Preliminary Findings of Weaker Executive Control Network Resting State fMRI Functional Connectivity in Opioid Use Disorder compared to Healthy Controls
Abstract
Objective: Resting state functional magnetic resonance imaging (fMRI) functional connectivity has been used as a tool to study brain mechanisms associated with addictions. Recent research in substance use disorders has focused on three brain networks termed the default mode network (DMN), salience network (SN), and executive control network (ECN). The purpose of this study was to examine the functional connectivity of those three networks in opioid use disorder (OUD) subjects compared to healthy control subjects (HC). Methods: The present study investigated functional connectivity differences between OUD subjects compared to HC using independent component analysis. This study also examined the relationship between functional connectivity and negative urgency scores, as well as compared the functional connectivity of severe OUD to mild or moderate OUD. Results: In OUD subjects (n=25) compared to HC (n=25), a cluster in the left dorsolateral prefrontal cortex within the left ECN had significantly weaker functional connectivity. No significant differences were found between groups for the functional connectivity of the DMN, SN, or right ECN. No significant associations were found between functional connectivity and negative urgency, and no differences were found between severe OUD and mild or moderate OUD. Conclusion: These novel preliminary results suggest that ECN functional connectivity may differ between OUD and HC. This finding is consistent with previous research showing altered executive function in OUD and supports further examination of ECN functional connectivity in association with treatment response in OUD. Given our relatively small sample size (50 subjects total; 25 subjects per group), our results should be treated as preliminary for hypothesis generation, and replication will be needed in future studies.
Keywords: Opioid use disorder; Functional connectivity; Independent component analysis; Executive control network; Resting state networks; Negative urgency; Functional magnetic resonance imaging
Introduction
Opioid Use Disorder (OUD) represents a significant public health issue, and there remains room for improvement in the understanding of the neurobiology underlying OUD. Among the tools used for studying OUD neurobiology are functional magnetic resonance imaging (fMRI) studies of brain neural functional connectivity. Functional connectivity measures the statistical dependence between brain regions of spontaneous fluctuations in the fMRI signal [1,2]. Resting state fMRI functional connectivity can provide information about how the brain is organized into functional networks and how those functional networks may differ in OUD. Zhang and Volkow (2019) have reviewed the addiction functional connectivity literature with focus on the Default Mode Network (DMN). That review reported differences in substance users’ functional connectivity within sub-systems of the DMN and between the DMN and other networks, such as the Salience Network (SN) and the Executive Control Network (ECN) [3]. The DMN, SN, and ECN are three networks possibly related to addiction and psychopathology in general [4,5]. It has been suggested that functional connectivity within and among the DMN, SN, and ECN is altered in substance use disorders, possibly reflecting salient environmental cues (SN) interacting more with circuits associated with craving (DMN) and less with circuits associated with executive control (ECN) [3,5,6].
Several studies of OUD have assessed functional connectivity within the DMN, with inconsistent findings. While several studies found decreased functional connectivity within the DMN, some studies found increased functional connectivity [7-11]. Fewer studies have assessed functional connectivity within the SN and ECN [3,12]. Both Upadhyay et al. (2010) and Wang et al. (2016) found decreased functional connectivity within the SN in OUD subjects relative to healthy controls. Li et al. (2018) found increased functional connectivity between the left ECN and the right ECN in OUD, but to our knowledge there are no published studies which have addressed functional connectivity within the left ECN or right ECN in OUD [13]. Furthermore, many of the above-referenced studies used seed-based functional connectivity methods which may be more prone to head motion confounds than independent component analysis (ICA) [1]. ICA allows for quantitative investigation of whole networks without the need for selection of a seed region of interest and can better account for artifacts compared to seed-based methods which can be influenced by spatial confounds [1]. ICA with subsequent analysis using the dual regression module of FSL software (www.fmrib.ox.ac. uk/fsl/) considers both amplitude and shape of the signal time-course of resting state networks [1,14]. Head motion can mimic amplitude effects, and dual regression can more accurately localize these amplitude effects of motion and thus avoid misinterpreting them as differences in connectivity [14].
The present study used ICA to test for functional connectivity differences between OUD subjects and non-drug using healthy control subjects (HC) within the DMN, SN, and ECN. Previous research using ICA has shown the ECN to be lateralized as left ECN (LECN) and right ECN (RECN) components, and therefore we studied both LECN and RECN components in our ICA analysis [15]. The present study also tested for associations of functional connectivity with negative urgency scores (NU) across all subjects. NU is an aspect of impulsivity, which has been linked to executive control, and is described as the tendency to act rashly in response to negative affect [16,17]. NU has been associated with greater opioid use and future opioid use in a chronic pain patient sample, but has not yet been studied in association with functional connectivity in OUD [18].
We hypothesized that OUD subjects would have lower within-network DMN and lower within-network SN functional connectivity relative to HC, based on previous findings in OUD individuals who were actively using illicit opioids and/or taking methadone or buprenorphine [19,20]. We hypothesized that OUD subjects would score higher in NU, similar to previous findings in opioid users and other substance users [18,21]. We also hypothesized that NU scores would negatively correlate with SN functional connectivity of both groups based on a previous study using tobacco users and the proposed associations of the SN with salience detection and evaluation [22,23]. We also performed exploratory analyses of associations between NU scores and the within- network functional connectivity of the DMN, LECN, and RECN. We also planned exploratory analyses to compare between groups the within-network functional connectivity of the LECN and RECN. All of these hypotheses were registered with the Open Science Framework (OSF) prior to any data analysis in this study [24]. The present study also compared post-hoc the functional connectivity of OUD subjects with different levels of OUD severity. To investigate the effects of heterogeneity of our OUD sample, we also performed post-hoc analyses of functional connectivity of OUD with urine drug screens positive for buprenorphine or methadone compared to OUD with urine drug screens positive only for illicit opioids. Also, to investigate the effects of heterogeneity, we examined the relationship between functional connectivity and time since last opioid use in the OUD subjects. Given our relatively small sample size (50 subjects total; 25 subjects per group), our results should be treated as preliminary for hypothesis generation, and replication will be needed in future studies.
Materials and Methods
Subjects and procedures
All study procedures were approved by the Virginia Commonwealth University Institutional Review Board. OUD subjects were recruited from the Richmond, Virginia, community outpatient setting by using flyers and in-person recruitment at addiction treatment clinics. To obtain a more representative sample of the OUD population that could be seen clinically, no restrictions regarding current drug use were imposed during recruitment. HC subjects were recruited by using flyers and other advertisements. Written informed consent and a thorough screening were obtained, including a medical history, physical examination, and psychiatric and substance use histories conducted using the Mini International Neuropsychiatric Interview (MINI) for Diagnostic and Statistical Manual version 5 (DSM-5) [25]. Inclusion criteria were DSM-5 diagnosed OUD (for OUD only) and age between 18 and 70 years. Exclusion criteria were any history of schizophrenia, seizure disorder, significant head trauma, any changes to psychoactive medications within 30 days prior to the study period, any other DSM- 5 Substance Use Disorder with a severity diagnosis greater than the subject’s DSM-5 OUD severity diagnosis (Mild, Moderate, Severe), or DSM-5 Severe Alcohol Use Disorder. In addition, HC subject exclusion criteria were any history of substance use disorder. Subjects were seen for a screening visit and three additional visits in which they completed several behavioral measures, an MRI screening and mock MRI session, and an MRI scan. Participants were asked to refrain from smoking 1 hour before and drinking caffeine 3 hours before their MRI scan. Urine drug screens (UDS) and alcohol breath screens were obtained during each visit. 25 OUD and 25 HC subjects were included in the final analysis. Greater detail regarding the subjects and procedures is presented in Supplementary File.
Behavioral measures
Negative urgency: NU scores were extracted from a short form of the UPPS-P scale (Urgency, lack of Premeditation, lack of Perseverance, Sensation Seeking, and Positive Urgency scale) [26,27].
Opioid use: The number of subjects with at least one UDS positive for different categories of opioids and non-opioid drugs are reported for descriptive purposes.
OUD severity: OUD severity was obtained from the DSM-5 severity diagnosis (Mild, Moderate, Severe).
Time since last opioid use in hours was obtained by a clinician interview conducted immediately prior to the participant’s MRI scan.
Behavioral and demographic data were analyzed using JMP statistical software (JMP, Version 14. SAS Institute Inc., Cary, NC, 1989-2019). Greater detail regarding the behavioral measures is presented in Supplementary File.
MRI acquisition
MRI scans were acquired using the Philips Medical Systems (Best, Netherlands) Ingenia wide-bore dStream 3T MRI scanner, with a 32-channel receive head coil. Single shot gradient-echo echoplanar imaging (EPI) was used for acquiring fMRI data. The fMRI acquisition parameters were: SENSE in-plane acceleration factor 1.5, multiband factor 3, repetition time 1625 ms, echo time 30 ms, flip angle 52°, field of view 240 mm (anterior-to-posterior) × 240 mm (left-to-right) × 125.70 mm (foot-to-head), in-plane resolution 2.5 mm × 2.5 mm, 45 axial slices, slice thickness 2.5 mm, interslice gap 0.30 mm, 420 repetitions per run after 12 dummy acquisitions, and total duration 11 minutes 22 seconds. Subjects completed the resting state fMRI scan with eyes open while looking at a black fixation cross on a white screen. Greater detail regarding MRI acquisition parameters is available in Supplementary File.
MRI preprocessing
Initial removal of signal outliers, heart rate physiologic noise correction, slice timing correction, spatial smoothing, and registration to a T1 anatomical scan were performed. Susceptibility-induced off-resonance field correction was conducted by FSL "topup" software (www.fmrib. ox.ac.uk/fsl/). Quality control for head motion was performed by eliminating fMRI runs which did not meet the Parkes et al. (2018) stringent criteria [28]. Head motion re-alignment and signal correction was performed using the FSL MCFLIRT motion-correction program and ICAAROMA, respectively [29-31]. Further denoising was performed using the aCompCor procedure implemented in CONN software (www.nitrc. org/projects/conn, RRID:SCR_009550), and ICA components with possible motion-related structured noise are regressed out during the FSL dual regression procedure [32,33]. The denoised fMRI timeseries was transformed into MNI space. High pass filtering (cutoff=125 s), but not low pass filtering, was performed. Greater detail in the preprocessing steps is presented in Supplementary File.
Functional connectivity analysis
Group ICA maps were created using all subjects in both groups by FSL MELODIC (Multivariate Exploratory Linear Decomposition into Independent Components) (https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/MELODIC) with 30 components, the output of which was visually inspected to identify the DMN, SN, LECN, and RECN networks [34]. Dual regression analysis was then performed in FSL to obtain the subjectspecific component maps of parameter estimates of functional connectivity, which were then used as the input to non-parametric permutations statistical tests using FSL’s Permutation Analysis of Linear Models (PALM) (https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/PALM) for comparison between groups [14,35]. The FSL standard Threshold Free Cluster Enhancement (TFCE) was used to identify statistically significant clusters of voxels while maintaining family-wise-error (FWE) control [36]. We analyzed the regression of the subject-specific functional connectivity on NU scores for all subjects in both groups, and we also compared the functional connectivity of subjects with severe OUD to subjects with mild or moderate OUD. All reported p-values of functional connectivity results are FWE corrected for the number of voxels, the number of contrasts, and the 4 networks examined.
To examine the effects of heterogeneity in our OUD sample, we also performed two post-hoc analyses. In the first analysis, we compared OUD subjects with at least one UDS positive for buprenorphine or methadone to OUD subjects with UDS positive for illicit opioids only. In the second analysis, we analyzed the regression of the subject-specific functional connectivity on self-reported time since last opioid use, measured in hours.
For baseline variables (NU, education, and mean framewise displacement (mFD)) that statistically significantly differed between the two groups, we conducted a preliminary analysis of the regression of functional connectivity on the baseline variable to determine whether that variable should be included as a covariate in an analysis of covariance (ANCOVA) based on recommendations from a standard statistics textbook [37]. Given that 17/25 OUD subjects were tobacco users while 0/25 HC subjects were tobacco users, we were unable to perform ANCOVA but instead compared the functional connectivity of tobacco using OUD subjects to non-tobacco using OUD subjects.
Greater details of our functional connectivity analysis steps are in Supplementary File.
Results
Demographic and behavioral results
Demographics: The mean, standard deviation, and range of age, years of education, and mFD of OUD and HC subjects are listed in Table 1. Age did not significantly differ between groups (t=1.19, df=48, p=0.240). Years of education were significantly lower by 2.5 years in the OUD subjects (t=5.08, df=48, p<0.001) relative to HC. mFD was significantly higher by 0.03 mm in the OUD subjects (t=3.53, df=48, p<0.001) relative to HC. 10 out of the 25 OUD subjects were female and 12 out of the 25 HC subjects were female. A Chi-Square Test determined the groups did not differ significantly in sex (𝑋2=0.33, df=1, p=0.569). 16 out of 25 OUD subjects self-reported smoking tobacco products compared to 0 of the 25 HC subjects.
| HC (n=25) | OUD (n=25) | Difference | |||
|---|---|---|---|---|---|
| Mean (SD) | Range | Mean (SD) | Range | Statistic | |
| Age | 34.2 (14.9) | 21 to 68 | 39 (10.5) | 21 to 57 | t=1.19, df=48, p=0.24 |
| Education | 15.2 (1.85) | 12 to 21 | 12.7 (1.65) | 10 to 18 | t=5.08, df=48, p<0.0001** |
| mFD | 0.08 (0.02) | 0.05 to 0.12 | 0.11 (0.03) | 0.06 to 0.15 | t=3.53, df=42, p=0.001** |
| NU | 6.5 (2.4) | 4 to 11 | 8.8 (3.5) | 4 to 16 | t=2.70, df=48, p=0.010* |
| Ethnicity | 10 AA, 11 C, 2 H, & 2 A | 16 AA, 9 C | |||
| Sex | 12 F | 10 F | 𝑋2=0.33, df=1, p=0.57 | ||
| Tobacco Use | 0/25 users | 16/25 users | |||
Table 1: Sample Demographic information. 𝑋2=Chi-Square statistic, mFD=mean framewise displacement, NU=negative urgency, C=Caucasian, H=Hispanic, AA=African American, A=Asian, F=female. *p<0.05 **p<0.01
OUD urine drug screens: All 25 OUD subjects had at least one UDS positive for any opioid (including buprenorphine or methadone). UDS results for OUD subjects are listed in Table 2.
| Number of subjects with at least one positive UDS | |
|---|---|
| Any Opioids including Bup or MTD | 25/25 |
| Opioids excluding Bup & MTD | 20/25 |
| Bup or MTD | 16/25 |
| Bup (7/12 self-reported in treatment) | Dec-25 |
| MTD (0/4 self-reported in treatment) | Apr-25 |
| Non-opioid illicit drug | 15/25 |
| Number of subjects with OUD severity diagnosis | |
| Severe | 18/25 |
| Moderate | Jun-25 |
| Mild | Jan-25 |
| Time since last opioid use (in hours) | |
| Median (IQR) | 24 (3.5-72) |
| Range | 2 - 2,352 |
Table 2: Drug use in Opioid Use Disorder subjects. UDS=urine drug screen,Bup=buprenorphine, MTD=methadone, Non-opioid illicit drugs were: cocaine, methamphetamine, marijuana, or barbiturates. The proportions do not add to unity because more than one drug could sometimes be detected per each screening sample. The median and interquartile range (IQR) are reported for time since last opioid use because the data were not normally distributed.
Behavioral results: The mean, standard deviation, and range of NU scores of OUD and HC subjects are listed in Table 1. OUD subjects scored significantly higher in NU than HC subjects (t=2.70, df=48, p=0.010). The proportions of OUD subjects with severe, moderate, and mild OUD are listed in Table 2. The median, interquartile range (IQR), and range of hours since last opioid use are listed in Table 2.
Functional connectivity between groups
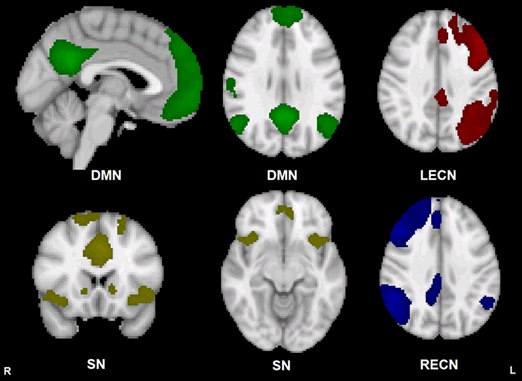
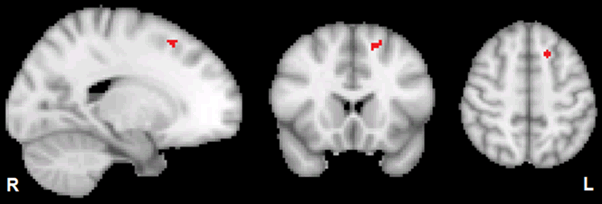
Component maps for the DMN, SN, LECN, and RECN, generated by FSL MELODIC from both groups combined, are displayed in Figure 1. As displayed in Figure 2, dual regression analysis showed that the OUD group had significantly weaker within-network functional connectivity relative to HC in a cluster (30 voxels; cluster peak at MNI coordinates (x=-16, y=19, z=52) mm) within the LECN (Cohen’s d=1.455, p=0.022 after FWE correction for voxels, contrasts, and the 4 networks examined). According to the Harvard-Oxford Cortical Structural Atlas (https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/Atlases), this cluster was in the left superior frontal gyrus. According to the histological study of Rajkowska and Goldman-Rakic (1995), the entire cluster was within the coordinates of their broadly defined dorsolateral prefrontal cortex (dlPFC), with approximately half of the voxels in the cluster falling within their conservatively defined dlPFC [38]. The mean functional connectivity parameter estimates output from stage 2 of the Dual Regression procedure for HC and OUD are listed in Table 3. OUD functional connectivity was not greater than HC (p greater than 0.998) within the LECN. OUD and HC did not significantly differ in the within- network functional connectivity for any of the other 3 networks (p greater than 0.562).
Figure 1: Group template maps generated from FSL MELODIC ICA for all subjects thresholded arbitrarily at Z ≥ 4 for display purposes. Units are Z-scores calculated by dividing the original component connectivity strength at each voxel by the standard deviation of the residual noise. The left side of the brain is on the viewer’s right side for the axial and coronal images. Color depictions and MNI coordinates (mm) of the slice location of each image: DMN – green [sagittal slice: x=-2], [transverse slice: z=29], SN – yellow [coronal slice: y=18], [transverse slice: z=-10], LECN – red [transverse slice: z=36], RECN – blue [transverse slice: z=36]
Figure 2: Displayed in red is the significant TFCE cluster within the left superior frontal gyrus (Harvard-Oxford Cortical Structural Atlas) within the LECN for the OUD HC contrast thresholded at FWE corrected p less than 0.05. MNI coordinates of cluster peak are [x=-16, y=19, z=52] mm; number of voxels in cluster=30. The left side of the brain is on the viewer’s right side of the axial and coronal images
| HC (n=25) | OUD (n=25) | Difference | Coordinates of Voxel with Peak T-value |
|
|---|---|---|---|---|
| Mean (Standard Deviation) Parameter Estimate | 9.06 (2.86) | 3.78 (2.42) | t=5.04, df=48, p=0.035* | x=53, y=72, z=62 |
Table 3: Mean functional connectivity parameter estimates for HC and OUD. Mean parameter estimates for the statistically significant cluster in the LECN with weaker functional connectivity in OUD relative to HC. The parameter estimate (beta value) of the regression coefficient at each voxel constitutes the subject-specific spatial map of relative functional connectivity across the network. The functional connectivity parameter estimates at each voxel are the output of stage 2 of the Dual Regression procedure and quantitatively represent the relative magnitude of functional connectivity of a given voxel with the subject specific fMRI timecourse that is characteristic of the entire network. T-statistic and p-value are the mean T-statistic and p-value, respectively, across the cluster. p-value is FWE-corrected for the number of voxels in the brain, contrasts (OUD>HC and HC>OUD), and number of networks examined (4). *p<0.05.
Regression with NU
The regression of functional connectivity on NU score was not significant for any of the 4 networks (p greater than 0.473) nor were there any significant NU x group interaction effects in any of the 4 networks (p greater than 0.454).
OUD severity functional connectivity comparison
Given that there was only one OUD subject with a DSM-5 diagnosis of mild severity, mild and moderate severity OUD subjects were combined into one subgroup. Severe OUD and mild/moderate OUD did not significantly differ in functional connectivity within any of the 4 networks (p greater than 0.696).
Post-hoc analysis
OUD with at least one UDS positive for buprenorphine or methadone (n=16) and OUD with UDS positive for only illicit opioids (n=9) did not significantly differ in functional connectivity within any of the 4 networks (p greater than 0.179). The regression of functional connectivity on time since last opioid use was not significant for any of the 4 networks (p greater than 0.313).
Head motion and education regressions
The regression of functional connectivity on mFD was not significant for any of the 4 networks (p greater than 0.246). The regression of functional connectivity on education was not significant for any of the 4 networks (p greater than 0.294). Thus, none of the baseline variables mFD, education, or NU met the criteria for inclusion as a covariate in ANCOVA, due to lack of a significant relationship of these variables with functional connectivity in this sample [37].
Discussion
Our results provide preliminary evidence that LECN functional connectivity may be weaker in OUD subjects compared to HC subjects. Differences in LECN resting state fMRI functional connectivity have been shown in cocaine use disorder and alcohol use disorder subjects relative to controls, but to our knowledge, this is the first published study to show differences in LECN resting state fMRI functional connectivity in OUD relative to HC [39-41]. These results need to be replicated, but if LECN functional connectivity can be shown to consistently differ in OUD subjects compared to HC, it may represent a neurobiological underpinning of impaired executive functioning observed in OUD [42]. Future studies should examine the association between LECN functional connectivity and addiction-related behaviors and treatment response in OUD.
The ECN is proposed to play a role in executing goal-directed behavior.
Dysfunction in the ECN has been linked to multiple psychopathologies, including substance use disorders [4,43]. Reese et al. (2019) found weaker functional connectivity within the LECN in cocaine use disorder subjects compared to healthy controls [39]. Two studies found contrasting results in alcohol use disorder – Weiland et al. (2014) found weaker functional connectivity within the LECN in alcohol use disorder subjects relative to healthy controls, while Zhu et al. (2017) found stronger functional connectivity in the LECN in alcohol use disorder subjects relative to healthy controls [40,41]. The opposing findings of Zhu et al. (2017) may be because their reported region within the LECN with increased functional connectivity was in the left posterior parietal cortex, while the region with significantly weaker LECN functional connectivity from our study was in the left dlPFC [41]. Although both Reese et al. (2019) and Weiland et al. (2014) found weaker functional connectivity in the LECN, they did not report which specific region within the LECN showed decreased functional connectivity [39,40].
The dlPFC node within the ECN has been proposed to play a key role in directing and sustaining attention during goal directed behavior and working memory [44,45]. While there is currently no universally accepted interpretation of stronger or weaker resting state fMRI functional connectivity, one suggestion proposed by some of the authors of the FMRIB Software Library (FSL) is that weaker within-network functional connectivity in a given set of voxels or regions may reflect weaker synchrony with the processes of a given network [33]. While speculative, weaker functional connectivity of the dlPFC within the LECN may be related to impaired recruitment of the dlPFC within the LECN during the initiation of goal directed behavior. This impaired recruitment may relate to the impaired attentional control and impulsivity found in substance use disorders [45]. Executive control deficits in OUD have been well documented and may be partially improved by drug abstinence and medication therapies [42]. If ECN functional connectivity is shown to relate to executive control deficits in OUD, it may serve as a target for OUD treatment studies.
Impulsivity is proposed to relate to increased substance use by an increased proclivity to act on the immediate reward associated with substance use without consideration of the long-term consequences of substance use [46]. NU may be related to increased drug use because of susceptibility to impulsive action in response to the stress of drug withdrawal and craving [21]. However, the results in the present study do not support a significant association between NU and functional connectivity of the LECN. It is possible that our sample size was too small to detect a subtle association between LECN functional connectivity and NU. Future work should further investigate whether LECN functional connectivity is associated with impulsivity and executive control task performance in larger samples of HC and OUD subjects.
DMN functional connectivity did not differ between groups. The lack of a significant difference in DMN functional connectivity may be related to previous findings suggesting that DMN functional connectivity may be influenced by recent drug use, although recency of last opioid use was unrelated to DMN functional connectivity in our post-hoc analysis [5]. It is also possible that different sub-systems and sub-regions of the DMN may be differentially affected by chronic drug use [3]. Our findings also showed that SN and RECN functional connectivity did not differ between OUD and HC, and we were unable to find previous published studies using ICA that compared OUD and HC within these networks. Our results do not support any statistically significant differences in functional connectivity of any of the 4 networks examined between severe OUD and mild or moderate OUD subgroups, although this subgroup analysis was underpowered due to small subgroup sample size.
All but 2 of the OUD subjects in this study were current users according to their DSM-5 diagnosis, and therefore this study was unable to compare former opioid users with current opioid users. Future studies should include subjects with past OUD to compare the effects of former vs. current OUD on network connectivity. On the other hand, the time since last opioid use was analyzed in the present study and showed no significant effects on functional connectivity in the networks analyzed. We also compared OUD subjects who were UDS positive for buprenorphine or methadone with OUD subjects who were UDS positive for only illicit opioids and found no statistically significant differences between the two subgroups. However, this subgroup analysis was also underpowered because of small subgroup sample size.
Limitations
A limitation of our study was the relatively small sample size. Recent research has highlighted the importance of large sample sizes in promoting reliability and reproducibility of resting state fMRI research [47]. Given our relatively small sample size, our results should be interpreted with caution and treated as preliminary for hypothesis generation. Replication of our results is needed. Another limitation of our study was the heterogeneity of our OUD sample. While our heterogenous OUD sample may be more representative of the OUD population, it is unclear exactly how differences in treatment adherence, recency of drug use, and polysubstance use may influence functional connectivity. Future studies with larger sample sizes should examine the effects of these factors systematically. Additionally, our sample size was underpowered to determine group interactions with sex; further work is needed to analyze the influence of sex on functional connectivity in OUD. Additionally, there was a range of time lag between the date of assessment of OUD severity and the date of the MRI scan that may have led to inaccuracy in estimating the actual OUD severity at the time of the scan.
Another limitation is that the OUD group had significantly higher mFD, education, and NU than the HC group. However, the mFD difference between groups was only 0.03 mm, and all subjects in both groups met the stringent motion criteria for inclusion in the analysis (Supplementary File) [28]. Furthermore, the effects of head motion on the fMRI signal had been corrected with the CompCor method and the ICAAROMA method, and ICA components with possible motion-related structured noise are regressed out during the dual regression procedure [31-33]. Despite all of these correction procedures and the very small difference in head motion between groups, it is crucial to rule out variations in head motion as driving group differences in functional connectivity, and if there are group differences in head motion, then investigators should perform a check whether motion is correlated with functional connectivity in their study [28]. In our sample, when conducting this check, there was no statistically significant correlation between mFD and functional connectivity in any of the 4 networks analyzed. Education and NU were also not statistically significantly related to the functional connectivity of any of the 4 networks analyzed. Another limitation is that 16 of the OUD subjects were tobacco users while none of our HC subjects were tobacco users. Tobacco-using OUD subjects did not statistically significantly differ from non-tobacco-using OUD subjects in functional connectivity of any of the 4 networks analyzed, although this analysis was underpowered. Future studies comparing OUD to HC should aim to balance subject groups for years of education attained, NU, and tobacco use. Due to the small sample size, difference in baseline demographic variables between OUD and HC subjects, and heterogeneity in drug use and treatment status in the OUD subjects, our results should be interpreted with caution and treated as preliminary. Furthermore, our results do not provide evidence regarding any specific behavioral correlates of the weaker LECN resting state fMRI functional connectivity in OUD relative to HC. Future studies should examine the addiction-related behavioral associations of LECN functional connectivity differences between OUD and HC.
Conclusion
These novel preliminary results suggest that ECN functional connectivity may differ between OUD and HC. This finding is consistent with previous research showing altered executive function in OUD and supports further examination of ECN functional connectivity in association with treatment response in OUD. Given our relatively small sample size (50 subjects total; 25 subjects per group), the statistically significant difference in demographic variables between groups, and the heterogeneity within our OUD group, our results should be treated as preliminary for hypothesis generation. Replication of these results will be needed in future studies.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
This research was supported by UG1DA050207 and by the National Institutes of Health through the NIH HEAL Initiative under award number: U54DA038999. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or its NIH HEAL Initiative.
Author Contributions
GM, LKM, JS, TR, and LM designed the phenotyping study from which subjects were sampled. JS, GM, LM, EZ, and KW designed the analyses for this study. ML reviewed anatomical scans for incidental pathology. TR was substantially involved in U54DA038999, consistent with her role as Scientific Officer. She had no substantial involvement in the other cited grants. KW wrote the original manuscript draft. All authors reviewed, contributed edits to, and approved the final manuscript
References
- Cole DM, Smith SM, Beckmann CF (2010) Advances and pitfalls in the analysis and interpretation of resting-state FMRI data. Front. Syst. Neurosci 4: 8.
- Sadraee A, Paulus M, Ekhtiari H (2019) fMRI as an outcome measure in clinical trials: A systematic review in clinicaltrials. gov. medRxiv 19002972.
- Zhang R, Volkow ND (2019) Brain default-mode network dysfunction in addiction. Neuroimage 200: 313-331.
- Menon V (2011) Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci 15(10): 483-506.
- Sutherland MT, McHugh MJ, Pariyadath V, Stein EA (2012) Resting state functional connectivity in addiction: lessons learned and a road ahead. Neuroimage 62(4): 2281-2295.
- Menon V, Uddin LQ (2010) Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct 214(5-6): 655-667.
- Ma N, Liu Y, Fu XM, Li N, Wang CX, et al. (2011) Abnormal brain default-mode network functional connectivity in drug addicts. PloS one 6(1): e16560.
- Ma X, Qiu Y, Tian J, Wang J, Li S, et al. (2015) Aberrant default mode functional and structural connectivity in heroin-dependent individuals. PLoS One 10(4): e0120861.
- Li Q, Li Z, Li W, Zhang Y, Wang Y, et al. (2016) Disrupted default mode network and basal craving in male heroin-dependent individuals: a resting-state fMRI study. J Clin Psychiatry 77(10): 1211-1217.
- Liu J, Qin W, Yuan K, Li J, Wang W, et al. (2011) Interaction between dysfunctional connectivity at rest and heroin cues-induced brain responses in male abstinent heroin-dependent individuals. PloS one 6(10): e23098.
- Qiang LI, Yang WC, Wang YR, Huang YF, Wei LI, et al. (2013) Abnormal function of the posterior cingulate cortex in heroin addicted users during resting-state and drug-cue stimulation task. Chin Med J. 126(4): 734-739.
- Ieong HFH, Yuan Z (2017) Resting-state neuroimaging and neuropsychological findings in opioid use disorder during abstinence: A review. Front. Hum. Neurosci 11: 169.
- Li Q, Liu J, Wang W, Wang Y, Li W, et al. (2018) Disrupted coupling of large-scale networks is associated with relapse behaviour in heroin-dependent men. J Psychiatry Neurosci 43(1): 48.
- Nickerson LD, Smith SM, Öngür D, Beckmann CF (2017) Using dual regression to investigate network shape and amplitude in functional connectivity analyses. Front. Neurosci 11: 115.
- Shirer WR, Ryali S, Rykhlevskaia E, Menon V, Greicius MD (2012) Decoding subject-driven cognitive states with whole-brain connectivity patterns. Cereb Cortex 22(1): 158-165.
- Neal LB, Gable PA (2016) Neurophysiological markers of multiple facets of impulsivity. Biol Psychol 115: 64-68.
- Cyders MA, Smith GT (2007) Mood-based rash action and its components: Positive and negative urgency. Personality and individual differences 43(4): 839-850.
- Vest N, Reynolds CJ, Tragesser SL (2016). Impulsivity and risk for prescription opioid misuse in a chronic pain patient sample. Addict Behav 60: 184-190.
- Upadhyay J, Maleki N, Potter J, Elman I, Rudrauf D, et al. (2010) Alterations in brain structure and functional connectivity in prescription opioid-dependent patients. Brain 133(7): 2098-2114.
- Wang PW, Lin HC, Liu GC, Yang YHC, Ko CH, et al. (2016) Abnormal interhemispheric resting state functional connectivity of the insula in heroin users under methadone maintenance treatment. Psychiatry Res Neuroimaging 255: 9-14.
- Zorrilla EP, Koob GF (2019) Impulsivity derived from the dark side: Neurocircuits that contribute to negative urgency. Front. Behav. Neurosci 13: 136.
- Menon V (2015) Salience Network. In: Arthur W. Toga, editor. Brain Mapping: An Encyclopedic Reference. Academic Press: Elsevier. 2: 597-611.
- Um M, Hummer TA, Cyders MA (2019) Relationship of negative urgency to cingulo insular and cortico-striatal resting state functional connectivity in tobacco use. Brain Imaging Behav 14(5):1921-1932.
- Woisard K (2020) Resting State Functional Connectivity in Opiate Users: Associations with Negative Urgency.
- Arlington VA (2013). American Psychiatric Association. Diagnostic and statistical manual of mental disorders (5th ed.).
- Lynam DR, Smith GT, Whiteside SP, Cyders MA (2006) The UPPS-P: Assessing five personality pathways to impulsive behavior. West Lafayette, IN: Purdue University.
- Lynam DR (2013) Development of a short form of the UPPS-P Impulsive Behavior Scale. Unpublished Technical Report.
- Parkes L, Fulcher B, Yücel M, Fornito A (2018) An evaluation of the efficacy, reliability, and sensitivity of motion correction strategies for resting state functional MRI. Neuroimage 171: 415-436.
- Jenkinson M, Bannister P, Brady M, Smith S (2002) Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17(2): 825-841.
- Woolrich MW, Behrens TEJ, Beckmann CF, Jenkinson M, Smith SM (2004) Multilevel linear modelling for FMRI group analysis using Bayesian inference. NeuroImage 21(4): 1732–1747.
- Pruim RH, Mennes M, Van Rooij D, Llera A, Buitelaar JK, et al. (2015) ICA-AROMA: A robust ICA-based strategy for removing motion artifacts from fMRI data. Neuroimage 112: 267-277.
- Behzadi Y, Restom K, Liau J, Liu TT (2007) A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage. 37(1): 90-101.
- Bijsterbosch J, Smith SM, Beckmann CF (2017) An introduction to resting state fMRI functional connectivity. Oxford University Press pp. 63-64, 64-65, 119.
- Beckmann CF, Smith SM (2004) Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Trans Med Imaging 23(2): 137-152.
- Winkler AM, Ridgway GR, Webster MA, Smith SM, Nichols TE (2014) Permutation inference for the general linear model. Neuroimage 92: 381-397.
- Smith SM, Nichols TE (2009) Threshold-free cluster enhancement: Addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage 44(1): 83-98.
- Kutner MH, Nachtsheim CJ, Neter J, Li W (2005) Applied linear statistical models. Boston: McGraw-Hill Irwin. 5:347, 919, 940.
- Rajkowska G, Goldman-Rakic PS (1995) Cytoarchitectonic definition of prefrontal areas in the normal human cortex: II. Variability in locations of areas 9 and 46 and relationship to the Talairach Coordinate System. Cereb Cortex 5(4): 323-337.
- Reese ED, Yi JY, McKay KG, Stein EA (2019) Triple Network resting state connectivity predicts distress tolerance and is associated with cocaine use. J Clin Med 8(12): 2135.
- Weiland BJ, Sabbineni A, Calhoun VD, Welsh RC, Bryan AD, et al. (2014) Reduced left executive control network functional connectivity is associated with alcohol use disorders. Alcohol Clin Exp Res 38(9): 2445-2453.
- Zhu X, Cortes CR, Mathur K, Tomasi D, Momenan R (2017) Modelâ€free functional connectivity and impulsivity correlates of alcohol dependence: a restingâ€state study. Addict Biol 22(1): 206-217.
- Pujol CN, Paasche C, Laprevote V, Trojak B, Vidailhet P, et al. (2018) Cognitive effects of labeled addictolytic medications. Prog Neuropsychopharmacol Biol Psychiatry 81: 306-332.
- Menon B (2019) Towards a new model of understanding The triple network, psychopathology and the structure of the mind. Med Hypotheses 133: 109385.
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, et al. (2007) Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 27(9): 2349-2356.
- Crews FT, Boettiger CA (2009) Impulsivity, frontal lobes and risk for addiction. Pharmacol Biochem Behav 93(3): 237-247.
- Kozak K, Lucatch AM, Lowe DJ, Balodis IM, MacKillop J, et al. (2019) The neurobiology of impulsivity and substance use disorders: implications for treatment. Ann N Y Acad Sci 1451(1): 71-91.
- Poldrack RA, Baker CI, Durnez J, Gorgolewski KJ, Matthews PM, et al. (2017) Scanning the horizon: towards transparent and reproducible neuroimaging research. Nat Rev Neurosci 18(2): 115-126.
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 4116
- [From(publication date): 0-2021 - Mar 31, 2025]
- Breakdown by view type
- HTML page views: 3380
- PDF downloads: 736