Research Article Open Access
Preimplantation Genetic Diagnosis (PGD) for Heart Disease Determined by Genetic Factors
| Anver Kuliev*, Tatiana Pakhalchuk and Svetlana Rechitsky | |
| Reproductive Genetic Innovations, Northbrook, IL | |
| Corresponding Author : | Anver Kuliev Reproductive Genetic Innovations 2910 MacArthur Blvd, Northbrook, IL 60062 Tel: 847 400-1515 E-mail: anverkuliev@hotmail.com |
| Received: September 30, 2015; Accepted: November 18, 2015; Published: November 28, 2015 | |
| Citation: Kuliev A, Pakhalchuk T, Rechitsky S (2015) Preimplantation Genetic Diagnosis (PGD) for Heart Disease Determined by Genetic Factors. Arrhythm Open Access 1:103. doi:10.4172/atoa.1000103 | |
| Copyright: © 2015 Kuliev A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
| Related article at Pubmed, Scholar Google | |
Visit for more related articles at
Abstract
The application of PGD has currently been extended to an increasing number of common disorders with genetic predisposition, including inherited heart disease, the prevention and treatment of which presents an important challenge. The major problem is that no pre-clinical diagnosis and preventive management exists, with high risk of premature or sudden death. We previously described the first series of 18 PGD cycles for 5 different inherited cardiac diseases, and showed feasibility and extremely high utility of preventing inheritance of genes predisposing to these conditions. The present paper summarizes the cumulative experience of 51 PGD cycles for 14 cardiac diseases, determined by 23 different gene mutations. This resulted in the embryo transfer in 44 of 51 PGD cycles, yielding 29 (66%) unaffected pregnancies and birth of 27 healthy, disease predisposition free children. This is the world’s largest PGD experience, demonstrating important clinical implications of PGD for preventing inheritance of predisposing genes for heart disease, as practical means for avoiding the risk of mortality or premature or sudden death in offspring of couples carrying a heart disease predisposing genes.
| Keywords |
| PGD; Inherited heart disease; Risk for inherited predisposition to premature or sudden death. |
| Introduction |
| PGD is gradually becoming a practical tool in medical practice for inherited disorders and assisted reproductive technology [1]. In addition to PGD application to the conditions presented at birth, it appeared also attractive to avoid the birth of carriers of those disorders that may or may not manifest in later life, including common diseases with genetic predisposition, such as inherited forms of heart disease [2,3]. The first PGD for heart disease was performed for Holt-Oram syndrome (HOS) [2], characterized by atrial septal defect and cardiac conduction disorder, although the clinical manifestations may be extremely variable, not usually being presented at birth, or presented only with a sinus bradycardia, as the only clinical sign which might be left unnoticed. So it is a typical condition for which PGD if very attractive option, allowing to reproduce without fear of producing offspring at risk of developing HOS with risk for sudden death. |
| The importance of PGD in this group of conditions is due to the fact that there is no current prospect of their treatment, as the disease may manifest despite pre-symptomatic diagnosis and follow up. So PGD seems to provide a possible relief for the at-risk couples to reproduce without much fear that their prospective children could be at high risk for premature or sudden death. This paper presents the world’s largest experience of PGD for inherited heart disease, performed for couples at risk who were able to avoid the risk and produce healthy children free of predisposition to inherited heart disease. |
| Material and Methods |
| A total of 51 PGD cycles for 30 couples at risk for producing an affected progeny with inherited heart disease were performed, which includes 18 cycles for 9 couples reported earlier [3] (Table 1). All PGD cycles were performed using a standard IVF protocol coupled with micromanipulation procedures for sequential first and second polar body (PB) (PB1 and PB2) sampling, and/or embryo biopsy, described elsewhere [4,5]. The biopsied PBs, blastomeres or blastocyst samples were tested by the multiplex nested PCR analysis, involving the above mutations and linked marker analysis in a multiplex heminested system [6,7]. The majority of cases were performed by embryo biopsy, with only a few by PB biopsy procedure [8]. |
| In cases of advanced reproductive age, 24-chromosome aneuploidy testing was performed (Figure 1), using next generation technologies (Illumina Inc), with a few described earlier done by FISH analysis [4]. Pregnancy outcome was defined as the presence of a gestational sac with foetal cardiac activity. |
| As per the informed consent, approved by Institutional Review Board, the embryos derived from the embryos free of genetic predisposition to heart disease, based on the mutation and polymorphic marker information, were pre-selected for transfer back to patients, while those with predisposing mutant genes were considered affected , and tested to confirm the diagnosis. |
| Results and Discussion |
| PGD cycle for one of the couples at risk for producing a progeny with Holt-Oram syndrome (HOS) is presented in Figure 1. |
| As can be seen from Table 1, cardiac disorder was either a primary problem, such as cardiomyopathy dilated (3 cycles for 3 couples) or hypertrophic (10 cycles for 8 couples), long QT (3 cycles for 3 couples) and Holt-Oram syndromes (5 cycles for 3 couples), or a part of other syndromes, such as of Emery-Dreifus muscular dystrophy (16 cycle for 6 couples), cardioencephalomyopathy (4 cycles for 2 couples), or Noonan (6 cycles for 3 couples), Bardet-Biedel (2 cycles for 1 couple) and Barth syndromes (1 cycle). As listed in Table 1, the above heart diseases tested were determined by 23 different mutations. Except one case of X-linked inheritance in the couple at risk for producing the offspring with Emery-Dreifuss muscular dystrophy, and 2 autosomal recessive cardio encephalomyopathy cases, all others were autosomal dominant, and the couples may have even no previous affected progeny, but had a family history of premature or sudden death. |
| Of 51 PGD cycles performed for 30 at risk couples 44 resulted in transfer of cardiac disease predisposition free embryos, yielding 29 clinical pregnancies (66% pregnancy rate per transfer) and birth of 27 disease or disease predisposition free children. |
| The overall efficiency of DNA testing was sufficiently high, resulting in diagnosis of 415 (89.0%) of 466 biopsied embryos, of which 208 were detected as normal or carriers, and 202 abnormal (only 5 with inconclusive results). A total of 208 embryos were tested also for aneuploidy (the results are not shown), which detected 54 aneuploid and 154 euploid embryos, so on the basis of mutation and aneuploidy testing only 65 embryos, overall, were preselected for transfer in 44 cycle (1.47 embryos per transfer cycle on the average). As a result, all but three couples became pregnant after the first or second PGD cycles and delivered a healthy child with no inheritance of predisposing gene mutations. It is also of note that no misdiagnosis was observed, suggesting that PGD for the inherited cardiac disease is a highly accurate procedure, which may be recommended for a wider clinical application. |
| As mentioned above, the majority of the cardiac diseases tested were dominant, conferring susceptibility to the disease in heterozygous status, except for cardioencephalomyopathy, which is recessive, and EDMD, which is X-linked. Accordingly, both latter conditions present very early after birth, badly requiring PGD to avoid the birth of affected child, and have a disease free offspring. As shown in Table 1, PGD for both of these conditions resulted in birth of unaffected children after first or second cycle. |
| Presented results show that PGD may be offered as a realistic option for couples at high risk for producing offspring with cardiac disease, to avoid inheritance of the predisposing genes from parents. So the couples at risk should be informed about such option, as if inheritance of these genes is not avoided, their offspring may be susceptible to a serious cardiac disease, which may manifest at the early childhood, or later in adult life, with the main clinical realization of premature or sudden death. |
| This makes important to incorporate the family history into the clinical settings, including any cardiac problems in the family members, such of heart attack, sudden death at young ages, any information about family members with pacemakers or internal cardiac defibrillators, arrhythmia or heart surgery, that may indicate to a possible candidates who might benefit from PGD. Of course, the chances of their offspring to develop the disease will differ depending on the mode of inheritance and also certain medications or activities, such as excessive exercise, but the risk that this will lead to cardiac arrest or sudden death cannot be excluded, justifying parents’ requests for PGD. In addition, the personal experience of the couple is of particular importance, altering the family's perception of severity of the cardiac disease, as the basis for their decision to undertake PGD. It may be predicted that one of the possible groups to benefit from such information are couples already undergoing IVF for fertility treatment, as they may represent immediate risk group who may avoid the inheritance of genetic susceptibility factors within the framework of IVF. |
| It should be mentioned, that symptoms of inherited cardiac disease may be easily overlooked, so the information on the family history may be the only reason to test for the presence of predisposing gene mutations and consideration about the need for PGD, which may appear the lifesaving procedure for individuals at risk. So with the future implementation of preconception screening programs for identification of carries of genes predisposing to inherited cardiac disease, PGD might appear a useful tool for avoiding the risk for producing offspring with inherited cardiac diseases with high probability of premature or sudden death at their lifespan. |
| As in other common disorders with genetic predisposition, PGD for cardiac disease has also important ethical implications, as most of these conditions are not present at birth, and may not be realized even during the lifetime. So the couples at risk could be reluctant to use prenatal diagnosis for cardiac disease, as pregnancy termination cannot be justified for this purpose, while PGD seems to be ethically more acceptable, allowing couples to reproduce, establishing only pregnancy free from predisposing genes. |
| References |
|
Tables and Figures at a glance
| Table 1 |
Figures at a glance
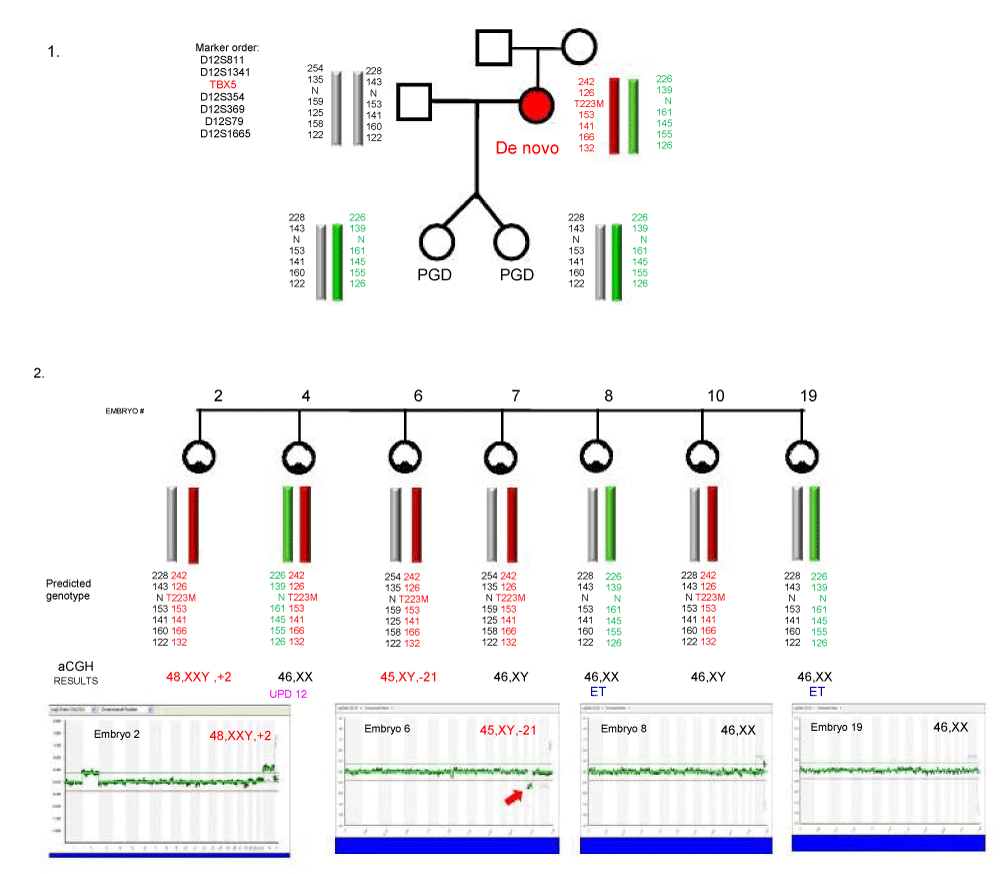 |
| Figure 1 |
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 10926
- [From(publication date):
March-2016 - Apr 19, 2025] - Breakdown by view type
- HTML page views : 10116
- PDF downloads : 810