Research Article Open Access
Preliminary Research of Off-Line Bioartificial Liver on Patients with Hbv Related Acute-On-Chronic Liver Failure
Liu Hongling1*, You Shaoli2, Zhu Bing2, Rong Yihui2, Zang Hong1, Liu Wanshu2, Mao Panyong2, Wan Zhihong2 and Xin Shaojie2*1Liver Transplantation Research Center, the 302 Military Hospital, Beijing, China
2Liver Failure Diagnosis and Treatment Center, the 302 Military Hospital, Beijing, China
- *Corresponding Author:
- Liu Hongling
Liver Transplantation Research Center
the 302 Military Hospital, Beijing 100039, China
Tel: +86-136-71113329
E-mail: lhl7125@sina.com
Xin Shaojie
Liver Failure Diagnosis and Treatment Center
the 302 Military Hospital, Beijing, China
Email: xinshaojie302@163.com
Received Date: September 07, 2016; Accepted Date: October 25, 2016; Published Date: October 27, 2016
Citation: Hongling L, Shaoli Y, Bing Z, Yihui R, Hong Z, et al. (2016) Preliminary Research of Off-Line Bioartificial Liver on Patients with Hbv Related Acute-On-Chronic Liver Failure. J Infect Dis Ther 6:303. doi:10.4172/2332-0877.1000303
Copyright: © 2016 Hongling L, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Infectious Diseases & Therapy
Abstract
Objective: The study aims to construct an off-line bioartificial liver support system (off-line BAL) with human liver cell line, and explore it’s safety and effect in patients suffering with HBV-related acute on chronic liver failure (ACLF).
Methods: The off-line BAL was constructed with cultured HepG2 cell. Twenty patients with HBV-related ACLF were randomly separated into the two groups. Patients in the treatment group were dealt with plasma exchange (PE) first and then BAL treatment. The control group received a therapy of PE only . The clinical parameters were assessed at different times and survival rate was evaluated at 3 months.
Results: In the treatment group, 9 patients’ general conditions and clinical symptoms were improved, total bilirubin decreased about 44.27%, MELD scores decreased to 21.71 from 24.26, prothrombin activity (PTA) increased to 48.97%, and there was a significant difference between pretreatment and post-treatment.
Compared to the control group, PTA increased dramatically (51.02% vs. 37.24%; P=0.0477) at 4 weeks and MELD score decreasedsignificantly (21.71 vs. 24.47; P=0.0409) at post-treatment in BAL groups. During the 12 weeks, the survival rates were 70% and 50% (P=0.3613) in the treatment and control groups. No severe adverse events occurred and no liver tumor was found following three years of observation.
Conclusions: The off-line BAL may be safe for patients with liver failure. It can improve the patients’ clinical conditions and laboratory parameters, but it has no obvious benefit compared to PE treatment. The routine clinical application still needs further evidence.
Keywords
Liver failure; Bioartificial liver; HepG2; Treatment
Introduction
Liver failure is a severe clinical syndrome characterized by hepatic encephalopathy, ascites, icterus, coagulation disorder due to a variety of acute or chronic injuries induced by various causes, such as hepatotoxic drugs, alcohol consumption, and hepatitis virus infection [1-3]. In China, Hepatitis B virus (HBV) infection accounts for the highest proportion of hepatitis cases. Some chronic hepatitis B patients may rapidly progress toward acute-on-chronic liver failure (ACLF), and liver transplantation is considered the standard treatment for these patients. However, several limitations, such as lack of donor organs, operative damage, risks of rejection and high costs have restricted the use of liver transplantation in many ACLF patients.
Therefore, new alternative therapy to delay or improve disease progression of ACLF is urgently required. Cell therapies have been suggested and extensively studied in the world [4]. Among them, hepatocytes based therapy for liver failure has been attracting great attention [4,5]. Several types of extracorporeal artificial livers have been used to treat liver failure patients including physical and bioartificial liver (BAL) support systems [6].
The physical artificial liver system can improve hyper-bilirubinemia and hepatic encephalopathy. However, these roles are temporary, and clinic trials have shown a benefit to the short-term survival rate of liver failure patients [7]. To acquire replacement of hepatic function, researchers have tried to develop BAL devices [2,3,8].
BAL is equipment through which blood plasma is circulated over living liver cells or liver cell line cultured in a bioreactor. The use of BAL with living cells can provide patients with functions of metabolism, detoxication and synthesis [2]. At present, on-line BAL is common in clinical trials in which the blood plasma separation devices and cell based bioreactor are linked; however, anticoagulant therapy may have impacts on patients’ coagulant system in the course of therapy. Up to now, no bioartificial liver has been applied for treating patients in clinics due to its unsatisfactory effect in improving the liver function [8,9].
In the present study, we successively established the cell line expressing human augmenter of liver regeneration (hALR). A total of 800 million HepG2 hALR cells were cultured in bioreactors for 96 hours, and found it can keep hepatocyte - specific synthesis and metabolism functions [5,10].
In this study, we performed a new therapy that was characteristic of the bioreactor located separately from the liver failure patient. The blood plasma was separated from the patient and returned after the purification in the cell- based bioreactor. Here, we observed the safety and validity of this therapy in patients suffered with HBV-related acute-on-chronic liver failure (ACLF).
Materials and Methods
Patients
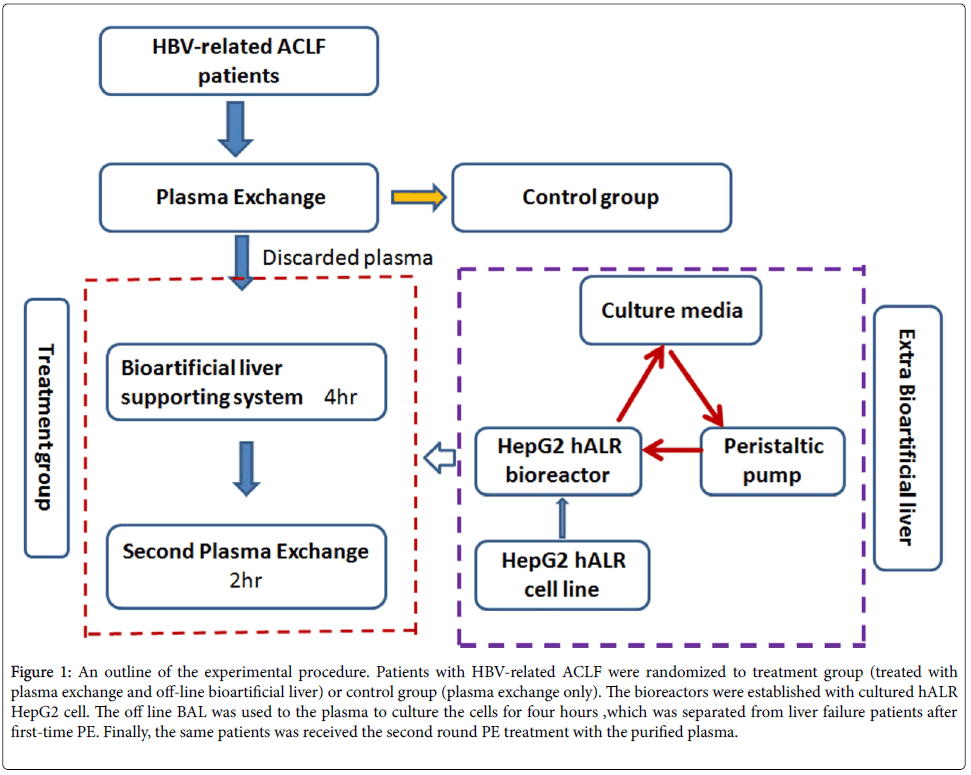
This open-labeled and controlled study was registered at the Chinese Clinical Trial Registry site (no. ChiCTR-TRC- 12002029) and was authorized by the Military Logistic Ministry of Health, China. Twenty patients with HBV-related ACLF, diagnosed according to the Chinese guideline of liver failure in 2010 [11], were enrolled between 2011 and 2012. They were randomized into BAL group or control group. Patients in BAL group were dealt with Plasma Exchange (PE) and off-line BAL therapy; whereas, patients in control group accept PE therapy [5]. Patients with severe bacterial peritonitis and gastrointestinal bleeding at enrollment were excluded from the study. An outline of the experimental procedure is summarized in Figure 1.
Figure 1: An outline of the experimental procedure. Patients with HBV-related ACLF were randomized to treatment group (treated with plasma exchange and off-line bioartificial liver) or control group (plasma exchange only). The bioreactors were established with cultured hALR HepG2 cell. The off line BAL was used to the plasma to culture the cells for four hours ,which was separated from liver failure patients after first-time PE. Finally, the same patients was received the second round PE treatment with the purified plasma.
Ethics
The protocol was approved by the Ethical Committee of the 302 Military Hospital of China. All procedures were done according to the ethical standards and the Helsinki Declaration. Written informed consent was acquired from all patients before admission to the study.
Management of patients
Every patient received standard treatment including intensive care monitoring, supporting treatment, plasma infusion, albumin supplement, antibiotics and terlipressin (if required). LamivudineGlaxo SmithKline,Tianjing, China and entecavir (Bristol-Myers Squibb, Shanghai, China) were utilized on the patients with HBV reactive replication (HBV DNA ≥ 102 copies/ml).
Off-line bioartificial liver procedure
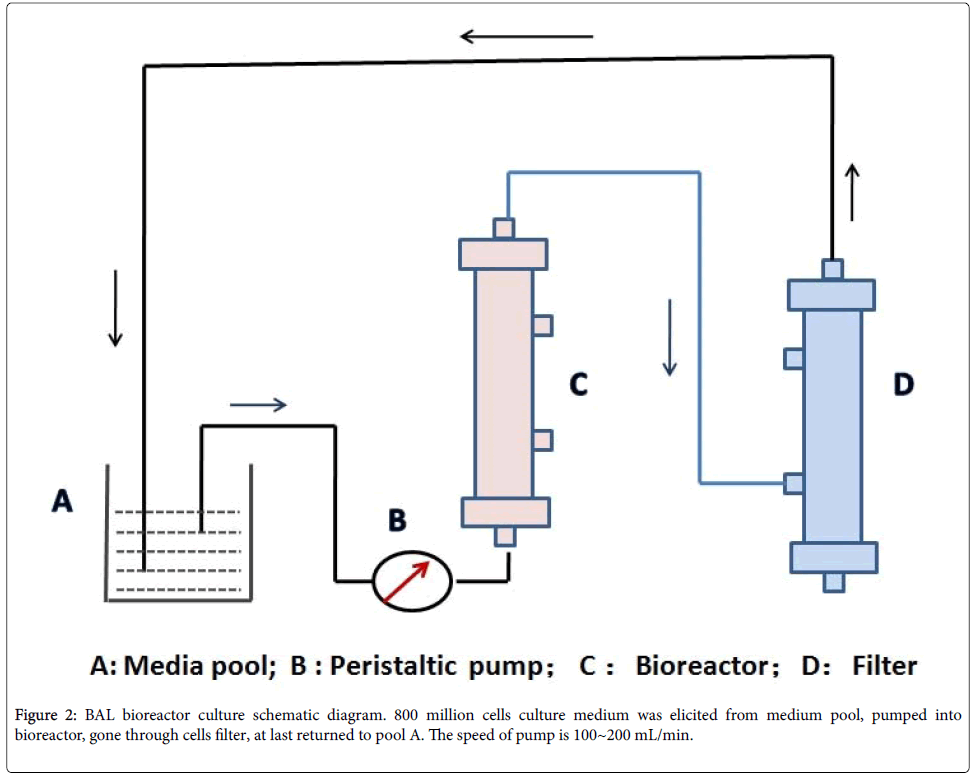
1. Bioreactor construction: Stable expressed hALR-HepG2 cell line and bioreactors containing approximately 800 million cells were established previously in our lab [10]. Prior to use, bioreactors were rinsed with 500 mL 0.9% sodium chloride for three times (Figure 2).
2. Plasma exchange for the first time: Blood access was set up through a double-lumen catheter (Arrow International, Inc. PA, US) via the jugular vein of the patient with liver failure. PE was manipulated with EC-40W plasma separator (Asahi Kasei Co., Japan) in artificial machine (EQUA-SMART, Italy) with blood flow velocity 100 mL/min and exchange rate 10~15 mL/min. The total volume of discarded blood plasma was about 2800 mL.
3. Extracorporeal bioartificial liver treatment: Extracorporeal bioartificial liver supporting system was constructed as shown in Figure 2. After the bioreactor with HepG2 hALR was cultured in media for 96 hours, the culture media were replaced with 1000mL 0.9% NaCl and rinsed three times. Then the 0.9% NaCl was replaced to the plasma from step 2, and continued to culture five hours. Plasma volume was 1500 mL; the flow velocity was 40 mL/min.
4. PE for the second time: The purified plasma from step 4 was used to perform the second PE for the patients with plasma separator EC-40W (Asahi Kasei Co., Japan) in artificial machine (EQUASMART, Italy). The blood flow velocity was 100 mL/min, exchange rate was 10~15 mL/min. The discarded blood plasma volume was near 1500 mL.
Control group treatment procedure
All the treatment procedure was same as the step 1: PE was performed in artificial machine with blood flow velocity 100 mL/min and exchange rate 10~15 mL/min. The exchanged plasma volume was about 2800 mL.
Biochemical parameters: Serum total bilirubin (TBil), aspartate aminotransferase (ALT), electrolytes, prothrombin activity (PTA), blood ammonia (BLA), serum creatinine (Cre) and α-fetal protein (AFP) were monitored through the therapy session. The blood biochemical indices were detected in Automatic Analyzer (Olympus, Japan).
MELD scoring system for patients was assessed as: MELD=0.378ln [total bilirubin (mg/dl)]+1.12 ln (INR)+0.95 ln [creatinine (mg/dl)] +0.64.
Prognostic evaluation: Improved- patients who were discharged from our hospital because clinical symptoms improved and hepatic function recovered (serum TBil level decreased ≥ 50%, PTA ≥ 40%).
Useless or deteriorated: Patients who died, or accepted liver transplantation, or didn’t meet the above criteria.
Statistical analysis: Statistical analysis was performed using GraphPad Prism 6 software. Numerical data was described by mean ( ± standard deviation) or median (minimum, maximum) of the group. Statistical significance between two groups was determined by the chi-square test for categorical data and Student’s T-Test for quantitative data, or if the data were heavily skewed (non-normal) then Wilcoxon Rank-Sum Test. If baseline measures were available then the T-test was performed using analysis of covariance (ANCOVA), adjusting for baseline. Statistical significance was established when P ≤ 0.05.
Results
Baseline characters
Change of clinical situation and biochemical indicators after BAL treatment
Clinical situation improvement After off-line BAL treatment, nine patients’ clinical symptoms including fatigue, nausea, anorexia, abdominal distention, and hepatic encephalopathy were improved.
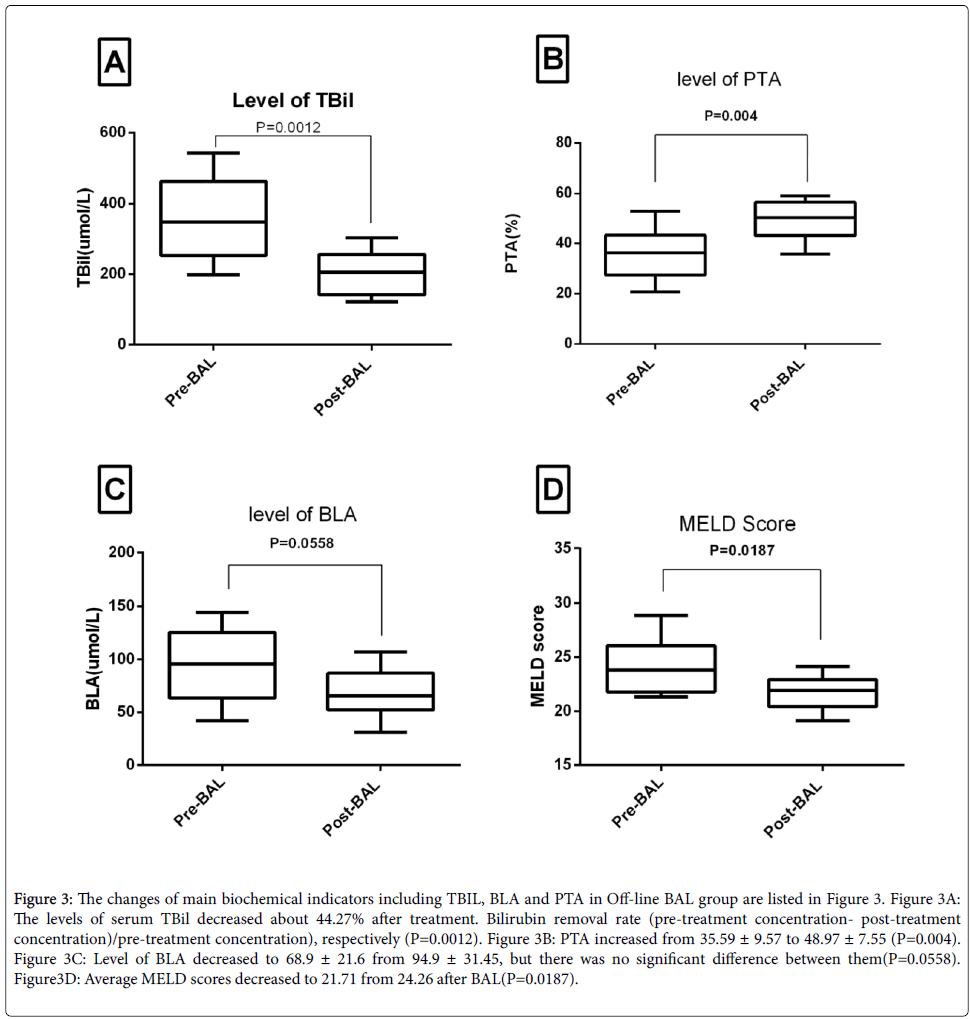
Changes of biochemical indicators The changes of main biochemical indicators including ALT, TBil and PTA in treatment groups are listed in Figure 3. The serum TBil level decreased about 44.27% after BAL. Bilirubin removal rate (pre-treatment-post-treatment)/pre-treatment), respectively (P<0.05). Average MELD scores were 24.26 and 21.71 in the pre-treatment and post-treatment of BAL.
Figure 3: The changes of main biochemical indicators including TBIL, BLA and PTA in Off-line BAL group are listed in Figure 3. Figure 3A: The levels of serum TBil decreased about 44.27% after treatment. Bilirubin removal rate (pre-treatment concentration- post-treatment concentration)/pre-treatment concentration), respectively (P=0.0012). Figure 3B: PTA increased from 35.59 ± 9.57 to 48.97 ± 7.55 (P=0.004). Figure 3C: Level of BLA decreased to 68.9 ± 21.6 from 94.9 ± 31.45, but there was no significant difference between them(P=0.0558). Figure3D: Average MELD scores decreased to 21.71 from 24.26 after BAL(P=0.0187).
Comparison between BAL and control group
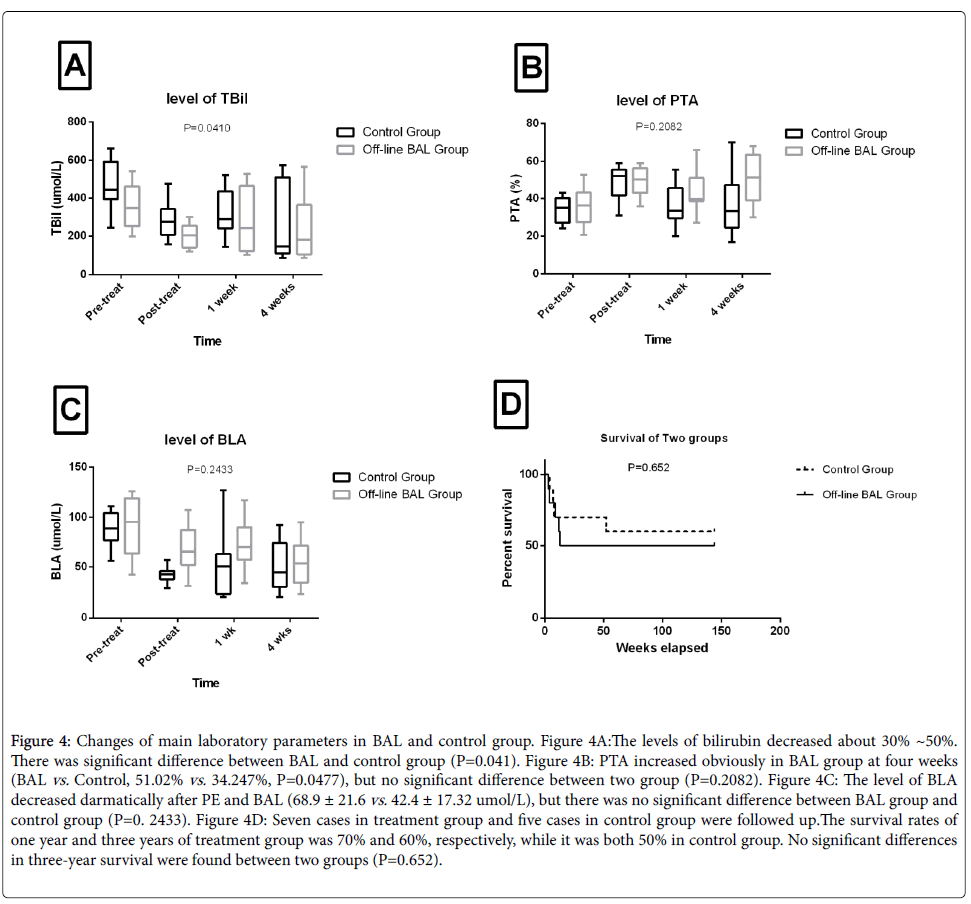
The changes of main laboratory parameters in BAL and control group are listed in Figure 4. The levels of serum total bilirubin decreased about 30%~50% in two groups. There was a significant difference between two groups (P=0.041). PTA increased significantly in the BAL group at 4 weeks (BAL group vs. Control group, 51.02% vs. 34.247%, P=0.0477), but no significant difference between two groups (P=0.2082). The level of BLA decreased obviously after PE and BAL treatment (68.9 ± 21.6 vs. 42.4 ± 17.32 umol/L), but there was no significant differences between BAL group and control group (P=0. 2433).
Figure 4: Changes of main laboratory parameters in BAL and control group. Figure 4A:The levels of bilirubin decreased about 30% ~50%. There was significant difference between BAL and control group (P=0.041). Figure 4B: PTA increased obviously in BAL group at four weeks (BAL vs. Control, 51.02% vs. 34.247%, P=0.0477), but no significant difference between two group (P=0.2082). Figure 4C: The level of BLA decreased darmatically after PE and BAL (68.9 ± 21.6 vs. 42.4 ± 17.32 umol/L), but there was no significant difference between BAL group and control group (P=0. 2433). Figure 4D: Seven cases in treatment group and five cases in control group were followed up.The survival rates of one year and three years of treatment group was 70% and 60%, respectively, while it was both 50% in control group. No significant differences in three-year survival were found between two groups (P=0.652).
Average MELD scores pretreatment, post-treatment, one week and four weeks in BAL group was 24.26, 21.71, 22.10 and 19.90, respectively, and were 24.71, 24.47, 25.08, and 18.79 in control group (P=0. 4938).
Serum albumin & lactate: The level of serum albumin & lactate were (29.4 ± 1.6) g/L, (1.62 ± 0.41) mmol/L in BAL group, and (28.9 ± 2.3) g/L, (1.71 ± 0.31) mmol/L in control group, there were no significant differences.
Safety
Short term outcomes
In the treatment group, survival rate of four weeks was 90% with nine patients were treated in hospital and one patient dying of hepatic encephalopathy. While in the control group, three patients died after plasma exchange, and survival rate of four weeks was 70% (P=0.1213). On the 12 weeks, the survival rate of the BAL and control groups were 70% and 50%, respectively (P=0.3613). In treatment group, one died of hemorrhage in the upper alimentary tract and one died of hepato-renal syndrome. At the same time, five patients survived, one patient received liver transplantation and four died of ACLF in control group (Table 2).
| Off-line BAL Group (n=10) | Control group(n= 0) | P value | |
|---|---|---|---|
| Age (years) | 41.4 ± 10.45 | 43.1 ± 11.27 | NS |
| Gender(Male/Female) | 10/0 | 09-Jan | NS |
| TB (umol/L) | 359.6 ± 114.6 | 371.98 ± 106.5 | NS |
| MELD score(R) | 24.26 ± 2.68 | 24.71 ± 2.94 | NS |
| PTA(%) | 35.59 ± 9.57 | 35.1 ± 5.41 | NS |
| Encephalopathy | 3 | 2 | NS |
| Ascite | 9 | 8 | NS |
| Cr(umol/L) | 91.32 ± 17.1 | 89.9 ± 10.4 | NS |
| BAL:BioartificialLiver; TB:Total Bilirubin; PTA:ProthrombinActivity; Cre: Creatinine; MELD: Model for End-Stage Liver Disease; R=0.378ln [total bilirubin (mg/dl)] +1.12 ln (INR)+0.95 ln [creatinine (mg/dl)]+0.64 | |||
Table 1: The baseline patient characteristics.
| Patient | Group | Gender | Yo | Diagnosis | Base MELD | prognosis |
|---|---|---|---|---|---|---|
| 1 | T | Male | 63 | ACLF | 21.31 | Diedï¼Â?6 days after BALï¼Â? |
| 2 | T | Male | 33 | ACLF | 21.36 | improvement |
| 3 | T | Male | 38 | ACLF | 25.31 | improvement |
| 4 | T | Male | 45 | ACLF | 21.92 | improvement |
| 5 | T | Male | 32 | ACLF | 25.05 | Diedï¼Â?45 days after BALï¼Â? |
| 6 | T | Male | 44 | ACLF | 23.06 | improvement |
| 7 | T | Male | 29 | ACLF | 22.86 | improvement |
| 8 | T | Male | 54 | ACLF | 24.61 | improvement |
| 9 | T | Male | 40 | ACLF | 28.85 | improvement |
| 10 | T | Male | 36 | ACLF | 28.23 | Diedï¼Â?35 days after BALï¼Â? |
| 11 | C | Female | 42 | ACLF | 19.71 | liver transplantation |
| 12 | C | Male | 29 | ACLF | 28.25 | Diedï¼Â?10 days after PEï¼Â? |
| 13 | C | Male | 43 | ACLF | 21.81 | Diedï¼Â?15 days after PEï¼Â? |
| 14 | C | Male | 33 | ACLF | 27.63 | improvement |
| 15 | C | Male | 63 | ACLF | 22.55 | improvement |
| 16 | Cl | Male | 51 | ACLF | 24.65 | Diedï¼Â?27 days after PEï¼Â? |
| 17 | C | Male | 44 | ACLF | 24.58 | improvement |
| 18 | C | Male | 30 | ACLF | 23.38 | improvement |
| 19 | C | Male | 39 | ACLF | 25.99 | improvement |
| 20 | C | Male | 57 | ACLF | 28.6 | Diedï¼Â?21 days after PEï¼Â? |
| T: Treatment group; C: Control group; PE: Plasma Exchange;BAL: Bioartificial Liver;ACLF: Acute-on-Chronic Liver Failure | ||||||
Table 2: Patients’ general condition and outcome on 12 weeks.
Follow-up
We followed up on seven cases (treatment group) and five cases (control group), all without liver transplantation. The survival rates of one year and three years of treatment group was 70% and 60%, respectively. It was both 50% in control group. No significant differences in three-year survival rate were found (Figure 3D, P=0.652).
All the patients in treatment group received abdominal B-ultrasonic or Magnetic Resonance Imaging examination at least twice each year, and six patients did not have were not found existing liver hepatic space occupying lesion in three years. The main biochemical indicators including ALT, TBil and PTA at one year and three years have no significant differences between BAL and control groups. The average AFP of one year and three years was (9.81 ± 3.52) ng/mL and (8.18 ± 2.27) ng/mL in treatment group, respectively.
Discussion
Severe liver disease is a major cause of death worldwide. The liver has the potential ability to regenerate; liver diseases ensue to meet the needs when the regeneration process is impaired [12]. Various therapies have been widely studied in China and other countries recently. Among them, liver cells or cell line based treatments for ACLF has been attracting more attention [13].
Off-line bioartificial liver support system was a novel concept in bioartificial area and its may have potent regenerative potential in liver failure patients. It was first reported in 2002, but there were no further reports in this [14,15]. Off-line BAL can purify the plasma, improve the microenvironment and supply the factors of promoting hepatocyte growth with liver failure. In this study, 10 patients with HBV related ACLF were treated with cell lines constructed in our department and shoved satisfactory safety [10]. The plasma from patients was further purified through cell therapy, which had the effect of detoxicfication and biosynthesis [16-18]. Moreover, HepG2 cell transfected with ALR was used to secrete ALR, and it may be beneficial in patients with ACLF [10,19]. After off-line BAL therapy, MELD scores improved significantly in patients with liver failure. Furthermore, no serious side effects were observed during the treatment period, and no tumors were found in living patients during the three years follow-up time [16].
Bilirubin and PTA were two critical indicators for prognosis of liver failure in the early stage. In the treatment group, statistical differences existed between pre-therapy and post-therapy. However, as for PTA, significant changes were observed four weeks post treatment between the BAL group and control group, which might be associated with the use of cell therapy.
Blood ammonia in BAL and control group showed significant improvement, and it was more obvious after PE in control group. We believe, the reason was the plasma used in off-line BAL needed 4~6 hours to be in vitro and that the hepatocytes could not synthesize enough metabolize ammonia [20-23]. In addition, insufficient hepatocyte amounts might have impacts on the patients’ outcome with liver failure [24]. Thus, we will focus on increasing hepatocyte amounts and constructing multi-component bioreactor in our later study.
In summary, the off-line bioartificial liver supporting systems we constructed have satisfactory safety and efficacy, but the routine clinical application still needs furth investigations.
Acknowledgments
We thank the 13th Five-Year National Science and Technology Major Project for Infectious Diseases (No.2016ZX10002004-005) and of 302 Military Hospital Project (YNKT 2014008) for their support.
References
- Chistiakov DA (2012) Liver regenerative medicine: advances and challenges. Cells Tissues Organs 196: 291-312.
- Inoue K, Kourin A, Watanabe T, Yamada M, Yoshiba M (2009) Artificial liver support system using large buffer volumes removes significant glutamine and is an ideal bridge to liver transplantation. Transplant Proc 41: 259-261.
- Sgroi A, Serre-Beinier V, Morel P, Buhler L (2009) What clinical alternatives to whole liver transplantation? Current status of artificial devices and hepatocyte transplantation. Transplantation 87: 457-465.
- Chamuleau RA, Deurholt T, Hoekstra R (2005) Which are the right cells to be used in a bioartificial liver? Metab Brain Dis 20: 327-335.
- RongYH, Liu HL, You SL, LiuWS, HuY, et al. (2010) Construction and experimental study on off-line hybrid bioartificial liver supporting system with human liver cell line. Chinese J ExpClinVirol 24: 193-195.
- Phua J, Lee KH (2008) Liver support devices. Curr Opinion Crit Care 14: 208-215.
- Chen JJ, Huang JR, Chen YM, Lu YF, li lJ (2012) A clinical study on the treatment of severe hepatitis by a combined artificial liver. Hepatogastroenterology 59: 2273-2275.
- Hassanein TI, Schade RR, Hepburn IS (2011) Acute-on-chronic liver failure: extracorporeal liver assist devices. CurrOpinCrit Care 17: 195-203.
- Sumeet KA, Jacqueline GO (2014) Acute-on-chronic liver failure. Clin Liver Dis 18: 561-574.
- Liu HL You SL, RongYH,Wu YC, Zhu B, et al. (2013) The newly established human liver cell line: a potential cell source for the bioartificial liver in the future. Human Cell 26: 155-161.
- Liver Failure and Artificial Liver Group, Chinese Society of Infectious Diseases, Chinese Medical Association; Severe Liver Diseases and Artificial Liver Group, Chinese Society of Hepatology, Chinese Medical Association (2012) Diagnostic and treatment guidelines for liver failure. Chin J Clin Infect Dis 5: 321-326.
- Hans LT, Keyur P (2014) Therapy of acute and fulminant hepatitis B. Intervirology 57: 181-188.
- Yu Y, Fisher JE, LillegardJB, Rodysill B, Amiot B, et al. (2012) Cell therapies for liver diseases. Liver transplant 18: 9-21.
- Ikeda T, Aoki T, Miyashita T,Kasuya K, Tsuchida A, et al. (2002) Experimental study of plasma recycling system by off-line bioartificial liver in rats. Transplant Proc 34: 2706-2710
- Enosawa S, Miyashita T, Endo M,Suzuki S, Amemiya H, et al. (2002) Off-line bioartificial liver: a novel concept of treatment and its potency of liver regeneration. Transplant Proc 34: 2711-2713.
- LillegardJB, Fisher JE, Nedredal G, Luebke-Wheeler J,Bao J, et al. (2011) Normal atmospheric oxygen tension and the use of antioxidants improve hepatocyte spheroid viability and function. J Cell Physiol 226: 2987-2996.
- Bao J, Fisher J, Lillegard J,Wang W, Amiot B, et al. (2013) Serum free medium and mesenchymal stromal cells enhance functionality and stabilize integrity of rat hepatocyte spheroids. Cell Transplant 22: 299-308.
- Thasler WE, Schlott T, Thelen P, Hellerbrand C,BatailleF, et al. (2005) Expression of augmenter of liver regeneration (ALR) in human liver cirrhosis and carcinoma. Histopathology1: 57-66
- Liu HL, Yu Y, Glorioso J, Shennen F, RodysilB, et al. (2014) Cold storage of rat hepatocytes spheroids. Cell Transplantation 23: 819-830.
- Yu Yue, Liu HL, Ikeda Y, Bruce A, Rinaldo P, et al.(2012) Hepatocyte-like cells differentiated from human induced pluripotent stem cells: Relevance to cellular therapies. Stem Cell Research 9: 196-207.
- Leckie P, Davenport A, Jalan R (2012) Extracorporeal liver support. Blood Purif 34: 158–163.
- Hoekstra R, Nibourg GA, van der Hoeven TV,Ackermans M T, Hakvoort T, et al. (2012) The effect of rat. acute-liver- failure plasma on HepaRG cells. Int J Artif Organs 35: 1006–1014.
- PoyckPP, van Wijk AC, van der Hoeven TV, de Waart DR,Chamuleau R, et al. (2008) Evaluation of a new immortalized human fetal liver cell line (cBAL111) for application in bioartificial liver. J Hepatol 48: 266–275.
- ÅÂ?ahintürk Y, Çekiç B, ZorluGörgülügil G, Harmandar FA, Uyar S, et al. (2016) Cirrhotic Ascites management via procalcitonin level and a new approach B-mode gray-scale histogram.Turk. J Gastroenterol 27: 47-54.
Relevant Topics
- Advanced Therapies
- Chicken Pox
- Ciprofloxacin
- Colon Infection
- Conjunctivitis
- Herpes Virus
- HIV and AIDS Research
- Human Papilloma Virus
- Infection
- Infection in Blood
- Infections Prevention
- Infectious Diseases in Children
- Influenza
- Liver Diseases
- Respiratory Tract Infections
- T Cell Lymphomatic Virus
- Treatment for Infectious Diseases
- Viral Encephalitis
- Yeast Infection
Recommended Journals
Article Tools
Article Usage
- Total views: 12011
- [From(publication date):
October-2016 - Apr 05, 2025] - Breakdown by view type
- HTML page views : 11106
- PDF downloads : 905