaf skull directly led to the prenatal diagnosis of craniosynostosis in nine cases (60%). The fetus with Pfeiffer syndrome type 3 had a normal-shaped head, although mild bilateral ventriculomegaly, frontal bossing and exophthalmos were observed at 29 weeks. The diagnosis was uncertain during the fetal period because major cranial sutures seemed to be open in the BPD view, although a postnatal 3D-CT scan detected lambdoid suture synostosis. A missense mutation on FGFR2: p.W290C (c.870G>C) was postnatally confirmed in the affected boy. In another case where skull deformation was not evident, the mother was referred for the assessment of mild ventriculomegaly at 21 weeks. This baby was examined by 3D-US to check all cranial sutures, which were normal. Although other related abnormalities including hypoplastic nasal bone, low-set ear, hypertelorism and exophthalmos were detected, the overall prenatal diagnosis was inconclusive and the mother carried on the pregnancy. The postnatal clinical diagnosis was Pfeiffer syndrome and the subtype was not reported (Figure 2).
Figure 2: A case of type 3 Pfeiffer syndrome (case 12). a) Cross section of the head at 29 weeks of gestation. Please note that cranial deformation was not evident. Only borderline ventriculomegaly was detected. b) Postnatal 3D-CT of the head at day 5. The fontanel was widely open and the lambdoid suture was partially synostotic.
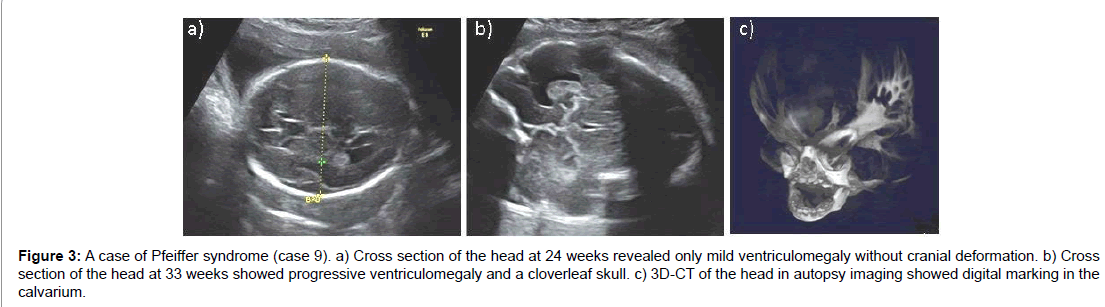
Ventriculomegaly was seen in 11 out of 15 cases (73%). In three cases, ventriculomegaly worsened progressively with rapid increases in BPD measurements. Development of hydrocephalus in utero was evident in case 9. Autopsy 3D-CT imaging showed digital marking in the calvarium indicating hydrocephalus with increased intracranial pressure. Polyhydramnios occurred in nine cases (60%) and a series of amnioreduction was necessary in three cases (Figure 3).
Figure 3:A case of Pfeiffer syndrome (case 9). a) Cross section of the head at 24 weeks revealed only mild ventriculomegaly without cranial deformation. b) Cross section of the head at 33 weeks showed progressive ventriculomegaly and a cloverleaf skull. c) 3D-CT of the head in autopsy imaging showed digital marking in the calvarium.
Extracranial anomalies associated with Pfeiffer syndrome included congenital heart diseases, genital anomalies and anal atresia, with VSD in three cases, anal atresia in three and hypospadias and bifid scrotum in one case each (Table 3).
| Case No. | GA at diagnosis (wk) | Deformation of skull | BPD>+3.0SD | Maximum width of Vp (mm) | Other related findings | Other unrelated findings | Polyhydroamnios | Type | Development to hydrocephalus | Anomaly, postnatal detected | Prognosis |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 29 | + | + | 37 | Cloverleaf skull, frontal bossing, exophthalmos | - | + | UK | + | - | Delayed |
| 2 | 27 | + | + | WNL | Cloverleaf skull | - | + | UK | + | VSD、PDA | Normal |
| 3 | 25 | + | + | 18 | Exophthalmos | CDH | + | UK | + | CDH | UK |
| 4 | 20 | + | + | 43 | Cloverleaf skull | - | + | typeⅡ | + | Bifid scrotum, anal stenosis | Lost follow-up |
| 5 | 27 | + | + | 13 | Cloverleaf skull, scoliosis | - | - | typeⅡ | + | VSD | Died at 4years |
| 6 | 32 | + | + | 14 | Cloverleaf skull, midfacial hypoplasia | - | Amnioreduction | typeⅡ | + | Abnoraml genitalia, imperforate anus | Delayed |
| 7 | 25 | + | <-3.0SD | 37 | Cloverleaf skull, exophthalmos, scoliosis | - | Amnioreduction | typeⅡ | + | - | Delayed |
| 8 | 31 | + | + | 17 | Cloverleaf skull, exophthalmos | - | - | typeⅡ | + | - | Delayed |
| 9 | 26 | + | + | 47 | Cloverleaf skull, exophthalmos, scoliosis | Hypospadias | Amnioreduction | typeⅡ | N.A. | Hypospadias | Neonatal death |
| 10 | 29 | + | + | WNL | Cloverleaf skull | - | - | type Ⅱ | + | - | Delayed |
| 11 | 21 | - | - | 10.3 | Increased NT, hypoplastic nasal bone, low-set ears, hypertelorism, exophthalmos | Single UA | + | UK | + | VSD,PDA | UK |
| 12 | 29 | - | + | 13 | Frontal bossing, exophthalmos | - | + | typeⅢ | + | Anal atresia | Died at 3years |
| 13 | Postnatal | UK | - | WNL | - | - | - | typeⅠ | - | - | Normal |
| 14 | Postnatal | UK | UK | UK | - | - | - | UK | + | Sacrococcygeal eversion | UK |
| 15 | Postnatal | + | - | 11 | Dolicocephaly | (DD twin) | - | typeⅡ | + | Cloverleaf skull, syndactyly, polydactyly | Died at 23 months |
*UK: Unknown, WNL: Within normal range, CDH: Congenital diaphragmatic hernia, UA: Umbilical artery, VSD: Ventricular septum defect, PDA: Persistent ductus arteriosus, N.A.: Not applicable.
Table 3: Details of Pfeiffer syndrome.
Apert syndrome
Four out of six cases were prenatally diagnosed as Apert syndrome. The cranial shape characterising Apert syndrome is unique, with a wide appearance in BPD view and acrocephaly. Syndactyly was noted subsequently after the detection of the abnormal shape of the head in all cases with prenatal diagnoses. Ventriculomegaly was not characteristic of Apert syndrome except in one case and no patients developed postnatal hydrocephalus. Associated anomalies with Apert syndrome included cleft palate in two cases, VSD in one case and agenesis of the corpus callosum in one case (Table 4 and Figure 4).
| Case No. | GA at diagnosis | eformation of skull | BPD>+3 SD | Ventriculomegaly | Other related findings | Other unrelated findings | Polyhydroamnios | evelopment to hydrocephalus | Anomalies, postnatal detected | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Apert S. | |||||||||||
| 16 | 32 | + | - | - | Acrocephaly, abnormal profile, syndactyly | - | - | - | Cleft palate | Speech delay | |
| 17 | 28 | + | UK | - | Acrocephaly, syndactyly, wide septum pellucida | VSD | - | - | Blephaophimosis, microphthalmia, midfacial hypoplasia | elayed | |
| 18 | 27 | + | + | Borderline | Syndactyly | Short extremities | Amnio reduction | - | Cryptorchidism | Normal | |
| 19 | 32 | + | - | - | Frontal bossing, exophthalmos,hypertelorism, syndactyly | - | + | - | Cleft palate | Normal | |
| 20 | Postnatal | - | + | - | - | - | - | - | Syndactyly | UK | |
| 21 | Postnatal | UK | UK | UK | - | - | - | + | ACC, polydactyly, syndactyly, cleft of soft palate | UK | |
| Crouzon S. | |||||||||||
| 22 | 27 | + | - | + | - | - | - | + | - | elayed | |
| 23 | 31 | + | <-3.0SD | - | Exophthalmos | - | - | - | - | Mildly delayed | |
| 24 | Postnatal | - | - | - | - | - | - | - | - | Normal | |
| Beare-Stevenson S. | |||||||||||
| 25 | 37 | - | - | + | Exophthalmos | Scoliosis | - | + | Sacrococcygeal eversion | Severely delayed | |
| 26 | Postnatal | - | + | - | - | - | - | + | Hemangioma, sacrococcygeal eversion, hyposparias, cryptorchism,thick fingers | Severely delayed | |
| Saethre-Chotzen S. | |||||||||||
| 27 | Postnatal | UK | UK | UK | - | - | - | - | PDA, anal atresia | UK | |
| Sensenbrenner S. | |||||||||||
| 28 | 16 | UK | - | - | - | Hygroma, short extremeties, bone fracture, polydactyly narrow chest | + | - | Low-set ear, brachydactyly, short rib bone | ied at 2 years | |
| Thanatophoric dysplasia | |||||||||||
| 29 | 20 | + | + | Borderline | Cerebellar hypoplasia | Short extremites, narrow chest | N.A. | N.A. | - | TOP at 21week | |
| 30 | 24 | UK | + | UK | Cerebellar hypoplasia | Short extremites, narrow chest | + | - | - | ied at 8months | |
Table 4: Details of other forms of syndromic craniosynostosis.
Crouzon syndrome
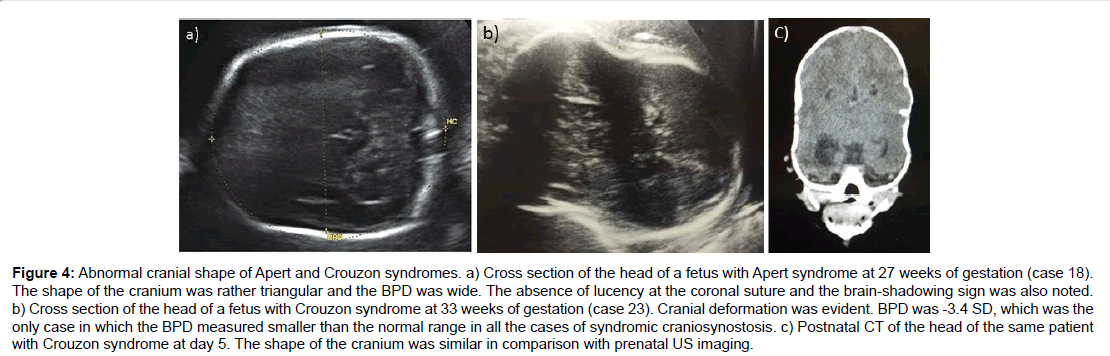
Three cases of Crouzon syndrome were reported. Related abnormalities were detected in two cases but were not specific enough to lead to the precise diagnosis of Crouzon syndrome. Cranial deformation was exceptional with smaller BPD measurements in case 23. The mother in case 24 had Crouzon syndrome, but no specific findings were detected in the fetus in the prenatal scan. All three cases were diagnosed based on clinical manifestations in the first year of life. No other extracranial anomalies were detected in the three children (Figure 4).
Figure 4:Abnormal cranial shape of Apert and Crouzon syndromes. a) Cross section of the head of a fetus with Apert syndrome at 27 weeks of gestation (case 18). The shape of the cranium was rather triangular and the BPD was wide. The absence of lucency at the coronal suture and the brain-shadowing sign was also noted. b) Cross section of the head of a fetus with Crouzon syndrome at 33 weeks of gestation (case 23). Cranial deformation was evident. BPD was -3.4 SD, which was the only case in which the BPD measured smaller than the normal range in all the cases of syndromic craniosynostosis. c) Postnatal CT of the head of the same patient with Crouzon syndrome at day 5. The shape of the cranium was similar in comparison with prenatal US imaging.
Other forms of syndromic craniosynostosis
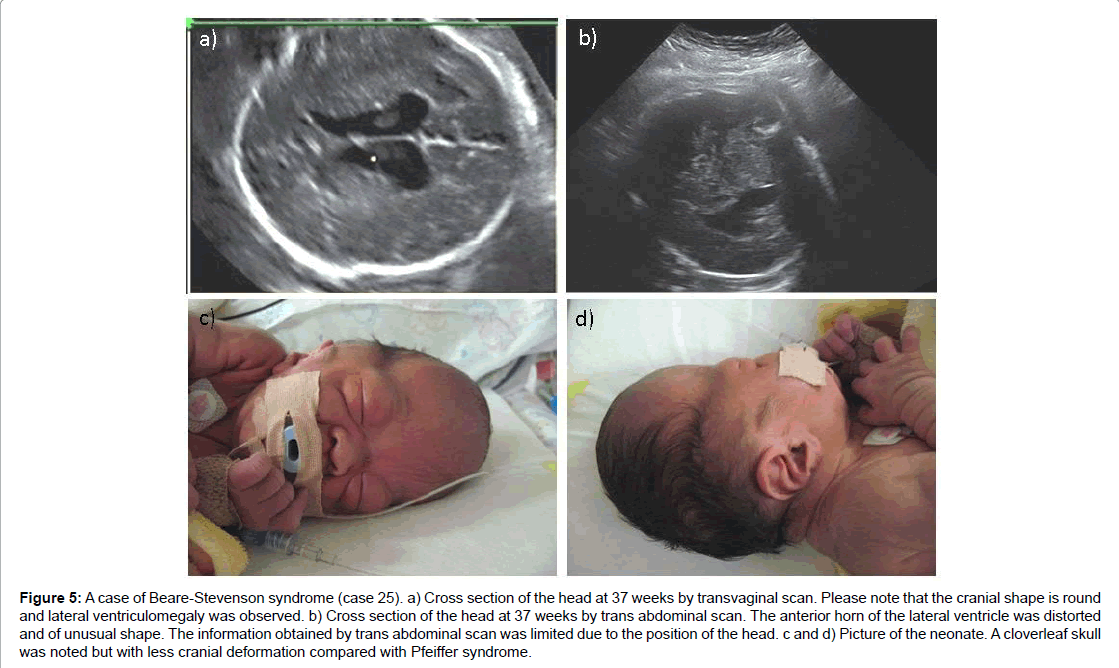
In one case of Beare-Stevenson syndrome, the fetus was referred for the investigation of mild ventriculomegaly at 37 weeks of gestation. On detailed examination combined with transvaginal scan, despite normal shaped skull, the combination of an irregular shape of the cerebral ventricle with exophthalmos and spinal anomaly indicated that the fetus had some type of craniosynostosis. In another case of Beare- Stevenson syndrome, abnormally large BPD after 30 weeks was noted, but a detailed scan denied any other related abnormalities. In both cases, a distinctive face was evident at birth and the infants experienced respiratory difficulties, needing immediate resuscitation and supportive care (Figure 5).
Figure 5:A case of Beare-Stevenson syndrome (case 25). a) Cross section of the head at 37 weeks by transvaginal scan. Please note that the cranial shape is round and lateral ventriculomegaly was observed. b) Cross section of the head at 37 weeks by trans abdominal scan. The anterior horn of the lateral ventricle was distorted and of unusual shape. The information obtained by trans abdominal scan was limited due to the position of the head. c and d) Picture of the neonate. A cloverleaf skull was noted but with less cranial deformation compared with Pfeiffer syndrome.
There was no abnormality detected throughout the prenatal period in a fetus with Seathre-Chotzen syndrome. Neonatologists reported no sign of the enlargement or deformation of ventricles at birth. Diagnostic confirmation by genetic assessment has not been offered to the parents so far.
One fetus had cranioectodermal dysplasia that was first detected at 16 weeks by a cystic hygroma and short extremities. The presentation was more consistent with fetal skeletal dysplasia such as short-rib polydactyly syndrome, with multiple bone fractures, a narrow chest and polydactyly. The BPD was growing within normal range and ventriculomegaly was not suspected. Postnatal 3D-CT revealed sagittal and lambdoid suture synostosis and the diagnosis was based on the following clinical manifestations: Craniosynostosis, low-set ears, short fingers, short limbs, renal dysfunction, hepatic fibrosis and retinal degeneration.
Both cases of thanatophoric dysplasia were diagnosed prenatally in the early second trimester because of short extremities. In case 28, the affected girl was the first twin of a dichorionic twin pregnancy and was clinically diagnosed as thanatophoric dysplasia type1 postnatally. In case 29, genetic testing revealed a mutation at c.1948A>G in the exon 14 of FGFR3, confirming the diagnosis of thanatophoric dysplasia type 2.
Non-syndromic craniosynostosis
Three cases were detected prenatally, including one case of sagittal suture synostosis and two cases of bilateral coronal suture synostosis. In the fetus with sagittal suture synostosis, the abnormal shape of the posterior part of skull and dolichocephaly (CI value less than 75%) were noted, leading to the detection of craniosynostosis after the investigation using fetal CT. Abnormal cranial shape was evident as well as ventriculomegaly or exophthalmos in both fetuses with bilateral coronal suture synostosis.
Craniosynostosis complicated with chromosomal aberration
Craniosynostosis can be complicated with secondary findings of maldevelopment in the size of the brain. All three fetuses required close observation due to fetal growth restriction, but none were diagnosed prenatally. One fetus with cranial deformation, mild nephrosis and VSD was found to have 11q deletion syndrome. The other two cases were diagnosed as 14q deletion syndrome. One case presented with a strawberry-shaped skull with ventriculomegaly and the other with cranial deformation with depressions at the bilateral coronal suture, ventriculomegaly and cardiomegaly.
Prognosis
The outcome of the cases of craniosynostosis depended on the underlying conditions. Four children with Pfeiffer syndrome had died at the time of writing due to complications of the disease, while the children with Apert and Crouzon syndromes were all still alive. In one case of the most severe type of Pfeiffer syndrome, neonatal death at birth was inevitable as he did not respond for resuscitation. The other three children died of seizure, infection and complications after surgery at 23 months, three years and four years, respectively. Seven children with Pfeiffer syndrome required tracheotomy for sustaining respiration (Table 5).
| Syndromic craniosynostosis (n=27) | Non-syndromic craniosynostosis (n=8) | Chromosomal abberation (n=3) | Total (n=38) | |
|---|---|---|---|---|
| Alive | 22 (81%) | 8 | 3 | 33 (87%) |
| Operation of craniosynostosis | 20 (74%) | 6 (75%) | 1 (33%) | 27 (71%) |
| VP/VA shunt | 17 (63%) | - | - | 17 (45%) |
| Developmental delay | 10/15 (66%) | 2 (25%) | 3 (100%) | 15/20 (75%) |
| (speech delay only) | 1 | 1 | - | 2 |
| Tracheotomy | 10 (42%) | - | - | 10 (26%) |
Table 5: Prognosis of craniosynostosis. Three cases of syndromic craniosynostosis were excluded from analysis: postnatal follow-up was lost in 1 case and there was one case each of neonatal death and termination of pregnancy.
One newborn with thanatophoric dysplasia type 1 needed continuous mechanical ventilation during his life and died at eight months due to heart failure. Another child with cranioectodermal dysplasia died at two years due to seizure and intestinal necrosis one week after his second open surgery for cranioplasty.
The children with non-syndromic craniosynostosis were all alive, but one child with right coronal and squamosal synostosis born with spastic quadriplegia was diagnosed with cerebral palsy at six months of age. All children with chromosomal deletion syndrome were alive but needed support for developmental delays.
Discussion
The present study examined the characteristic US findings observed in fetuses with craniosynostosis in low-risk populations without a family history. First, abnormal BPD may represent a candidate for a detailed scan because it showed a strong association between skull deformity, especially when BPD above +3.0 SD or below -3.0 SD. Second, ventricular deformity may be helpful in detecting craniosynostosis when fetal skull deformation is minimal. Four cases were prenatally suspected, although skull deformation was not recognised as the fetuses presented with exophthalmos and ventriculomegaly with an unusual shape.
Craniosynostosis contains variable phenotype conditions, so the prenatal findings are several US characteristic findings include the brain shadowing sign, absence of lucency at cranial suture, 3D sonography to evaluate open cranial suture and the CI, but none of these were universal findings commonly seen in all cases of craniosynostosis [4,9,14,15]. We also found cases without the brain shadowing sign, which has previously been reported to have 100% sensitivity in detecting craniosynostosis [4]. Alternatively, we observed specific tendencies in each syndrome. For instance, the considerable frequency of polyhydramnios in Pfeiffer syndrome was firstly described in our study. The fetuses with Pfeiffer syndrome presented with more prominent severe skull deformities beginning earlier in development compared to other types of craniosynostosis. The pathogenesis of developing polyhydramnios in Pfeiffer syndrome remains unknown, however, decreased swallowing function caused by CNS abnormality were speculated to be one of the reasons in other type of craniosynostosis [18]. Conversely, Crouzon syndrome was more difficult to detect because of milder skull deformities without specific associated anomalies during gestation, which could explain why far fewer cases were detected than cases of Pfeiffer syndrome in this study, even though Crouzon syndrome is the most common form of syndromic craniosynostosis.
Cranial shape in craniosynostosis is determined by the location of the premature fusion and cannot be used to distinguish between syndromes. It is also known that the premature sutural zone varies even within the same syndrome [19]. Compensatory expansion of a nonfused cranial suture is the main cause of abnormal cranial biometry in syndromic craniosynostosis. A midline calvarial defect or a wide gap at the parietal lesion, is an example observed in Apert syndrome [19,20]. In addition, bilateral bulging of the temporal lobes is seen in a cloverleaf skull [11]. These examples are the some of the causes of increased BPD or a CI value above the normal range. In sagittal synostosis, growth at the lambdoid suture forms an elongated cranium with occipital bulging [21]. The CI is abnormal and decreased in these cases. Our study also shows that both patterns can occur and abnormal biometry can be an indication of detailed examination with a differential diagnosis of craniosynostosis.
The difficulty of prenatal diagnosis or suspicion of fetal craniosynostosis with the lack of cranial deformity is noteworthy. Even experienced obstetricians failed to identify craniosynostosis although they were high-risk cases selected for abnormal findings. No cranial deformity during prenatal period was confirmed in two cases of Pfeiffer syndrome and two cases of BSS in our series. Without the synostosis of major sutures such as coronal, sagittal and metopic suture, direct observation of these cranial sutures using surface rendering of 3D-US will also fail to detect cranial synostosis. These four patients shared the manifestation of distinctive face, which could be detected with careful observation of the balance between calvarium and face (frontal bossing) or other facial features, especially the position of eyes. These small findings would be the only signs indicating fetal craniaosynostosis in such cases. Type 3 Pfeiffer syndrome shares common characteristics with type 2 Pfeiffer syndrome but lacks the cloverleaf skull [22,23]. Severe proptosis is one of the characteristics of type 3 Pfeiffer syndrome, but the fetus with this condition tends to be missed or categorised as “unknown” despite the presence of multiple other anomalies [23]. BSS is characterised by craniosynostosis and cutis gyrate in most patients, with acanthosis nigricans, choanal anomalies and prominent umbilical stumps representing other clinical features [24,25]. In the fewer than 30 cases reported to date, BSS with FGFR2 mutation typically exhibits a cloverleaf skull. However, Ron et al. presented an unusual case that presented all of the classic features except for craniosynostosis at birth [26]. The head shapes of both of our cases were also normal, indicating that a dysmorphic change of the cranium is not always sufficiently severe as to be detected as a cloverleaf skull phenotype of BSS in a prenatal scan.
Moreover, ventriculomegaly has a strong association with or is the result of brain distortion secondary to skull deformation [27]. It is very common, especially in syndromic craniosynostosis and never seen in cases of single suture synostosis other than coincidental cases [28,29]. This finding may derive from the fact that the occurrence of ventriculomegaly is more related to the size of the skull base. In craniosynostosis, the skull base is smaller, which leads to the stenosis of the jugular foramen and crowding of the posterior fossa. As a result, venous pressure rises and higher CSF pressure is required to maintain CSF outflow balance [30]. A sudden rise in CSF pressure may sometimes provoke subsequent progressive hydrocephalus in severe cases [28]. Patients with craniosynostosis, who developed hydrocephalus, may induce periventricular atrophy which causes the change of the shape of surrounding brain structures as well as the ventricles [31]. All of our cases of ventricular deformity were present with ventriculomegaly, which was inferred to be one of the characteristic findings of fetal craniosynostosis, although the number of experienced cases was limited.
Abnormality of the corpus callosum and holoprosencephaly can be also detected prenatally in craniosynostosis, although corresponding cases were not observed in our series [32,33]. These CNS to be more related to a primary disorder linked with genetic alterations rather than mechanical effects secondary to the anomalies are associated with poorer clinical outcomes and are a major cause of headaches, muscle weakness, hearing problems, extreme fatigue, poor motor coordination and cognitive and social disabilities [34]. At the histological level, malformation of the hippocampus and amygdala were also detected in individuals diagnosed with Pfeiffer syndrome and Apert syndrome or thanatophoric dysplasia also shared some of these neuropathological features [35]. These characteristics are thought skull deformity [36-38].
The prognosis of the fetus depends on the underlying conditions. Generally, good outcomes can be expected in non-syndromic craniosynostosis. Wilkie et al. also reported that non-syndromic craniosynostosis and children with chromosomal abnormalities experience fewer surgeries (with only 17% requiring repeat surgeries) and fewer complications, including ventricular shunting or tracheostomy, than those with syndromic craniosynostosis [39].
In contrast, the biggest concern of syndromic craniosynostosis is the complications, which sometimes determines their life expectancy or quality of life [9]. According to our cases, children died as a result of Pfeiffer syndrome, thanatophoric dysplasia and cranioectodermal dysplasia and developmental delays were severe in both cases of BSS. A precise diagnosis in cases of syndromic craniosynostosis would help in the prediction of adverse outcomes. Some cases of craniosynostosis are complicated with chromosomal structural abnormalities and the lesions of chromosomal involvement are reported to be diverse. Deletions of 9p and 11q or duplication of 13q and 15q are known to have an association with metopic synostosis [2,39,40]. Correlation between the 14q deletion and craniosynostosis has not been clearly shown except in one case report, although craniosynostosis might be a possible secondary phenotype in any child with compromised brain development [41].
Regarding the supplementary imaging for prenatal diagnosis, either MRI or CT or sometimes both, were completed once fetal craniosynostosis was suspected after US examination. From a diagnostic point of view, 3D-CT has the advantage over MRI that it allows the observation of not only the cranial sutures as well as the cranial shape, but also other skeletal abnormalities such as elbow synostosis. MRI facilitates the evaluation of brain abnormalities or small changes in hands and feet, while the cranial sutures cannot be directly visualised and some indirect signs such as the notch at the level of the coronal sutures or the thickening of calvarium could be assessed [1]. However, there are some concerns about radiation exposure and it remains controversial whether fetal CT is justified for the diagnosis of craniosynostosis. Radiohumeral synostosis, for examples, was indirectly confirmed by 2D-US with fixed flexed elbow position which remained motionless for several hours [42]. Otherwise, the synostosis of the elbow, a primary piece of information for the diagnosis of Pfeiffer syndrome, will be missed by either MRI or US. Fetal CT could be a good option when a more specific diagnosis is necessary for perinatal management, but not in all cases.
The limitations of this study include the varied technical skills of the individuals performing the scanning as well as the US equipment used. The impact of negative findings is likely to be underestimated. Most cases detected in our series were acquired from a low-risk population as a result of general obstetricians performing the fetal scan. Also, molecular confirmation of postnatal genetic diagnosis was not commonly conducted in our series. One possible explanation would be that the investigation of pathogenic genetic changes was more commonly performed when the underlying causes of the anomalies were nonspecific or unknown, rather than to confirm the clinical diagnosis. The cohort study was difficult to design, although we attempted to collect as many cases as possible and contacted all major hospitals treating cases of craniosynostosis. Approximately one million babies are born in Japan each year. Pfeiffer syndrome, for example, occurs in one in 100,000 birth and 50 affected babies should therefore be born in Japan over five years [1]. The collected cases in our study were of craniosynostosis in which the diagnosis was apparent from the prenatal course or facial features at birth, with more cases diagnosed after the infancy period.
Conclusion
In conclusion, not all fetuses with craniosynostosis present with characteristic features in utero and the findings observed by fetal scan may occur at some point over the course of dysmorphic changes. Being aware of abnormal head biometry and ventricular deformity could raise the prenatal detection rate of craniosynostosis by adding value to previously reported sonographic findings, which may be particularly useful in fetuses whose cranial deformation is not evident.
Acknowledgements
We deeply express our gratitude for providing the descriptions of the corresponding cases to Dr. Tomomi Kotani, Dr. Katsuhiko Naruse, Dr. Masakatsu Sase, Dr. Katsusuke Ozawa, Dr. Osamu Samura, Dr. Toru Funakoshi, Dr. Masayuki Endo, Dr. Masaki Ogawa, Dr. Kazuharu Tanaka, Dr. Nobuhiro Hidaka, Dr. Kenji Hayata, Dr. Takeshi Kanagawa, Dr. Yutaka Kozuma, Dr. Takashi Matsuoka, Dr. Takeshi Nagamatsu, Dr. Ichiro Yasuhi, Dr. Akito Miyauchi, Dr. Takuji Kyoya, Dr. Makoto Nomiyama, Dr. Masahito Mizuuchi and Dr. Hironobu Hyodo. We would like to thank all of other members of Perinatal Research Network Group who cooperated and participated in this study.
References
- Helfer TM, Peixoto AB, Tonni G, Araujo Júnior E (2016) Craniosynostosis: Prenatal diagnosis by 2D/3D ultrasound, magnetic resonance imaging and computed tomography. Med Ultrason 18: 378-385.
- Kimonis V, Gold JA, Hoffman TL, Panchal J, Boyadjiev SA (2007) Genetics of craniosynostosis. Semin Pediatr Neurol 14: 150-161.
- Rubio EI, Blask A, Bulas DI (2016) Ultrasound and MR imaging findings in prenatal diagnosis of craniosynostosis syndromes. Pediatr Radiol 46:709-718.
- Haratz KK, Leibovitz Z, Svirsky R, Drummond CL, Lev D, et al. (2016) The 'brain shadowing sign': A Novel marker of fetal craniosynostosis. Fetal Diagn Ther 40: 277-284.
- McGlaughlin KL, Witherow H, Dunaway DJ, David DJ, Anderson PJ (2010) Spectrum of Antley-Bixler syndrome. J Craniofac Surg 21: 1560-1564.
- Koga H, Suga N, Nakamoto T, Tanaka K, Takahashi N (2012) Clinical expression in Pfeiffer syndrome type 2 and 3: Surveillance in Japan. Am J Med Genet A 158A: 2506-2510.
- Fujimoto T, Imai K, Matsumoto H, Sakamoto H, Nakano T (2011) Tracheobronchial anomalies in syndromic craniosynostosis with 3-dimensional CT image and bronchoscopy. J Craniofac Surg 22: 1579-1583.
- Vargervik K, Rubin MS, Grayson BH, Figueroa AA, Kreiborg S, et al. (2012) Parameters of care for craniosynostosis: Dental and orthodontic perspectives. Am J Orthod Dentofacial Orthop 141: S68-S73
- Choi JW, Lim SY, Shin HJ (2016) Craniosynostosis in growing children: Pathophysiological changes and neurosurgical problems. J Korean Neurosurg Soc 59: 197-203.
- Johnson D, Wilkie AO (2011) Craniosynostosis. Eur J Hum Genet 19: 369-376.
- Miller C, Losken HW, Towbin R, Bowen A, Mooney MP, et al. (2002) Ultrasound diagnosis of craniosynostosis. Cleft Palate Craniofac J 39: 73-80.
- Jeanty P, Romero R (1984) Obstetrical Ultrasound. McGraw Hill, England. Pp: 91-92.
- Delahaye S, Bernard JP, Rénier D, Ville Y (2003) Prenatal ultrasound diagnosis of fetal craniosynostosis. Ultrasound Obstet Gynecol 21: 347-353.
- Chaoui R, Levaillant JM, Benoit B, Faro C, Wegrzyn P, et al. (2005) Three-dimensional sonographic description of abnormal metopic suture in second and third-trimester fetuses. Ultrasound Obstet Gynecol 26: 761-764.
- Benacerraf BR, Spiro R, Mitchell AG (2000) Using three-dimensional ultrasound to detect craniosynostosis in a fetus with Pfeiffer syndrome. Ultrasound Obstet Gynecol 16: 391-394.
- Renier D, Lajeunie E, Arnaud E, Marchac D (2000) Management of craniosynostoses. Childs Nerv Syst 16: 645-658.
- The Japan Society of Obstetrics and Gynecology/Japan Association of Obstetricians and Gynecologists (2017) Guideline for obstetrical practice in Japan 2017. Kyorinsha, Tokyo. Pp: 1-4.
- Filkins K, Russo JF, Boehmer S, Camous M, Przylepa KA, et al. (1997) Prenatal ultrasonographic and molecular diagnosis of Apert syndrome. Prenat Diagn 17: 1081-1084.
- Cohen MM Jr, Kreiborg S (1996) Suture formation, premature sutural fusion and suture default zones in Apert syndrome. Am J Med Genet 62: 339-344.
- Cohen MM Jr, Kreiborg S (1993) An updated pediatric perspective on the Apert syndrome. Am J Dis Child 147: 989-993.
- Cohen MM Jr, MacLean RE (2000) Craniosynostosis diagnosis, evaluation and management. 2nd edition, Oxford University Press, New York. Pp: 354-360.
- Blaser SI, Padfield N, Chitayat D, Forrest CR (2015) Skull base development and craniosynostosis. Pediatr Radiol 45: S485-S496.
- Cohen MM Jr (1993) Pfeiffer syndrome update, clinical subtypes and guidelines for differential diagnosis. Am J Med Genet 45: 300-307.
- Cohen MM Jr, MacLean RE (2000) Craniosynostosis diagnosis, evaluation and management. 2nd edition, Oxford University Press, New York, Pp: 388-392.
- Barge-Schaapveld DQ, Brooks AS, Lequin MH, Van-Spaendonk R, Vermeulen RJ, et al. (2011) Beare-Stevenson syndrome: Two dutch patients with cerebral abnormalities. Pediatr Neurol 44:303-307
- Ron N, Leung S, Carney E, Gerber A, David KL (2016) A case of beare-stevenson syndrome with unusual manifestations. Am J Case Rep 17: 254-258.
- Tokumaru AM, Barkovich AJ, Ciricillo SF, Edwards MS (1996) Skull base and calvarial deformities: Association with intracranial changes in craniofacial syndromes. AJNR Am J Neuroradiol 17: 619-630.
- Cinalli G, Sainte-Rose C, Kollar EM, Zerah M, Brunelle F, et al. (1998) Hydrocephalus and craniosynostosis. J Neurosurg 88: 209-214.
- Collmann H, Sörensen N, Krauss J (2005) Hydrocephalus in craniosynostosis: A review. Childs Nerv Syst 21: 902-912.
- Sainte-Rose C, LaCombe J, Pierre-Kahn A, Renier D, Hirsch JF (1984) Intracranial venous sinus hypertension: Cause or consequence of hydrocephalus in infants? J Neurosurg 60: 727-736.
- Rijken BF, Leemans A, Lucas Y, Van-Montfort K, Mathijssen IM, et al. (2015) Diffusion tensor imaging and fiber tractography in children with craniosynostosis syndromes. AJNR Am J Neuroradiol 36: 1558-1564.
- Raam MS, Solomon BD, Shalev SA, Muenke M (2010) Holoprosencephaly and craniosynostosis: A report of two siblings and review of the literature. Am J Med Genet C Semin Med Genet 154C: 176-182.
- Camera G, Lituania M, Cohen MM Jr (1993) Holoprosencephaly and primary craniosynostosis: The Genoa syndrome. Am J Med Genet 47: 1161-1165.
- Tunçbilek G, Alanay Y, Uzun H, Kayikçioğlu A, Akarsu NA, et al. (2010) Intracranial and extracranial malformations in patients with craniofacial anomalies. J Craniofac Surg 21: 1460-1464.
- Khonsari RH, Delezoide AL, Kang W, Hebert JM, Bessieres B, et al. (2012) Central nervous system malformations and deformations in FGFR2-related craniosynostosis. Am J Med Genet A 158A: 2797-2806.
- Finckh U, Schröder J, Ressler B, Veske A, Gal A (2000) Spectrum and detection rate of L1CAM mutations in isolated and familial cases with clinically suspected L1-disease. Am J Med Genet 92: 40-46.
- Demyanenko GP, Tsai AY, Maness PF (1999) Abnormalities in neuronal process extension, hippocampal development and the ventricular system of L1 knockout mice. J Neurosci 19: 4907-4920.
- Raybaud C, Di Rocco C (2007) Brain malformation in syndromic craniosynostoses, a primary disorder of white matter: A review. Childs Nerv Syst 23: 1379-1388.
- Wilkie AO, Byren JC, Hurst JA, Jayamohan J, Johnson D, et al. (2010) Prevalence and complications of single-gene and chromosomal disorders in craniosynostosis. Pediatrics 126: e391-e400.
- Bernstein PS, Gross SJ, Cohen DJ, Tiller GR, Shanske AL, et al. (1996) Prenatal diagnosis of type 2 Pfeiffer syndrome. Ultrasound Obstet Gynecol 8: 425-428.
- Lemyre E, Lemieux N, Décarie JC, Lambert M (1998)Del(14)(q22.1q23.2) in a patient with anophthalmia and pituitary hypoplasia. Am J Med Genet 77: 162-165.
Citation: Harada A, Miyashita S, Nagai R, Makino S, Murotsuki J (2018) Prenatal Sonographic Findings and Prognosis of Craniosynostosis Diagnosed during the Fetal and Neonatal Periods. J Preg Child Health 5:377. DOI: 10.4172/2376-127X.1000377
Copyright: © 2018 Harada A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 6564
- [From(publication date): 0-2018 - Dec 18, 2024]
- Breakdown by view type
- HTML page views: 5769
- PDF downloads: 795