Research Article Open Access
Pregerminated Brown Rice Enhanced NMDA Receptor/CaMKIIα Signaling in the Frontal Cortex of Mice
| Takayoshi Mamiya1*, Keiko Morikawa2 and Mitsuo Kise2 | ||
| 1Department of Chemical Pharmacology, Faculty of Pharmacy, Meijo University, Japan | ||
| 2FANCL Institute, FANCL Corporation, Yokohama, Japan | ||
| Corresponding Author : | Takayoshi Mamiya Department of Chemical Pharmacology Faculty of Pharmacy, Meijo University 150 Yagotoyama, Tempaku-ku, Nagoya 468-8503, Japan Tel: +81-52-839-2737 Fax: +81-52-834-8090 E-mail: mamiya@meijo-u.ac.jp |
|
| Received February 13, 2014; Accepted March 17, 2014; Published March 19, 2014 | ||
| Citation: Mamiya T, Morikawa K, Kise M (2014) Pregerminated Brown Rice Enhanced NMDA Receptor/CaMKIIa Signaling in the Frontal Cortex of Mice. J Rice Res 2:123. doi: 10.4172/jrr.1000123 | ||
| Copyright: © 2014 Mamiya T, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | ||
Related article at Pubmed Pubmed  Scholar Google Scholar Google |
||
Visit for more related articles at Rice Research: Open Access
Abstract
We previously reported that the continuous feeding of mice with pellets of pregerminated brown rice (PGBR; Hatsuga genmai in Japanese) enhances their spatial learning. Here, we show the possible relationships of the enhancement of learning and memory with the glutamatergic system in the brain of PGBR-pellet-fed mice. The enhancement of learning and memory in the novel object recognition and Y-maze tests after 28-day-feeding of PGBR pellets was inhibited by dizocilpine (10 μg/kg s.c.), an N-methyl-D-aspartate (NMDA) receptor antagonist, whereas the extracellular glutamate level and the glutamate content were not affected in the frontal cortex and hippocampus. In the frontal cortex of mice fed PGBR pellets, the phosphorylation of calcium calmodulin kinase IIα (CaMKIIα), one of the important events after NMDA receptor activation, was facilitated compared with that of mice fed control pellets. This facilitation was inhibited by dizocilpine (10 μg/kg s.c.), whereas the phosphorylation of extracellular signal-regulated protein kinases (ERKs), another index of memory formation was not affected by PGBR pellets. On the other hand, in the hippocampus, there was no significant difference in the phosphorylation of CaMKIIα and ERKs between the control and PGBR pellets-fed mice. Taken together, these results suggest that PGBR enhances the NMDA receptor/CaMKIIα signaling in the frontal cortex, leading to enhanced learning and memory in mice.
| Keywords | |
| Learning and memory; Pregerminated brown rice; NMDA receptor/CaMKIIα cascade; Frontal cortex | |
| Introduction | |
| A rice grain consists of the endosperm, bran layer, and germ layer, and is an important energy source for humans. Polished rice (PR), the staple food of Asians, is produced by eliminating the fiber-rich bran layer from unpolished rice, namely, brown rice (BR). Pregerminated brown rice (PGBR) is produced by soaking BR in water to induce partial germination. PGBR contains abundant amino acids, dietary fiber, vitamins, and minerals in the bran layer and embryo, and it tastes better than BR. We have reported the beneficial effects of PGBR on learning and memory in mice and raised a possibility that the glutamatergic system is involved in the enhancement of learning and memory by PGBR [1]. | |
| The glutamatergic system in the central nervous system is one of most important neuroregulatory systems in learning and memory [2]. For example, the dysfunction of the N-methyl-D-aspartate (NMDA) receptor induced by pharmacological or genetic techniques impairs learning and memory [3,4]. In contrast, overexpression of NMDA receptor 2B in the forebrain leads to enhanced activation of the NMDA receptor and better learning and memory in mice [5]. These findings suggest that continuous feeding with PGBR pellets may regulate learning and memory via the glutamatergic system in the brain, but the details remain unclear. | |
| In this study, firstly we examined whether the glutamatergic system plays a significant role in the PGBR-induced enhancement of learning and memory using an NMDA receptor antagonist in two behavioral tests. Next, we assessed the extracellular glutamate level by in vivo brain microdialysis experiments and glutamate content in the hippocampus and frontal cortex. Additionally, we examined the phosphorylation of calcium calmodulin kinase IIα (CaMKIIαα and extracellular signalregulated protein kinases (ERKs), because NMDA receptor activation leads to their phosphorylation, which is an important intracellular event related to learning and memory [6-8]. | |
| Materials and Methods | |
| Animals and foods | |
| Five-week-old male ICR mice (Nihon SLC Co., Shizuoka, Japan) were purchased. We received the mice from 11h00 and 13h00, then put them into the home cage and gave adequate food pellets (day 0). The animals were housed in a controlled environment (23 ± 1°C, 50 ± 5% humidity) and given access to water ad libitum. Room lights were on between 7:30 and 19:30. We used commercial pellets (AIN- 93G; Oriental Yeast, Tokyo, Japan) as the control, the ingredients of which are as follows: 39.7% cornstarch, 20% casein, 0.3% L-cystine, 13.2% α-cornstarch, 10% sucrose, 7% bean oil, 5% cellulose powder, 3.5% minerals, 1% vitamins, 0.25% choline bicitrate, and 0.0014% butylhydroquinone. The rice we used was grown in Hokkaido area in Japan [Oryza sativa subsp. japonica (Hoshino-yume)]. PGBR was prepared at 25–30% water content to induce germination and dried to 15% according to a patented procedure (Patent No. 3738025, JP, November 4, 2005). PGBR was manufactured as powdered feed by Oriental Yeast. The nutrients in the PGBR pellets were the same as those in AIN-93G, except that cornstarch was replaced with PGBR, as reported previously [1,9]. All experiments were conducted in accordance with the Guidelines for Animal Experiments of Meijo University (Approval number: Yaku-Jitsu-12) and the Guiding Principles for the Care and Use of Laboratory Animals approved by the Japanese Pharmacological Society (2007). Each experiment was carried out independently on the 29th day. | |
| Y-maze test: Short-term memory was examined by monitoring spontaneous alternation behavior in the Y-maze [1,9,10]. We used 13 mice in each group. The maze was made of wood painted black and each arm was 40 cm long, 12 cm high, 3 cm wide at the bottom, and 10 cm wide at the top. The arms converged at an equilateral triangular central area that was 4 cm at its longest axis. The apparatus was placed on the floor of the experimental room and illuminated with a 100 W bulb from 200 cm above. Each mouse was placed at the end of one arm and allowed to move freely through the maze during an 8-min session, and the series of arm entries was recorded visually. The alternation behavior was defined by the successive entry into the three arms, on overlapping triplet sets, and such behavior (%) was expressed as the ratio of actual alternations to possible alternations (defined as the total number of arm entries minus two), multiplied by 100. Dizocilpine (0- 30 μg/kg) was dissolved in 0.9% physiological saline and administered subcutaneously to mice 20 min before the test. | |
| Novel object recognition test: For the novel object recognition test, a mouse was habituated to a black plastic cage (30 x 30 x 50 cm) for 15 min for consecutive 3 days. In the training trial, two same objects (white film cases, Ø 3 x 6 cm) were placed in the cage, and the mouse was allowed to explore freely for 5 min. The time spent on approaching each object was recorded manually. In the retention trial immediately after the training trial, the mouse was removed once and then placed back in the same cage, in which one of the familiar objects used in the training trial was replaced by a novel object (black dry battery cell, Ø3 x 6 cm), and allowed to explore for 5 min. Exploratory preference, the ratio of time spent on approaching one of the two same objects in the training trial or the novel one in the retention trial over the total time spent approaching both objects, was used to measure recognition memory [approach duration (%)] [9,11]. We used 10 mice in each group. Dizocilpine (10 μg/kg) was administered subcutaneously to mice 20 min before the retention trial. | |
| In vivo brain microdialysis: One day before the microdialysis, mice were anesthetized with pentobarbital-Na (50 mg/kg, i.p.) and the dialysis probe (D-I-3-01, Eicom Corp., Kyoto, Japan) was implanted into the prefrontal cortex (+1.7 mm anteroposterior, -0.3 mm mediolateral from the bregma, -2.5 mm dorsoventral from the skull) and hippocampus (-1.6 mm anteroposterior, +1 mm mediolateral, -1.5 mm dorsoventral from the skull) according to the brain atlas [12]. The dialysis probe was perfused with artificial cerebrospinal fluid (aCSF; mM: NaCl, 127.6; KCl, 2.5; CaCl2, 1.3, pH 7.4) at a rate of 1 μL/min and connected to a microinfusion pump (Microsyringe Pump, ESP- 64, Eicom) via a single-channel liquid swivel. The mice were placed in individual acrylic cages (20 × 30 × 25 cm high) and allowed to adapt for at least 60 min before the experiment was started. About 3 h after the probe was inserted, samples (10 μL) were collected at 10-min intervals. The locations of dialysis probes were confirmed after the experiments. | |
| Glutamate in the dialysate was quantified using an HPLC-ECD system (HTEC-500, Eicom) with an immobilized enzyme column (E-Enzympak, Eicom). The mobile phase consisted of 0.1 M ammonium chloride buffer (pH 7.2) containing 10% ammonia solution, and 685 μM hexadecyltrimethylammonium bromide was delivered at a flow rate of 400 μL/min. To protect the analytical column from impurities in the mobile phase and samples, a precolumn (CH-GEL, Eicom) was placed between the pump and the injector, and the column temperature was maintained at 25°C. Aliquots (10 μL) of the perfusate samples were injected into the HPLC system and separated by a column of Eicompak Glu-Gel (Ø 4.6 × 150 mm, Eicom). The column containing glutamate oxidase catalyzed the formation of hydrogen peroxide from glutamate and oxygen. The resultant hydrogen peroxide was detected by ECD with a platinum working electrode at +450 mV. | |
| Glutamate content: Mice were sacrificed by focused microwave irradiation for 1.4 sec at 5 kW. The brains were quickly removed, and the hippocampus and frontal cortex were dissected out on an ice-cold glass plate as in our previous report [13]. Each frozen brain sample was weighed and homogenized with an ultrasonic processor in 0.1 M ammonium chloride buffer (pH 7.2) containing 10% ammonia solution and 685 μM hexadecyltrimethylammonium bromide. The homogenates were centrifuged at 15,000 x g for 15 min at 4°C. The supernatants were centrifuged by ultrafiltration (Ultrafree-MC, 5000 NMWL, Millipore, USA) at 5,000 x g for 20 min at 4°C, then aliquots (10 μL) were injected into the HPLC-ECD system (HTEC-500, Eicom). The HPLC-ECD system condition was similar to that in the microdialysis analysis. | |
| Western blot analysis of CaMKIIα and ERK phosphorylation: The mice were decapitated, and the hippocampus and frontal cortex were dissected (5 mice in each group). Each brain tissue was homogenized by sonication in ice-cold buffer (50 mM Tris-HCl, 150 mM NaCl, 10 mM NaF, 10 mM EDTA, 1 mM sodium orthovanadate, 2 μg/mL pepstatin, 2 μg/mL leupeptin, 2 μg/mL aprotinin, 1 mM PMSF, and 1 mM DTT, pH 7.4). The samples were boiled in Laemmli sample buffer, separated on 7.5% polyacrylamide gel and subsequently transferred to PVDF membranes (Millipore). The membranes were blocked with an ECL Blocking Agent (ECL Plus Western Blotting Detection Reagents, GE Healthcare, USA) for 2 h at room temperature and probed with a monoclonal antibody to the phospho-CaMKIIα subunit at Thr286 [0.2 μg/ml, Affinity BioReagents, Inc. (Thermo Fisher Scientific), USA] or a polyclonal antibody to phospho-p44/42 MAP kinase at Thr202/Tyr204 (0.1 μg/mL, Cell Signaling Technology, USA) overnight at 4°C. The membranes were washed with TBST buffer (10 mM Tris-HCl, 150 mM NaCl, and 0.1% Tween 20, pH 7.4) and subsequently incubated with a goat anti-rabbit horseradish peroxidase-conjugated secondary antibody for 2 h at room temperature. The immune complexes were detected by chemiluminescence immunoassay (GE Healthcare) and exposed to an X-ray film. The band intensities of the film were analyzed by densitometry (GelDoc 2000, BioRad, USA). To confirm equal loading of each protein, the membranes were stripped with stripping buffer (62.5 mM Tris-HCl, 100 mM 2-mercaptoethanol, and 2% SDS, pH 6.7) at 50°C for 5 min. They were then incubated with a monoclonal antibody to the CaMKIIα subunit [0.2 μg/mL, Affinity BioReagents, Inc. (Thermo Fisher Scientific)] or an anti-p44/42 MAP kinase polyclonal antibody (0.1 μg/mL, Cell Signaling Technology) and analyzed as described above [7,14]. | |
| Data analysis: All results were expressed as means ± SEM for each group. All data were analyzed by one-way ANOVA, followed by Tukey’s or Student’s t-tests. The level of significant difference was taken at P<0.05. | |
| Results | |
| Y-maze test | |
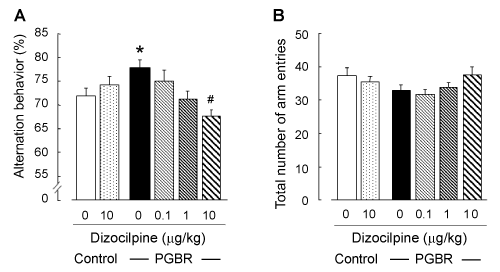
| As shown in Figure 1A, there was a significant enhancement of alternation behavior in the PGBR- pellet-fed mice compared with the control pellets-fed mice (F5,77=8.04, P<0.001, Tukey’s test; P<0.01). In the control-pellet-fed mice, dizocilpine (10 μg/kg) did not affect this behavior (Figure 1A), but at 30 μg/kg, it induced remarkable reduction (data not shown), as previously reported [10]. In the PGBR-pellet-fed mice, dizocilpine antagonized the effects of PGBR pellets in a dosedependent manner (Tukey’s test; P<0.05). On the other hand, statistical analysis revealed that the total number of arm entries was not affected by PGBR pellets or dizocilpine (F5,77=1.58, P=0.176, Figure 1B). | |
| Novel object recognition test | |
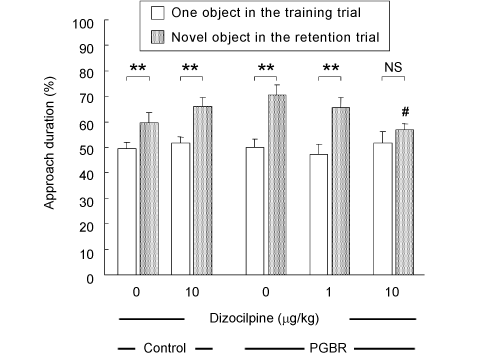
| There was no significant difference in approach duration (exploratory preference) in the training trial among the groups (white columns; F4,49=0.265, P=0.89), indicating that these groups essentially had the same levels of curiosity and/or motivation to explore the two same objects. In the retention trial, however, all groups exhibited greater preference toward the novel object than the familiar object (dotted columns). Approaching a novel object for a longer duration was inhibited by dizocilpine (10 μg/kg) in the PGBR-fed mice but not in the control-pellet-fed mice (F2,29=3.92, P=0.031, Tukey’s test; P<0.05) (Figure 2). | |
| Extracellular glutamate level | |
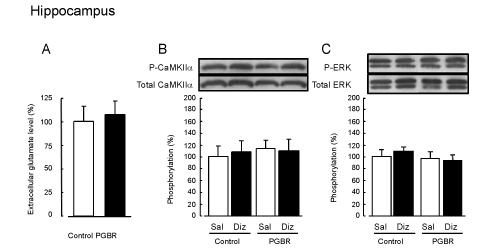
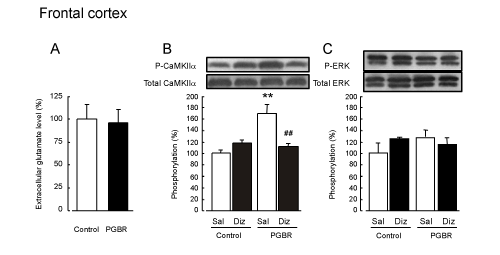
| The average basal levels of glutamate were 1.45 ± 0.19 and 6.97 ± 0.96 pmol/min in the hippocampus and frontal cortex of the controlpellet- fed mice respectively. There were no significant differences in accumulation for 60 min between the control- and PGBR-pellets-fed mice in both brain regions (Figures 3A and 4A). | |
| Glutamate content | |
| There were no significant differences in glutamate content between the control- and PGBR- pellet-fed mice in both brain regions [hippocampus: Control, 1912.9 ± 77; PGBR, 1803.9 ± 52 nmol/g wet tissue (F1,9=1.61, P>0.05); frontal cortex: Control, 1674.3 ± 43; PGBR, 1560.7 ± 22 nmol/g wet tissue (F1, 9=4.38, P>0.05)]. | |
| Western blot analysis of CaMKIIα and ERK phosphorylation | |
| CaMKIIα: There were no significant differences in CaMKIIα phosphorylation in the hippocampus between the groups we examined (F3,19=0.12, P=0.95, Figure 3B). On the other hand, in the frontal cortex of the Sal-treated mice, PGBR enhanced significantly CaMKIIα phosphorylation compared with that of the control-pellet-fed mice (F3,19=14.3, P<0.01, Tukey’s test; P<0.01, Figure 4B). In the dizocilpinetreated mice, the enhanced CaMKIIα phosphorylation was inhibited to the same level as that in the control treated with saline, whereas dizocilpine did not affect the phosphorylation in the control. | |
| ERK: On the other hand, we observed no marked changes in ERK phosphorylation in both the hippocampus (F3,19=1.58, P=0.23) and frontal cortex (F3,19=0.68, P=0.58) of the four groups (Figure 3C and 4C). | |
| Discussion | |
| Here, we confirmed the effects of continuous PGBR feeding on learning and memory in mice in two independent behavioral tests. We also investigated the implication of the glutamatergic system in PGBRinduced enhancement of learning and memory in the brain. | |
| As expected from our previous study [1], the PGBR-pellet-fed mice showed a more enhanced learning and memory in the two tests than in the control-pellet-fed mice. Therefore, to investigate whether the NMDA receptor is involved in those enhancements, we injected dizocilpine, an NMDA receptor antagonist, to the mice and subjected them to the Y-maze and novel object recognition tests. Dizocilpine (10 μg/kg) antagonized these enhancements in the PGBR-pellet-fed mice without exerting any abnormal behaviors by itself in the control. Taken together, it is possible that the PGBR-enhanced learning and memory in both tests may be accompanied by an increase in the activity of the endogenous glutamatergic system. Therefore, we assessed the glutamate release by in vivo brain microdialysis and the glutamate content in the frontal cortex and hippocampus. In both regions, there was no significant difference between the control- and PGBR-fed groups, suggesting that the synthesized and/or stored glutamate in presynapses and presynaptic releasing function may be similar in both groups and that postsynaptic changes may occur in the PGBR-fed group. | |
| Accordingly, we examined the effects of PGBR on NMDA receptor function and signal transduction through the NMDA receptor. The two potential targets for immediate activation mediated by the NMDA receptor are CaMKII [15,16] and ERK [7,17]. Therefore, we measured CaMKIIα and ERK phosphorylation to assess the postsynaptic NMDA receptor function. CaMKIIα phosphorylation in the frontal cortex was markedly potentiated in the PGBR-fed mice compared with the control, but not ERK phosphorylation. Dizocilpine inhibited this increased phosphorylation in the PGBR-fed mice. In the hippocampus, we could not find any significant difference in the phosphorylation of CaMKIIα or ERK between the two groups. On the basis of the behavioral and biochemical results, it is likely that the NMDA receptor/CaMKII cascade, but not the NMDA receptor/ERK cascade, may be enhanced functionally in the frontal cortex of PGBR-fed mice. | |
| Previously, we reported that continuous intake of PGBR also increases the serotonin content in the learned helplessness paradigm [18]. It has been reviewed that one of main energy sources in PGBR, glucose, modifies the brain function [19]. In addition to the function of PGBR in the brain, it decreases blood cholesterol level in rats [20], and it has been clarified that acylated steryl glucosides function as bioactive constituents in PGBR [21]. As our next step, we will try to examine the roles of PGBR constituents in the brain. | |
| We conclude that PGBR has the potential to enhance the learning and memory capabilities; in particular, it activates the glutamatergic system in the frontal cortex. Taken together, these results suggest that PGBR activates the NMDA receptor/CaMKIIα cascade in the brain, which leads to the enhancement of learning and memory ability in mice. | |
| Acknowledgement | |
| This work was supported by JSPS KAKENHI (Grant Numbers 24590304 and 22790233); the Nakatomi Foundation; the Takeda Science Foundation; the Sasakawa Scientific Research Grant from the Japan Science Society; and the Aichi Health Promotion Foundation. We are thankful to the late Professor Makoto Ukai, and Messrs. Keita Onogi, Yuya Hasegawa, Yoshiaki Kawai and Takamasa Asanuma for help in the experiment. | |
| Conflict of Interest | |
| The authors declare no conflict of interest. | |
References |
|
|
|
Figures at a glance
 |
 |
 |
 |
|||
| Figure 1 | Figure 2 | Figure 3 | Figure 4 |
Relevant Topics
- Basmati Rice
- Drought Tolerence
- Golden Rice
- Leaf Diseases
- Long Grain Rice
- Par Boiled Rice
- Raw Rice
- Rice
- Rice and Aquaculture
- Rice and Nutrition
- Rice Blast
- Rice Bran
- Rice Diseases
- Rice Economics
- Rice Genome
- Rice husk
- Rice production
- Rice research
- Rice Yield
- Sticky Rice
- Stress Resistant Rice
- Unpolished Rice
- White Rice
Recommended Journals
Article Tools
Article Usage
- Total views: 14528
- [From(publication date):
August-2014 - Apr 01, 2025] - Breakdown by view type
- HTML page views : 9940
- PDF downloads : 4588
