Predictive and Prognostic Value of ALK Gene Rearrangement in Non- Small Cell Lung Cancer
Received: 03-Dec-2013 / Accepted Date: 26-Jan-2014 / Published Date: 28-Jan-2014 DOI: 10.4172/2161-1165.1000146
Abstract
Anaplastic Lymphoma Kinase (ALK) is a relatively new as an oncogenic driver and a therapeutic target in Non-Small Cell Lung Cancer (NSCLC); the prognostic and predictive implications of ALK-positivity in NSCLC is far from clear and no large-scale studies have been reported to date. In the current review, we summarize published data examining the variation in prognostic and predictive effect of ALK-positivity on clinical outcomes in NSCLC patients, based on the extent of control of or adjustment for known confounding factors such as smoking status, disease stage, and age by study design or analyses. ALK rearrangement in NSCLC did not appear to be predictive of improved outcomes with chemotherapy but was predictive of poor response to EGFR TKI therapy. Overall, ALK rearrangement was found to be a negative prognostic factor in NSCLC in studies controlling for known confounding factors. In addition to highlighting the importance of controlling for confounding factors in retrospective studies evaluating outcomes, our review also summarizes evidence of an unmet need in terms of poor response amongst ALK-positive NSCLC patients to standard therapies that do not target ALK.
Keywords: Anaplastic Lymphoma Kinase (ALK); Non-Small Cell Lung Cancer (NSCLC); Survival; Clinical Outcomes; Prognostic; Predictive; Review
161370Introduction
Anaplastic Lymphoma Kinase (ALK) is relatively new in terms of an oncogenic driver and a drug target in Non-Small Cell Lung Cancer (NSCLC); however, little is known about the “natural history” of ALKrearranged NSCLC. Some investigators have speculated that it may represent a more indolent disease [1,2] or be an independent positive prognostic factor [3]. Others have suggested that ALK rearrangement may be a negative prognostic factor when controlling for known factors such as age, sex, smoking status, stage/grade, and histology [4-6]. With the advent of ALK-specific therapies and crossover in clinical trials, it is unlikely that the natural history of ALK-rearranged (ALKpositive) NSCLC can be examined in an unbiased manner moving forward. However, a handful of retrospective studies examining the outcomes with conventional therapy in ALK-positive NSCLC have been published or presented at scientific meetings. Here we review data from these retrospective studies, exclusive of those involving ALK inhibitor therapy, with the goal to evaluate historical survival outcomes and treatment outcomes from chemotherapy, EGFR Tyrosine Kinase Inhibitor (TKI) therapy, surgical therapy, and thoracic radiotherapy in ALK-positive NSCLC.
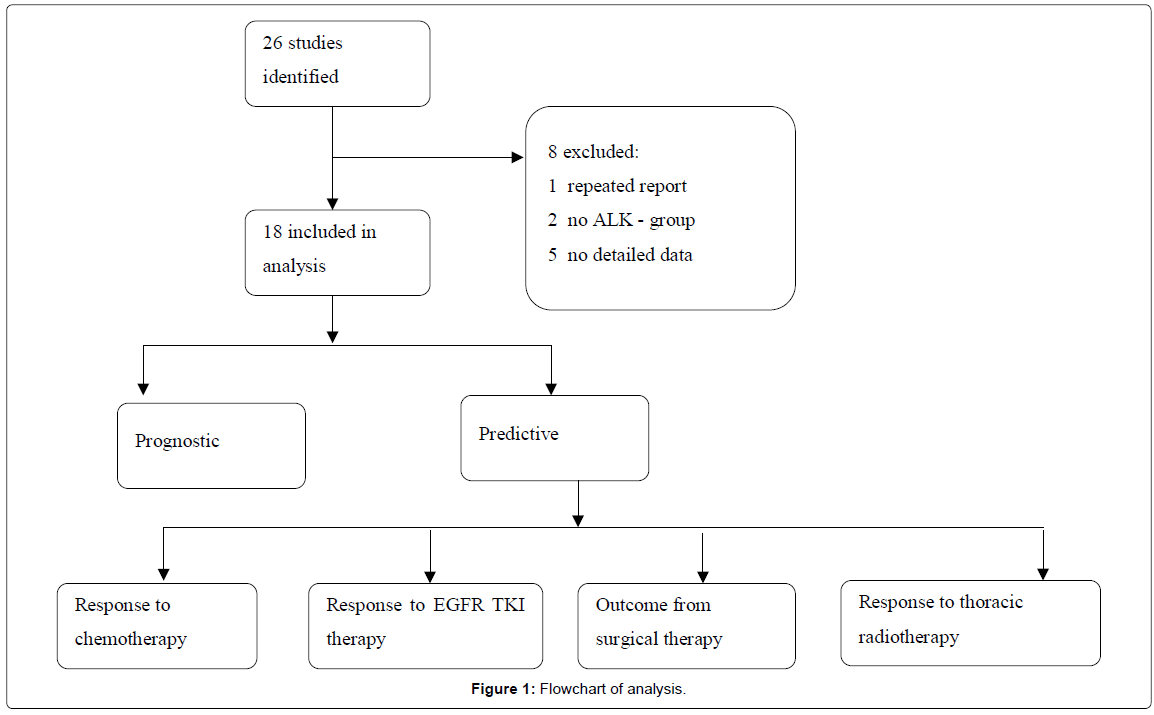
Methods
We searched published literature in English in peer- reviewed journals indexed in Pub Med, Google Scholar and presentations at conferences from July 2007 to Nov 2013 that had an observational study design assessing the predictive and prognostic value of ALK in NSCLC, and that tested for ALK status using various diagnostic tests including fluorescent in situ hybridization (FISH), Immunohistochemistry (IHC), or polymerase chain reaction (PCR). A total of 26 publications were identified and 8 were excluded, where two were reported from the same cohort [7,8]. Five studies reported only the outcome or gave a conclusion but did not have enough study description or data details [3,9-13]. Two studies had no confirmed ALK-negative comparator groups [1,14]. Aggregate data are summarized, comparing survival outcomes between ALK-positive versus ALK-negative NSCLC patients followed by an evaluation of responses with current non-ALK-targeted therapies (Figure 1). Clinical outcomes considered were Overall Survival (OS), Progression-Free Survival (PFS), Recurrence-Free Survival (RFS), Disease-Free Survival (DFS), Time to Progression (TTP), and Objective Response Rate (ORR). Studies were reviewed with a focus on the use of techniques within the study to control by study design and/or adjust with statistical methods for confounding factors that could impact the outcomes being investigated.
Results
ALK gene rearrangement as a prognostic biomarker in NSCLC
Summary of studies with no reported control of or adjustment for confounding factors: Six studies have examined OS in ALKpositive compared with ALK-negative, EGFR wild type (WT/WT) cases and two other studies examined OS in ALK-positive versus ALKnegative cases in which EGFR status was unknown. With a median follow-up time of 13 months at the time of analysis, Shaw et al. reported a median OS of 20 months in ALK-positive cases and 16 months in WT/WT cases (p=0.152; Table 1) [15]. In another study, which was an indirect comparison of OS between ALK-positive, crizotinib-naive cases and WT/WT controls, Shaw et al., reported the median OS from time of metastatic diagnosis as similar for the ALK-positive and WT/ WT cases at 20 and 15 months, respectively (unadjusted HR 0.77; p=0.244) [2]. Although an additional OS subset analysis conducted on cases ≤ 60 years old who were never- or light-smokers accounted to some degree for age and smoking status and showed median OS of 20 versus 24 months for ALK-positive and WT/WT cases, respectively (HR=1.01; p=0.978) [2], it was not formally controlled for all potential confounding variables including histology and treatment. With a median follow-up time of 10.8 months, Takeda et al., reported a median OS of 15.7 months in ALK-positive cases and 15.2 months in WT/ WT cases (HR=0.83, p=0.591; Table 1) [16]. Wang et al., reported a median OS of 19.27 months in 9 ALK-positive cases and 18.93 months in 45 WT/WT cases (p=0.481, Table 1) [17]. Martinez et al., reported a median OS of 4.5 months for WT/WT cases (n=65) versus a median OS not reached for 7 ALK-positive cases (p=0.103) and 15.7 months in 13 EGFR mutant cases (p=0.018) [8]. No statistically significant differences were found in median OS between groups in all these studies.
| Study | ALK+ (N)/Total (N) | Efficacy |
|---|---|---|
| Studies with no control or adjustment for confounding factors | ||
| Shaw et al. [15] | 17 ALK+ /96 never/light smokers, stage IV |
Median OS: • 20 months in ALK+a • 32 months in EGFR mu (p = 0.468 vs.ALK+) • 16 months in ALK−/EGFR WT (p = 0.152 vs.ALK+) |
| Shaw et al. [2] | 36 ALK+/356 advanced |
Median OS: • 20 months in ALK+ • 15 months in ALK−/EGFR WT (p = 0.244 vs.ALK+) |
| Takeda et al. [16] | 18 ALK+/200 advanced non squamous cases |
Median OS† : • 15.7 months in ALK+ • 24.8 months in EGFR mu (p = 0.135 vs. ALK+) • 15.2 months in ALK−/EGFR WT (p = 0.591 vs. ALK+) |
| Wang et al. [17] | 9 ALK+/113 stage IV |
Median OS: • 19.27 months in ALK+ • 23.13 months in EGFR mu • 18.93 months in ALK−/EGFR WT (p = 0.481 vs. ALK+ and EGFR mu) |
| Hayashi et al. [18] | 3 ALK+/37 locally advanced adeno cases |
Median OS: • 7.7 months in ALK+ • 67.5 months in EGFR mu • 42.6 months in ALK−/EGFR WT (p = 0.007 vs. ALK+) |
| Martinez et al. [8] | 7 ALK+/99 Non squamous ,all stages |
Median OS: • not reached in ALK+b • 15.7 months in EGFR mu • 4.5 months in ALK−/EGFR WT (p =0.103 vs. ALK+) |
| Paik et al. [19] | 28 ALK+/735 stage I-III |
Median OS: • 97.7 months in ALK+ • 78.9 months in ALK− (p = 0.10 vs. ALK+) |
| Fukui et al. [20] | 28 ALK+/720 Adeno resected cases, all stages |
5-year OS rate: • 81% in ALK+ • 77% in ALK− (p = 0.76 vs. ALK+) |
| Studies with control or adjustment for confounding factors | ||
| Lee et al. [6] | 23 ALK+ /262 non-squamous EGFR WT or TKI non-responders, stage IIIb–IVb |
Median OS: • 12.23 months in ALK+ • 29.63 months in EGFR mu (p = 0.001 vs. ALK+) • 19.33 months in WT/WT (p = 0.127 vs. ALK+) |
| Yang et al. [4] | 22 ALK+/296never-smoker, adenocasesc 9=stage I/II 7=stage III 6=stage IV |
DFS not reported in either ALK+ or ALK− groups (2-fold greater risk of progression or recurrence within 5 yrs of diagnosis reported in ALK+ vs.ALK− cases, p=0.004) |
| Kim et al. [5] | 13 ALK+/229 never-smokers, all stagesd |
Median OS: • 14.3 months in ALK+ • 37.2 months in EGFR mu (p = 0.001 vs. ALK+) • 33.3 months in ALK−/EGFR WT/KRAS WT (p = 0.016 vs. ALK+) |
| Wu et al. [21] | 39 ALK+/116 adeno cases, stage IVe |
Median OS: • 14.7 months in ALK+ • 10.3 months in ALK−/EGFR WT (p = 0.011 vs. ALK+) |
Table 1: Overall Survival.
Hayashi et al., reported a median OS in locally advanced adenocarcinoma patients of 7.7 months in 3 ALK-positive cases and 42.6 months in 23 WT/WT cases (p=0.007; Table 1) [18]. Fukui et al. selected adenocarcinoma cases who underwent pulmonary resection and reported the 5-year OS rate for ALK-positive patients was 81%; whereas, the ALK-negative (EGFR status unknown) was 77% (p=0.76) [19,20].
In early stage lung cancer patients, Paik et al. reported a median OS in stage I-III NSCLC patients of 97.7 months in ALK-positive cases and 78.9 months in ALK-negative (EGFR status unknown) cases (p=0.10) [19].
Summary of studies with reported control of or adjustment for confounding factors: Four studies to date have, a priori, matched or controlled for important independent prognostic factors. Three of them suggest or clearly demonstrate a shorter OS or DFS for ALK-positive versus ALK-negative cases and one stated a prolonged OS in ALKpositive cases. The case-matched analysis by JK Lee et al., reported a median OS in stage IIIb-IV cases of 12.23 months in ALK-positive (n=23), 29.63 months in EGFR mutant (n=46) and 19.33 months in WT/ WT (n=46) cases (p=0.001 versus EGFR mutant; p=0.127 versus WT/ WT) [6]. Yang et al., with selection of never-smoker, adenocarcinoma cases and control for age, sex, stage, and treatment, showed more than a 2-fold greater risk of recurrence or progression within 5 years of diagnosis in ALK-positive (n=22) versus ALK-negative (EGFR status unknown) cases (n=274; p=0.004; Table 1) [4]. In the same study, a higher rate of extra-thoracic metastasis was observed among ALKpositive cases compared with ALK-negative cases, (HR=2.44, p=0.03); albeit, the number of later stage patients in this analysis was limited (n=13). With selection of never-smokers and comparator groups which were balanced in terms of age, sex, histology, stage and performance status (PS), Kim et al., reported a shorter median OS of 14.3 months in ALK-positive cases compared with 33.3 months in ALK-negative, EGFR WT and KRAS WT (triple WT), and 37.2 months in EGFR mutant (ALK-negative) cases (p=0.016 for ALK-positive versus triple WT) [5]. In the same study, in multivariate analysis, ALK-positivity was associated with a lower OS in patients with resected NSCLC (adjusted HR, 4.162; p=0.005). The authors suggested that ALK-positivity may be a negative prognostic factor for early stage NSCLC. Wu et al., examined survival outcomes in lung adenocarcinoma patients with malignant pleural effusions and wild type EGFR in which ALK-positive and ALKnegative comparator groups were balanced in terms of age, sex, smoking history, PS, and treatment, and reported a longer median OS of 14.7 months in ALK-positive cases (n=39) compared with 10.3 months in WT/ WT cases (n=77, HR=0.53, p=0.011) [21]. The authors concluded that ALK translocation is associated with longer overall survival in lung adenocarcinoma EGFR–WT patients.
ALK gene rearrangement as a predictive biomarker
Six studies published to date report response to platinum-based chemotherapy in ALK-positive NSCLC (Table 2) [5,6,15-17,22]. Two of these studies controlled for or matched cases on potential confounding factors, while the other four did not. Four other studies, representing varying degrees of balance or control for confounding factors, reported response to pemetrexed either as a single agent [23,24], or in combination (Table 3) [25,26]. Five studies to date reported the efficacy of EGFR TKI therapy in ALK-positive NSCLC and four of them showed a 0% response rate [5,6,15,22]. Four studies reported survival outcomes in ALK-positive compared with ALK-negative patients who underwent surgical resection [5,19,20,27]. One of these studies balanced on clinically relevant factors [5].
| Chemotherapy regimens | EGFR TKI regimensa | |||||
|---|---|---|---|---|---|---|
| Study | ALK+ (N) | Regimen/ Line of treatment | Efficacy | ALK+ (N) | Response rate | Efficacy |
| Studies with no control or adjustment for confounding factors | ||||||
| Shaw et al. [15] | 12 ALK+ metastatic cases evaluable for chemo | 1st line platinum-based chemo | Median TTP reported as “in the range of 8–10 months” across ALK+, EGFR mu, and WT/WT | 10 ALK+ stage IV |
• 0% in ALK+ • 70% in EGFR mu • 13% in WT/WT |
Median TTP: • 5 months in ALK+ • 16 months in EGFR mu • 6 months in WT/WT |
| Koh et al. [22] | 32 ALK+ advanced adeno cases | 1st line platinum-based doublet chemo | Median PFS: • 6.2 months in ALK+ • 5.4 months in EGFR mu • 7.3 months in WT/WT |
16 ALK+ advanced adeno | • 0% in ALK+ • 50% in EGFR mu • 6.9% in WT/WT |
Median PFS: • 4.3 weeks in ALK+ • 19.6 weeks in EGFR mu • 6.0 weeks in WT/WT |
| Wang et al. [17] | 4 ALK+ stage IV | 1st and 2nd line platinum-based doublet chemo | Median PFS:c • 8.3 months in ALK+ • 4.1 months in EGFR mu • 4.9 months in WT/WT |
9 ALK+ stage IV |
• 33.3% in ALK+ • 46.9% in EGFR mu • 16.3% in WT/WT |
Median PFS: • 2.1 months in ALK+ • 8.8 months in EGFR mu • 2.2 months in WT/WT |
| Takeda et al. [16] | 18 ALK+ advanced non squamous cases |
1st line platinum-based chemo | Median PFS:c • 6.5 months in ALK+ • 6.0 months in EGFR mu • 4.3 months in WT/WT |
Not studied | ||
| Studies with control or adjustment for confounding factors | ||||||
| Lee et al. [6] | 21 ALK+ matched to 34 EGFR mu and 37WT/WT cases all stage IIIb–IVd | 1st line chemob | Median PFS • 3.87 months in ALK+ • 4.93 months in EGFR mu • 3.73 months in WT/WT |
10 ALK+ matched to 42 EGFR muand 27 WT/WTd | • 0% in ALK+ • 80.9% in EGFR mu • 14.8% in WT/WT |
Median PFS: • 1.37 months in ALK+ • 9.80 months in EGFR mu • 2.07 months in WT/WT |
| Kim et al. [5] | 12 ALK+ never-smokers, all stagese |
1st-line platinum-based chemo | Median PFS: • 5.0 months in ALK+ • 7.1 months in EGFR mu • 7.2 months in KRAS mu • 5.9 months in WT/WT/WT |
8 ALK+ never-smokers, all stagese | • 0% in ALK+ • 65.5% in EGFR mu • 0% in KRAS mu • 10.3% in WT/WT/WT |
Median PFS: • 1.6 months in ALK+ • 12.8 months in EGFR mu (p<0.001 vs.ALK+) • 2.1 months in KRAS mu • 6.3 months in WT/WT/WT (p=0.001 vs.ALK+) |
Table 2: Median PFS or TTP with Chemotherapy and EGFR TKI Regimens.
| Study | ALK+ (N)/ALK− (N) | Regimen and Line of Treatment | Efficacy | |
|---|---|---|---|---|
| ALK+ | ALK− | |||
| Studies with no control or adjustment for confounding factors | ||||
| Camidge et al. [25] | 19/37 | Pemetrexed alone or in combination any line | Median PFS: • 9 months |
Median PFS:b • 4 months |
| Lee et al. [23] | 15/37 | Pemetrexed mono therapy ≥ 2nd line | Median TTP: • 9.2 months (overall) • 9.2 months (2nd-line) • 6.4 months (≥ 3rd-line) |
Median TTP:a • 2.9 months (overall) • 2.7 months (2nd-line) • 4.0 months (≥ 3rd-line) |
| Lee et al. [24] | 32/unknown | Pemetrexedmonotherapy ≥ 2nd line | Median PFS: • 4.0 months |
Median PFS: • 1.6 months |
| Shaw et al. [26] | 70/112 | Platinum-pemetrexed any line | Median PFS: • 7.3 months |
Median PFS:b • 5.9 months |
| 58/99 | Platinum-pemetrexed any line, ≤ 65 yr | Median PFS: • 8.1 months |
Median PFS:a • 6.0 months |
|
| 64/45 | Platinum-pemetrexed any line, never/light smoking | Median PFS: • 7.3 months |
Median PFS:a • 7.5 months |
|
| 56/44 | Platinum-pemetrexed 1st line | Median PFS: • 8.5 months |
Median PFS:b • 5.4 months |
|
| 53/40 | Platinum-pemetrexed 1st line, never/light smoking | Median PFS: • 8.5 months |
Median PFS:a • 7.4 months |
|
| 51/75 | Pemetrexed alone or no-platinum combination any line | Median PFS: • 5.5 months |
Median PFS:b • 3.9 months |
|
| 41/57 | Pemetrexed alone or no-platinum combination any line, ≤ 65 yr | Median PFS: • 5.1 months |
Median PFS:a • 4.4 months |
|
| 44/34 | Pemetrexed alone or no-platinum combination any line, never/light smoking | Median PFS: • 5.5 months |
Median PFS:a • 5.3 months |
|
| 30/27 | Pemetrexed alone any line, never/light smoking | Median PFS: • 4.8 months |
Median PFS: a • 4.6 months |
|
| 31/39 | Pemetrexed alone or no-platinum combination ≥ 2nd line | Median PFS: • 4.4 months |
Median PFS:b • 3.8 months |
|
Table 3: Response to pemetrexed.
Summary of studies with no reported control of or adjustment for confounding factors
Analyses by Koh et al. [22] and Shaw et al. [15] showed similar ORR, PFS or TTP on chemotherapy in retrospectively-identified cohorts of patients with ALK-positive versus WT/WT NSCLC cases (Table 2). Koh et al. [22] reported median PFS with first-line platinumbased doublet therapy of 6.2 months in ALK-positive (n=32) versus 7.3 months in WT/WT cases (n=57), with ORR of 18.8% and 40.4%, respectively. Both PFS and ORR were reported as statistically nonsignificant. This analysis was not balanced for age, with patients in the ALK-positive cohort being statistically significantly younger than WT/WT patients (median 49 versus 61 years, respectively; p<0.001). Similarly, Shaw et al., reported a median TTP with first-line platinum-based chemotherapy in the range of 8-10 months for patients with ALKpositive, EGFR mutant, and WT/WT disease [15]. The chemotherapy ORR in this study was 25% for ALK-positive and 35% for WT/WT cases with no statistically significant difference between these groups (p=0.723). This cohort was not balanced for age, sex, smoking history or exposure to ALK inhibitor therapy, which would have had an impact on clinical outcome. In addition to these two studies, Takeda et al reported the median PFS with first-line platinum-based chemotherapy of 6.5 months in 18 ALK-positive versus 4.3 months in 151 WT/WT cases (p=0.437), with an ORR of 44% and 39%, respectively [16]. Both PFS and ORR were reported as statistically non-significant. Similarly, this analysis was not balanced for age, with patients in the ALK-positive cohort being statistically significantly younger than WT/WT patients (median 46 versus 64 years, respectively; p<0.001). The regimens were also not balanced between the comparator groups. The proportion of patients in ALK-positive and WT/WT groups treated with platinum plus pemetrexed were 39% and 17%, respectively (p=0.049). These would have had an impact on clinical outcome. Wang et al reported median PFS with first-line and second-line platinum-based doublet therapy of 8.3 months in ALK-positive versus 4.9 months in WT/ WT cases (p=0.25) [17]. The chemotherapy ORRs were 25.0% and 32.4%, respectively (p=0.762). Both ORR and PFS were not statistically significant. This study was not balanced in age, smoking status and other important prognostic factors between ALK-positive and WT/ WT groups.
Response to pemetrexed-based therapy: Camidg et al., Lee et al. and Lee et al. each retrospectively studied ALK-positive NSCLC response to pemetrexed-based therapy, yielding similar results (Table 3) [23-25]. Lee et al. examined single-agent pemetrexed response in second-line therapy or beyond and reported an overall median TTP of 9.2 months (95% CI 4.65-13.74 months) in ALK-positive (n=15) versus 2.9 months (95% CI of 0.51- 5.28 months) for WT/WT (n=37) and 1.4 months (95% CI of 1.27-1.52 months) in EGFR mutant cases (n=43, p=0.001) [23]. In the treatment-line stratified multivariate analysis, ALK-positivity was an independently significant predictor for TTP (HR=0.44; p=0.005). However, there were imbalances in the ALKpositive versus WT/WT groups in this cohort with respect to: (1) the majority (60%) of ALK-positive cases were in their third line of therapy or beyond compared with 29.7% of WT/WT cases and (2) the median age of ALK-positive cases was the youngest at 52 years. The authors did take the line of therapy into consideration by stratifying TTP by second or ≥ 3 lines of therapy; however, this step reduced the ALK-positive sample sizes to 6 and 9 patients, respectively [23].
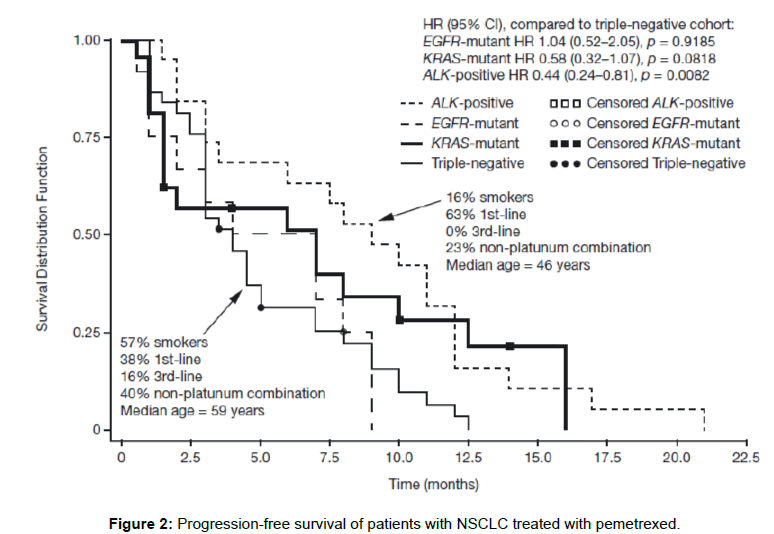
Camidge et al. assessed response to pemetrexed as single agent or in combination in metastatic NSCLC across all lines of therapy. A median PFS of 9 months (95% CI 3-12 months) in 19 ALK-positive cases compared with 4 months (95% CI 3-5 months) in 37 triple WT cases was observed [25]. Similar to the analysis of Lee et al., the only significant variable associated with prolonged PFS in a multivariate analysis was ALK-positivity (HR=0.36; p=0.0051). Important imbalances in the comparator groups in this analysis are depicted in Figure 2, Camidge et al. were careful to state that multiple other confounding factors may contribute to the longer PFS observed in the ALK-positive group [25].
Lee et al. examined single-agent pemetrexed response in secondline therapy or beyond and reported a median PFS of 4.0 months (95% CI 2.2-5.8 months) in 32 ALK-positive cases compared with 1.6 months (95% CI 1.0-2.2 months) in WT/WT cases [24]. Compared with the ALK-negative patients, ALK-positive patients were significantly younger (median age, 62 versus 50 years, respectively), with a higher proportion of never or light smokers (32.9% versus 73.9%, respectively; p=0.002). The authors stated that the difference in PFS between the ALK-positive and WT/WT cases should be interpreted cautiously.
Recently, Shaw et al., studied ALK-positive NSCLC response to pemetrexed-based therapy stratified for treatment line, age, smoking status, and different regimen [26]. The authors assessed response to pemetrexed-platinum combination regimen and reported the median PFS of 7.3 months in ALK-positive versus 5.9 months in WT/WT cases (p=0.182). Although an additional PFS subgroup analysis was conducted on first line treatment patients age ≤ 65 years old with never/ light smoking history accounted to some degree for confounders; it was not formally controlled for all potential confounding variables in the same sub group (Table 3). Only in the subset of patients who received pemetrexed–platinum combination as first line treatment, was there a statistically significant difference in median PFS among ALK-positive and WT/WT patients (p=0.018). All other subsets had no statistically significant differences between ALK-positive and WT/WT groups. The authors also examined single-agent pemetrexed or no-platinum combination response and reported a median PFS of 5.5 months in ALK-positive cases compared with 3.9 months in WT/WT cases (p=0.409). An additional PFS subgroup analysis was conducted on second or third line treatment, age ≤ 65 years old, never/light smoking history and pemetrexed alone with never/light smoking history (Table 3). In all the subsets of patients who received single-agent pemetrexed orno-platinum combination, no statistically significant differences were found in median PFS between ALK-positive and WT/WT groups.
Response to EGFR TKI therapy: Koh et al. reported a median PFS with EGFR TKI therapy of 4.3 weeks in ALK-positive (n=16) versus 6.0 weeks in WT/WT (n=29) and 19.6 weeks in EGFR mutant (n=18) cases (p<0.001) [22]. Shaw et al. measured median TTP as 5 months in ALK-positive, 6 months in WT/WT, and 16 months in EGFR mutant cases (p=0.004 for ALK-positive versus EGFR mutant; Table 2) [15]. Wang et al. reported a median PFS of 2.1 months in ALK-positive versus 2.2 months in WT/WT and 8.80 months in EGFR mutant cases (p=0.696 for ALK-positive versus WT/WT; p=0.032 for ALK-positive versus EGFR mutant) [17]. As mentioned above, these three studies had imbalances relative to age and smoking status, which may have confounded median PFS/TTP estimates. Additionally, in the study of Wang et al, there were only 9ALK-positive cases among the patients received EGFR TKI therapy
Outcome from surgical therapy: With selection of surgically resected stage I-III NSCLC patients, Paik et al. reported a median DFS of 76.4 months in ALK-positive and 71.3 months in ALK-negative (EGFR status unknown) cases (p=0.66) [19]. The authors suggested ALK-positivity may not be a predictive factor in the early (surgically resectable) stages of NSCLC. However, this analysis was not balanced for gender, age, smoking status and histology. Fukui et al. selected adenocarcinoma patients with primary lung cancer who underwent pulmonary resection and reported the 5-year DFS rate for ALKpositive patients as 74%; whereas, the DFS rate for ALK-negative (EGFR status unknown) was 68% (p=0.45) [20]. No significant difference was observed between the ALK-positive and ALK-negative groups. The analysis was not balanced for age and smoking history. Zhou et al. analyzed outcomes of patients with NSCLC who underwent radical surgical resection stratified into specific clinical stages [27]. In stage IA, ALK-positive cases had significantly longer DFS than ALK-negative (EGFR status unknown) cases (5-year DFS rate, 100% versus 29.5%, p=0.04). In stage IIIA, ALK-positive patients had poorer DFS than ALKnegative patients (median DFS, 6 months versus 16 months, p=0.0057, Table 4). In a multivariate analysis, the ALK-positivity was the only significant variable associated with poor survival in stage IIIA NSCLC (HR=4.0, p<0.001). Although stratified subset analysis accounted for stage to some degree; it was not controlled for age and gender.
| Study | ALK+ (N)/Total(N) | Efficacy |
|---|---|---|
| Studies with no control or adjustment for confounding factors | ||
| Paik et al. [19] | 28 ALK+/735 stage I-III |
Median DFS: • 76.4 months in ALK+ • 71.3 months in ALK- ( p =0.66 vs.ALK+) |
| Fukui et al. [20] | 28 ALK+/720 adeno cases all stages |
5-year DFS rate: • 74% in ALK+ • 68% in ALK- (p = 0.45 vs.ALK+) |
| Zhou et al. [27] | 12 ALK+/134 stage IA |
5-year DFS rate: • 100% in ALK+ • 29.5% in ALK- (p = 0.04 vs.ALK+) |
| 9 ALK+/165 stage IIIA |
Median DFS: • 6 months in ALK+ • 16 months in ALK- ( p =0.0057 vs.ALK+) |
|
| Studies with control or adjustment for confounding factors | ||
| Kim et al. [5] | 11 ALK+/119 never-smokers, all stagesa |
Median RFS: • 20.0 months in ALK+ • 39.7 months in EGFR mu • 21.4 months in KRAS mu • 26.8 months in WT/WT/WT |
Table 4: Median DFS or RFS with Surgical Therapy.
Response to thoracic radiotherapy: Hayashi et al. reported outcomes of patients with locally advanced adenocarcinoma NSCLC who underwent thoracic radiotherapy (TRT) alone or together with chemotherapy. The median PFS with TRT in 3 ALK-positive cases was 4.2 months compared with 18.6 months in 23 WT/WT and 13.1 months in 11 EGFR mutant cases (p=0.037 for ALK-positive versus WT/WT) [18]. The authors concluded the ALK ALK-positive patients had a poorer outcome after TRT treatment. However, this study was not matched or controlled for important prognostic factors. Additionally, there were only 3 ALK-positive patients who treated with TRT.
Summary of studies with control of or adjustment for confounding factors: In order to account for imbalances in clinical or patient characteristics between patient subgroups, a few investigators matched cases or applied statistical adjustment (control) in their survival analyses, accounting for age, sex, smoking status, histology, and stage of disease (Table 2).
Response to chemotherapy: Lee et al. retrospectively identified stage IIIb-IV cases of non-squamous histology and created a case cohort of ALK-positive cases matched 2:1 to both EGFR mutant and WT/WT cases on age at diagnosis, sex, stage, and smoking status. They observed a median PFS with first-line chemotherapy (none of which included pemetrexed) of 3.87 months in ALK-positive versus 4.93 months in EGFR mutant (p=0.825), and 3.73 months in WT/WT NSCLC cases (p=0.474) [6]. The first-line chemotherapy ORRs in this study were 28.6%, 32.4%, and 35.1% for ALK-positive, EGFR mutant, and WT/ WT groups, respectively (p=0.857 versus EGFR mutant; p=0.695 versus WT/WT).
Kim et al. [5] examined outcomes in NSCLC patients who were never-smokers, controlling for age, sex, histology, and PS in a multivariate analysis. In this analysis, the ORR to first-line platinumbased chemotherapy was 0% for ALK-positive (n=12) and 23% for triple WT cases (n=61). The difference in ORRs between groups was not statistically significant, nor was the median PFS estimate of 5.0 months for ALK-positive and 5.9 months for triple WT cases. The comparator groups, particularly the ALK-positive and triple WT cases, in this study were well-balanced in terms of age, sex, histology, PS, stage, and smoking status. Thus, even though unadjusted statistically, the Kaplan–Meier survival curves in this study represent well-balanced comparisons.
Response to EGFR TKI therapy: The matched case cohort analysis by Lee et al. [6] demonstrated a median PFS of 1.37 months in ALKpositive versus 2.07 months in WT/WT and 9.80 months in EGFR mutant cases (p=0.037 for ALK-positive versus WT/WT; p<0.001 for ALK-positive versus EGFR mutant).In the analysis by Kim et al. [5], median PFS with EGFR TKI therapy in ALK-positive cases was 1.6 months compared with 6.3 months in WT/WT and 12.8 months in EGFR mutant cases (p<0.001 for ALK-positive versus EGFR mutant; p=0.001 for ALK-positive versus WT/WT; Table 2). Thus, these results corroborate the findings of Shaw et al.; Koh et al., and Wang et al., and show worse response to EGFR TKI therapy in ALK-positive NSCLC cases.
Outcome from surgical therapy: Kim et al. examined outcomes in surgically resected NSCLC patients who were never-smokers, controlling for age, sex, histology, stage, and PS in comparator groups [5]. The median RFS with radical surgery in ALK-positive cases was 20.0 months compared with 26.8 months in WT/WT and 39.7 months in EGFR mutant cases (p=0.344). Although in a multivariate analysis, ALK-positivity was associated with a lower OS in patients with resected NSCLC (adjusted HR, 4.162; p=0.005) and the authors suggested that ALK-positivity may be a negative prognostic factor for early stage NSCLC.
Discussion and Conclusion
Lessons learned from the ALK NSCLC experience emphasize the importance of early molecular and clinical epidemiology research needed to understand the prognostic and predictive value of candidate biomarkers or drug targets. Overall, studies that controlled for potential confounding factors either by study design or in the analyses suggest worse or equivalent prognosis for ALK-positive NSCLC cases. Only one analysis, studied by Wu et al., concluded that ALK rearrangement is a favorable predictive factor for OS in ALK-positive NSCLC [21]. One observation in this study was the percentage of cases who received platinum-based doublet chemotherapy, second- or subsequent-line therapy, pemetrexed treatment and EGFR TKI therapy were all higher in ALK-positive group than in the ALK-negative group even though individually there was no significant treatment difference between ALK-positive cases and ALK-negative cases. These multiple favorable factors, when combined, in the ALK-positive group could skew the overall results and in addition, the absence of significant differences could have possibly been due to too small a sample size.
Both non-balanced and balanced/controlled analyses demonstrated statistically significantly shorter PFS or TTP amongst ALK-positive (including EGFR WT or EGFR unknown but EGFR TKI resistant) cases compared with EGFR mutant and WT/WT cases treated with EGFR TKI therapy. All studies except one reported a 0% ORR to EGFR TKI therapy in ALK-positive cases (Table 2). Although a small total number of patients were studied, these data suggest that ALK is a negative predictive factor for EGFR TKI therapy outcomes in ALKpositive NSCLC. All studies included in this review, regardless of whether they were balanced/controlled or not, suggested that PFS with platinum-based chemotherapy might not be significantly influenced by ALK status in patients with NSCLC. Although in two of the studies the patients received pemetrexed as a platinum partner, the difference between ALK-positive and WT/WT were not found to be statistically significant. Therefore, ALK rearrangements might not be a predictive marker for PFS with conventional platinum-based chemotherapy. It is important to note though, that sample size which could influence statistical significance of comparisons is a limitation in most of these studies.
The analysis by Camidge et al. exploring PFS with pemetrexedbased therapy in groups defined by ALK, EGFR, and KRAS status has important imbalances to consider. Specifically, the ALK-positive and triple WT groups represented considerably different treatment populations, with 63% of the former being 1st-line-treated patients versus 38% of the latter. There were also third-line treatment patients in the triple WT group (16%) but none in the ALK-positive group in addition to differences in smoking status and age. Thus, the median PFS difference observed of 9 months versus 4 months for ALK-positive versus triple WT cases can, at least in part, be explained by a differential prognosis of the two groups based upon the above-mentioned factors. The study by Lee et al.; however, did demonstrate some degree of balance between patient and clinical characteristics for ALK-positive and WT/WT cases treated with single-agent pemetrexed; however, there were still important imbalances for age and line of therapy, with more ALK-positive patients being younger and in a third- or greater line of therapy [23]. With stratifying by important clinical characteristics such as line of therapy, age, smoking status, and different regimen, the study by Shaw et al stated similar PFS between ALK-positive and WT/ WT groups with pemetrexed-based therapy [26]. None of the studies examining response to pemetrexed matched cases or controlled for known confounding factors when comparing the survival curves. Thus, despite the multivariate analyses identifying ALK as the only significant predictive variable in response to pemetrexed, the point estimates of median TTP or PFS and the accompanying Kaplan–Meier curves do not account for these same potentially confounding variables used in the multivariate analyses.
Retrospective studies to date on ALK-positive NSCLC highlight the importance of controlling for already known independent prognostic or predictive factors when trying to evaluate the impact of a new NSCLC biomarker. For ALK-positive NSCLC, opposing conclusions can be drawn from aggregate balanced/controlled versus non-balanced analysis. Smoking status can be an important confounding factor. For example, across several previous studies, the hazard ratio (HR) for survival is consistently lower for never-smokers versus smokers (HR across studies approximately 0.8) [28-32]. Thus, smoking status is a critical factor to consider in analyses assessing the effect of a new biomarker on NSCLC treatment response or survival.
Combining all published analyses of observational data to date, with the understanding of limitations of small sample sizes, degree of control of confounding factors, and retrospective study designs, ALK rearrangement in NSCLC appears to be (1) not predictive of improved outcomes with standard chemotherapy, (2) predictive of poor response to EGFR TKI therapy, and (3) not a favorable prognostic factor in NSCLC [2,4-6,15,22]. In fact, the majority of controlled or case matched analyses suggest that ALK positivity is a negative prognostic factor in NSCLC [4-6]. Thus, the example of ALK translocation serves as a good model of the need for attention to careful control or adjustment for clinically relevant confounding factors when evaluating the prognostic and predictive value of newly identified candidate biomarkers in NSCLC.
Conflict of interest statement
Kimary Kulig is a former employee of Pfizer Inc. Shrividya Iyer is a current employee of Pfizer. Yi Wang and Ping Yang have declared no conflicts of interest.
Acknowledgements
Editorial assistance was provided by Martin Quinn at ACUMED® (Tytherington, UK) and funded by Pfizer Inc.
We thank Susan Ernst at Mayo Clinic (Rochester, MN, USA) for her technical assistance with the manuscript.
Author Contributions
Contributors: Kimary Kulig and Yi Wang contributed the conception, design, literature search, and review. Kimary Kulig, Yi Wang, Shrividya Iyer, and Ping Yang contributed to data analysis interpretation, manuscript writing, and final approval of the manuscript.
References
- Paik PK, Arcila ME, Fara M, Sima CS, Miller VA, et al. (2011) Clinical characteristics of patients with lung adenocarcinomas harboring BRAF mutations. J Clin Oncol 29: 2046-2051.
- Shaw AT, Yeap BY, Solomon BJ, Riely GJ, Gainor J, et al. (2011) Effect of crizotinib on overall survival in patients with advanced non-small-cell lung cancer harbouring ALK gene rearrangement: a retrospective analysis. Lancet Oncol 12: 1004-1012.
- . Â Martinez P, Hernandez-Losa J, Castellvi J, Tallada N, Cedres S et al. (2011). ALK rearrangements in a selected population of advanced non-small cell lung cancer patients.FISH and immunohistochemistry diagnostic methods, prevalence, and clinical outcomes. J Clin Oncol 29: 7566.
- Yang P, Kulig K, Boland JM, Erickson-Johnson MR, Oliveira AM, et al. (2012) Worse disease-free survival in never-smokers with ALK+ lung adenocarcinoma. J Thorac Oncol 7: 90-97.
- Kim HR, Shim HS, Chung JH, Lee YJ, Hong YK, et al. (2012) Distinct clinical features and outcomes in never-smokers with nonsmall cell lung cancer who harbor EGFR or KRAS mutations or ALK rearrangement. Cancer 118: 729-739.
- Lee JK, Park HS, Kim DW, Kulig K, Kim TM, et al. (2012) Comparative analyses of overall survival in patients with anaplastic lymphoma kinase-positive and matched wild-type advanced nonsmall cell lung cancer. Cancer 118: 3579-3586.
- Martinez P, Hernandez-Losa J, Castellvi J, Tallada N, Cedres S, et al. (2011) Diagnostic methods, prevalence and clinical outcomes of patients with ALK positive non-small cell lung cancer. J Thorac Oncol 6: S293.
- Martinez P, Hernández-Losa J, Montero MÃ, Cedrés S, Castellvà J, et al. (2013) Fluorescence in situ hybridization and immunohistochemistry as diagnostic methods for ALK positive non-small cell lung cancer patients. PLoS One 8: e52261.
- . Yang P, Kulig K, Oliviera AM, Wampfler J, Boland-Froemmin JM, et al. (2011) Anaplastic lymphoma kinase(ALK) status and clinical outcomes by IHC and FISH: a retrospective study of never-smoker, adenocarcinoma lung cancer cases. Lung Cancer 71: S26-S28.
- Zhang X, Zhang S, Yang X, Yang J, Zhou Q, et al. (2010) Fusion of EML4 and ALK is associated with development of lung adenocarcinomas lacking EGFR and KRAS mutations and is correlated with ALK expression. Mol Cancer 9: 188.
- Guggisberg NV (2012) Clinicopathological incidence, significance, and survival outcomes of EML4-ALK translocation in lung cancer. J Thorac Oncol 7: S128.
- Yang P, Oliveira A, Wampfler J, Boland JM, Stoddard SM, et al. (2010) Reduced disease-free survival associated with anaplastic lymphoma kinase translocation (ALK+) in lung adenocarcinoma patients with no cigarette smoking history. J Thorac Oncol 5: S384.
- . Altavilla G, Santarpia M, Arrigo C, Rizzo M, Galletti G, et al. (2010) EML4-ALK fusion gene in lung adenocarcinoma: A retrospective analysis of the outcome of cisplatin plus pemetrexed treated patients. J Clin Oncol 28: 7610.
- Paik PK, Johnson ML, D'Angelo SP, Sima CS, Ang D, et al. (2012) Driver mutations determine survival in smokers and never-smokers with stage IIIB/IV lung adenocarcinomas. Cancer 118: 5840-5847.
- Shaw AT, Yeap BY, Mino-Kenudson M, Digumarthy SR, Costa DB, et al. (2009) Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J Clin Oncol 27: 4247-4253.
- Takeda M, Okamoto I, Sakai K, Kawakami H, Nishio K, et al. (2012) Clinical outcome for EML4-ALK-positive patients with advanced non-small-cell lung cancer treated with first-line platinum-based chemotherapy. Ann Oncol 23: 2931-2936.
- Wang Z, Zhang X, Bai H, Zhao J, Zhuo M, et al. (2012) EML4-ALK rearrangement and its clinical significance in Chinese patients with advanced non-small cell lung cancer. Oncology 83: 248-256.
- Hayashi H, Okamoto I, Kimura H, Sakai K, Nishimura Y, et al. (2012) Clinical outcomes of thoracic radiotherapy for locally advanced NSCLC with EGFR mutations or EML4-ALK rearrangement. Anticancer Res 32: 4533-4537.
- Paik JH, Choi CM, Kim H, Jang SJ, Choe G, et al. (2012) Clinicopathologic implication of ALK rearrangement in surgically resected lung cancer: a proposal of diagnostic algorithm for ALK-rearranged adenocarcinoma. Lung Cancer 76: 403-409.
- Fukui T, Yatabe Y, Kobayashi Y, Tomizawa K, Ito S, et al. (2012) Clinicoradiologic characteristics of patients with lung adenocarcinoma harboring EML4-ALK fusion oncogene. Lung Cancer 77: 319-325.
- Wu SG, Kuo YW, Chang YL, Shih JY, Chen YH, et al. (2012) EML4-ALK translocation predicts better outcome in lung adenocarcinoma patients with wild-type EGFR. J Thorac Oncol 7: 98-104.
- Koh Y, Kim DW, Kim TM, Lee SH, Jeon YK, et al. (2011) Clinicopathologic characteristics and outcomes of patients with anaplastic lymphoma kinase-positive advanced pulmonary adenocarcinoma: suggestion for an effective screening strategy for these tumors. J Thorac Oncol 6: 905-912.
- Lee JO, Kim TM, Lee SH, Kim DW, Kim S, et al. (2011) Anaplastic lymphoma kinase translocation: a predictive biomarker of pemetrexed in patients with non-small cell lung cancer. J Thorac Oncol 6: 1474-1480.
- Lee HY, Ahn HK, Jeong JY, Kwon MJ, Han JH, et al. (2013) Favorable clinical outcomes of pemetrexed treatment in anaplastic lymphoma kinase positive non-small-cell lung cancer. Lung Cancer 79: 40-45.
- Camidge DR, Kono SA, Lu X, Okuyama S, Barón AE, et al. (2011) Anaplastic lymphoma kinase gene rearrangements in non-small cell lung cancer are associated with prolonged progression-free survival on pemetrexed. J Thorac Oncol 6: 774-780.
- Shaw AT, Varghese AM, Solomon BJ, Costa DB, Novello S, et al. (2013) Pemetrexed-based chemotherapy in patients with advanced, ALK-positive non-small cell lung cancer. Ann Oncol 24: 59-66.
- Zhou JX, Yang H, Deng Q, Gu X, He P, et al. (2013) Oncogenic driver mutations in patients with non-small-cell lung cancer at various clinical stages. Ann Oncol 24: 1319-1325.
- Yano T, Haro A, Shikada Y, Maruyama R, Maehara Y (2011) Non-small cell lung cancer in never smokers as a representative 'non-smoking-associated lung cancer': epidemiology and clinical features. Int J Clin Oncol 16: 287-293.
- Yano T, Miura N, Takenaka T, Haro A, Okazaki H, et al. (2008) Never-smoking nonsmall cell lung cancer as a separate entity: clinicopathologic features and survival. Cancer 113: 1012-1018.
- Toh CK, Gao F, Lim WT, Leong SS, Fong KW, et al. (2006) Never-smokers with lung cancer: epidemiologic evidence of a distinct disease entity. J Clin Oncol 24: 2245-2251.
- Nordquist LT, Simon GR, Cantor A, Alberts WM, Bepler G (2004) Improved survival in never-smokers vs current smokers with primary adenocarcinoma of the lung. Chest 126: 347-351.
- Kawaguchi T, Takada M, Kubo A, Matsumura A, Fukai S, et al. (2010) Gender, histology, and time of diagnosis are important factors for prognosis: analysis of 1499 never-smokers with advanced non-small cell lung cancer in Japan. J Thorac Oncol 5: 1011-1017.
Citation: Kulig K, Wang Y, Iyer S, Yang P (2014) Predictive and Prognostic Value of ALK Gene Rearrangement in Non-Small Cell Lung Cancer. Epidemiol 4:146. DOI: 10.4172/2161-1165.1000146
Copyright: © 2014 Kulig K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 18074
- [From(publication date): 2-2014 - Apr 03, 2025]
- Breakdown by view type
- HTML page views: 13301
- PDF downloads: 4773