Prediction of Gastro-Oesophageal Reflux Using the Shape of Sleeve Gastrectomy Observed On Postoperative Gastrografin Swallow
Received: 07-Jan-2022 / Manuscript No. JGDS-22-51329 / Editor assigned: 10-Jan-2022 / PreQC No. JGDS-22-51329(PQ) / Reviewed: 24-Jan-2022 / QC No. JGDS-22-51329 / Revised: 28-Jan-2022 / Manuscript No. JGDS-22-51329(R) / Accepted Date: 02-Jan-2022 / Published Date: 04-Feb-2022 DOI: 10.4172/2161-069X.1000667
Abstract
Introduction: Sleeve gastrectomy is a commonly performed metabolic and bariatric procedure associated with exacerbating or precipitating gastro-oesophageal reflux disease (GORD). It is a common belief that the apparent shape and dimensions of the stomach seen on postoperative gastrografin swallow may be predictive of GORD.
Methods: All procedures were performed by a single surgeon within a single center who routinely conducted early postoperative gastrografin swallow. One independent assessor evaluated the apparent shape and dimensions of the gastric sleeve. Another assessor used a questionnaire to assess clinical reflux and quality of eating. Together, this data was systemically analyzed to determine whether the gastric sleeve’s apparent shape could predict GORD.
Results: Routine post-operative gastrografin swallow of 50 patients did not predict GORD at an average of 28 months from surgery. Post-operative reflux is weakly correlated preoperative anti-reflux medication use (r=0.34, p=0.02) and preoperative regurgitation (r=0.32, p=0.03).
Conclusion: The apparent shape of the sleeve pictured on early routine post-gastrografin swallow post-surgery was not a predictor of reflux in this group of patients with at least 18 months of follow up.
Keywords: Sleeve gastrectomy; large fundus; reflux; Postoperative swallow
Introduction
Sleeve gastrectomy (SG) is a common metabolic and bariatric procedure associated with an increased rate of gastroesophageal reflux disease (GORD), either de novo or further aggravation of pre-existing symptoms [1,2]. Current evidence is conflicting given that GORD is prevalent within the obese population [3-5]. Perhaps the current data fails to account for the effect of weight loss or nuances within the surgical technique itself, including the shape of the gastric sleeve, failure of the lower oesophageal sphincter complex, and adjunctive hiatal hernia repair.
Given there are concerns that the sleeve gastrectomy may be refluxogenic, clinicians are increasingly faced with the dilemma of case selection and appropriate preoperative counseling. Early postoperative detection will allow clinicians to curtail the complications of GORD. It is a common belief that the apparent shape of the tubular sleeve may be predictive of reflux, attributed to inadequate fluid flow dynamics. A previous radiographic study suggested that a dilated upper sleeve, particularly with a narrowed mid-body, is associated with reflux [6]. This study aims to evaluate the association between the shape of the gastric sleeve and GORD.
Materials and Methods
The study
A retrospective analysis of a cohort of fifty patients who underwent sleeve gastrectomy by a single surgeon within a single centre, as per the surgeon’s routine management, all patients underwent a routine postoperative gastrografin study.
All patients participating in the study had a minimum of 18 months follow up post-surgery. This time frame allows for much of the weight loss to occur within the first year and subsequently plateau and stabilize. It also gives patients the time to adjust and tolerate different food textures and portion sizes. We routinely prescribed an acid suppressant for the first three months post-surgery.
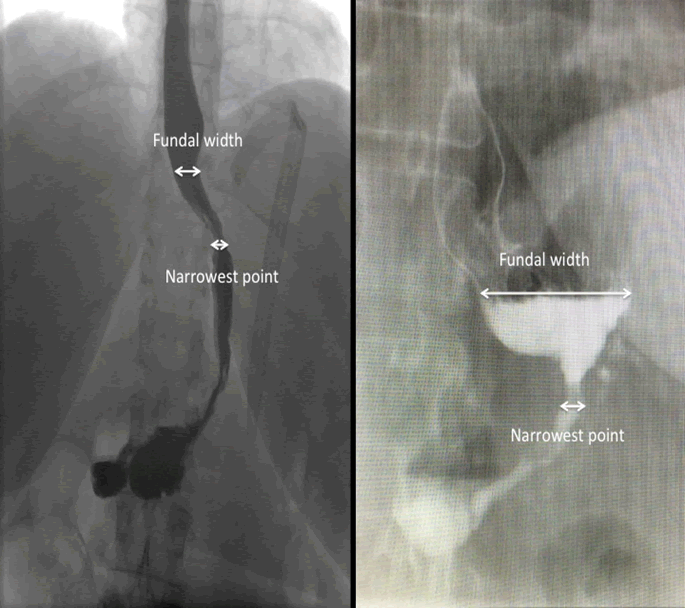
The first independent assessor would objectively measure the dimensions of the sleeve and designated anatomical landmarks, including vertebral body, oesophageal, fundal, and gastric body widths. These measurements account for and correct any possible magnification with the image intensifier. Measurements were performed with a digital screen ruler (Screen Ruler, Version 4.1, by Sprightly Software) (Figure 1).
The second independent assessor would contact the patients to organise a “self-administered” questionnaire. This questionnaire included a ‘return paid’ envelope. In broad terms, the study evaluated clinical reflux and associated clinical descriptors of reflux, including anti-reflux medication use, regurgitation, and dysphagia. The DAKAK scoring system for dysphagia and Visual Analogue Scales (VAS) are widely used and accepted, therefore not described in detail [7-10].
The sleeve gastrectomy
A 36 F calibration tube is routinely used to guide a tubular sleeve formation. There are familiar landmarks consistently used throughout the operation. The sleeve is started 2 cm from the pylorus, running wide at the incisura angularis and ends proximally at the angle of HIS, with a 1 to 2 cm cuff of the lateral stomach to avoid encroachment of the oesophagus.
In achieving confidence in identifying the angle of HIS and as not to encroach the gastro-oesophageal junction, we routinely identified the left crus in its entirety. The posterior gastric artery (a branch of the splenic artery) is routinely divided as it assists in complete posterior fundal mobilisation. If hiatal hernias were encountered or known preoperatively, this would mandate a formal hiatal dissection (demonstrating both pillars of the crura and repair over a 36 F calibration tube). The peri-gastric fat pad is often reflected medially away from the intended staple line.
Once the sleeve is complete, a series of interrupted sutures (2/0 prolene) is used to secure the freshly formed staple line to the omentum with the associated gastro-epiploic arcade. We believe this would mitigate the risk of gastric tubular torsion or spiralling of the staple line.
The postoperative contrast swallow
All patients are permitted to have oral fluids immediately post-surgery. The day following surgery, patients underwent a radiographic contrast swallow utilising gastrografin (Bayer, Australia. The active ingredient includes diatrizoate, meglumine and diatrizoate sodium) as a safe contrast medium. The volume of ingested contrast varies between 50 mls to 100 mls of contrast.
Fluoroscopic images were obtained in frontal and oblique projections with the patient in semi-recumbent and upright positions. Spot images were saved and retrospectively evaluated by an independent assessor unaware of the patients’ GORD and clinical symptoms.
Statistical analysis
Data were checked for normality. The Chi-squared test was utilised for categorical data. Spearman’s Rho was used to determine the association between two variables. Where appropriate, a student T-test was used to compare means between groups. Unless otherwise stated, all values given were mean+standard deviation (SD) and p<0.05 was considered significant. Statistical software SPSS version 22 (IBM Corporation) performed data analysis.
Results
Fifty participants were divided into two groups: Smaller Fundus (Quartile 1 and 2) and Larger Fundus (Quartile 3 and 4), with 25 participants in each. Demonstration of the outcomes between the two groups is shown in (Table 1). The two groups had no significant differences in age, height, and excess body weight loss across all follow up periods.
Table 1: Smaller Fundus vs Larger Fundus Outcomes.
| Measurement | Smaller Fundus (Mean ± SD) | Larger Fundus (Mean ± SD) |
Significance |
|---|---|---|---|
| (p- value) | |||
| Age | 47.1 (12.2) | 49.2 (12.8) | 0.552 |
| Height | 167.4 (8.0) | 166.4 (9.2) | 0.684 |
| Bariatric Outcomes | |||
| Pre-operative Weight | 128.3 (36.0) | 137.4 (28.4) | 0.325 |
| Pre-operative BMI | 45.8 (13.0) | 49.5 (8.7) | 0.248 |
| Weight at 3 months | 105.4 (33.1) | 111.9 (23.5) | 0.441 |
| BMI 3 months | 37.6 (12.1) | 40.6 (7.9) | 0.309 |
| % EBWL 3 months | 48.9 (22.4) | 37.6 (13.3) | 0.044 |
| % TWL 3 months | 17.7 (5.2) | 17.4 (5.5) | 0.835 |
| Weight at 6 months | 98.7 (33.7) | 103.8 (22.0) | 0.562 |
| BMI 6 months | 35.1 (12.2) | 37.4 (8.2) | 0.47 |
| % EBWL 6 months | 65.2 (31.2) | 53.4 (18.0) | 0.14 |
| % TWL 6 months | 23.7 (7.5) | 24.7 (6.6) | 0.638 |
| Weight at 12 months | 97.0 (33.7) | 92.6 (24.2) | 0.62 |
| BMI 12 months | 34.7 (12.3) | 33.9 (8.7) | 0.799 |
| % EBWL 12 months | 72.0 (36.9) | 68.2 (24.8) | 0.69 |
| % TWL 12 months | 27.0 (8.7) | 31.3 (9.7) | 0.279 |
| Weight at 24 months | 88.5 (29.3) | 89.6 (24.6) | 0.905 |
| BMI 24 months | 32.5 (10.8) | 32.3 (6.5) | 0.962 |
| % EBWL 24 months | 81.1 (38.5) | 89.6 (24.6) | 0.453 |
| % TWL 24 months | 28.2 (7.4) | 34.3 (10.2) | 0.056 |
| Gastrografin Swallow Outcomes | |||
| Vertebral body width (T9) | 25.7 (2.3) | 26.3 (2.8) | 0.458 |
| Maximal oesophageal width | 14.6 (3.6) | 14.0 (2.6) | 0.504 |
| Maximal fundal width | 13.7 (2.2) | 23.0 (5.1) | *<0.001 |
| Narrowest point (gastric body) | 10.0 (2.4) | 10.0 (3.6) | 0.972 |
| Vertebral body: Oesophageal width | 1.8 (0.5) | 1.9 (0.4) | 0.475 |
| Fundal width: Vertebral body | 1.2 (0.3) | 1.9 (0.3) | *<0.001 |
| Oesophageal width: Fundal width | 1.0 (0.3) | 0.7 (0.2) | *<0.001 |
| Fundal width: Narrowest point | 1.5 (0.5) | 2.7 (0.8) | *<0.001 |
| Gastro-oesophageal Reflux Outcomes | |||
| Interval since Surgery to Questionnaire (Months) | 28.2 (6.6) | 28.6 (6.7) | 0.737 |
| Heartburn (VAS=Visual Analogue Score) (0=no symptoms; 10=severe symptoms) | 1.0 (1.9) | 1.1 (1.9) | 0.871 |
| Dysphagia Liquid (VAS) | 0.3 (1.3) | 0.7 (2.2) | 0.744 |
| Dysphagia Solid (VAS) | 0.8 (2.1) | 1.1 (2.0) | 0.678 |
| Dysphagia Score (DAKKAK) (Score 45=no dysphagia) Higher score means less dysphagia | 41.4 (4.9) | 40.1 (6.0) | 0.421 |
| Pre-operative Medication (Proton pump inhibitors) | 5/25 | 7/25 | 0.678 |
| Post-operative Medication (Proton pump inhibitors) | 7/25 | 7/25 | 1 |
| Post-operative Reflux | 7/25 | 7/25 | 1 |
| Smaller Fundus=Quartile 1&2 Fundal Width. Larger Fundus=Quartile 3 and 4. BMI-Body mass index. TWL-Total Weight loss. EBWL-Excess Body Weight Loss. (Values are stated as Mean ± SD unless specified). *p<0.005=significance. Note: Higher Dakkak scores means less dysphagia. | |||
There was no significant correlation between fundal size and post-operative reflux (r=0.13, p=0.38). Examples of fundal width measures are shown in (Figure 1). In a sub-group analysis (independent of fundal size) of 12 patients reporting preoperative reflux, 5 (42%) had regression of reflux, whilst 7 (58%) reported ongoing postoperative reflux.
Post-operative reflux was weakly correlated with a few clinical indicators of reflux. These indicators are preoperative anti-reflux medication use (r=0.34, p=0.02) and preoperative regurgitation (r=0.32, p=0.03). Not surprisingly, there was a strong correlation between post-operative reflux (n=14) and regular post-operative anti-reflux medication use (n=13), (r=0.77, p=<0.001). There was a non-significant and weak association between postoperative reflux and preoperative reflux (r=0.38, p=0.07).
As a group, there is an incidence of “de-novo/spontaneous” development of reflux. In matching the same patient at different time points (preoperative and postoperative), of the 38 patients who did not report reflux before surgery, 7 (18%) had reported reflux afterwards (de-novo).
Discussion
This study aims to clarify whether a clinician should be concerned if a postoperative gastrografin swallow conducted shortly after sleeve gastrectomy (SG) demonstrated an “apparent large fundal pouch”. At the average follow up of 28 months (range 19 to 42), we have found no difference in the measured outcomes of gastroesophageal reflux disease (GORD) or weight loss.
To date, the literature remains unclear regarding the significance of the size of the fundal pouch observed on the immediate postoperative gastrografin swallow. Some authors determined that a larger fundus led to more reflux, whilst others determined the opposite effect [6,11-13]. In 2010, Keidar et al determined that a large fundus on postoperative swallow was associated with a higher incidence of reflux [6]. Keidar et al postulated that a large fundus enables the stomach to distend and produce more acid that can reflux into the oesophagus [6]. Unfortunately, Keidar et al did not report on the appearance of postoperative swallows on patients who did not have reflux [6]. We believed that despite such an intriguing observation, it could be within the realm of “ad-hoc rationalisation” for the observed phenomenon. Ultimately this may be inaccurate; as in our series, many patients have an apparent dilated fundus without reflux. Like Triantafyllidis et al and Lazoura et al, our study found 25% to 30% of sleeve gastrectomies may have similar radiological appearances (large fundus) without clinical consequences of reflux [12,13].
Lazoura et al indicated it was the “ideal” tubular sleeve that had a higher incidence of regurgitation and vomiting [13]. Unlike previous publications, it was evident that the author’s methodology and the study type (consecutive, case-matched, prospective) leads to less selection bias [13].
The claims that a large fundus leads to reflux stem from two known characteristics. The gastric fundus is responsible for receptive relaxation and food accommodation. The gastric antrum is responsible for trituration and emptying. Chambers et al. stated that a narrow sleeve gastrectomy results in loss of receptive relaxation and increased gastric pressure, resulting in accelerated gastric emptying [14]. Simultaneously, a narrow gastric sleeve reduces parietal cell mass and acid secretion. Hence, in theory, the larger the fundus, the slower the stomach empties and more acid can reflux up to the oesophagus.
However, these views are not consistently supported. Our study and that described by Lazoura et al postulates that the presence of a superior pouch may increase the stomach's ability to distend and accommodate food so that less gastric content is available for reflux [13]. Additionally, weight loss that results from surgery also reduces intra-abdominal pressure and reduces GORD.
As described by Lazoura et al., provided that the native anti-reflux mechanism is intact, some degree of the gastric fundus results in better accommodation of food [13]. Hence, a larger gastric fundus is less likely to lead to regurgitation and vomiting than those of the ideal tubular pattern.
We know that the anti-reflux mechanisms are multifactorial. It is not limited to the diaphragmatic pinch of the hiatal pillars but also the phrenooesophageal ligaments and intrinsic muscular fibres of the lower oesophagus. The sleeve gastrectomy can potentially alter the angle of HIS and result in partial resection of the sling fibres located at the junction of the stomach and lower oesophagus, but other mechanisms may be sufficiently protective against reflux.
We believe in the importance of the “native anti-reflux mechanism” in protecting against reflux. That is why Samakar et al. found that despite a concomitant hiatal repair with a sleeve gastrectomy, only about a third will have regression of reflux (ours 42%) [15]. Their de-novo reflux rate, even with concomitant hiatal hernia repair, was 15.6%, lower than our prevalence of 18%.
Limitation of study
There is the possibility of “selection bias” as it is known that patients that are generally satisfied with their outcomes tend to respond with enthusiasm. However, on the general perusal of our data, we observed a heterogeneous group, so the generalisation of “selection bias” on “generally satisfied patients” could not be determined with any confidence.
We understand this is a dynamic study with static images. It is the static images that our measurements are made. We attempted to minimise any observation error in two ways. Firstly by obtaining an impression of the sleeve. Secondly, we undertake an objective measurement (using the width of the T9 vertebral body to account for magnification). At this stage, we determine this result is easily reproducible.
The evaluation of gastric anatomy by postoperative swallow is indeed subjective. All the work has been based on a “fluid medium”. Perhaps, our thinking might change if we included a “solid medium” to understand how a dynamic tubular organ handles solid food textures differently from liquids.
Finally, reflux or GORD is a heterogeneous disease. It presents with varying clinical symptoms, changes with time and is diagnosed using various criteria. This includes the presence of typical symptoms and response to medication (known as clinical reflux), endoscopic findings (known as endoscopic reflux) or 24 hour pH monitoring in combination with manometric evaluation of the esophagus (known as laboratory reflux). Knowing the limitations of each, practicality, and cost, we adopted “clinical reflux” for this study as this reflected what patients would report to us in real life.
Conclusion
We would conclude the apparent shape of the sleeve noted on early routine post-gastrografin swallow following sleeve gastrectomy is not a predictor of reflux in this group of patients with at least 18 months of follow up. Preoperative clinical indicators of reflux (medication and regurgitation) were significantly correlated with GORD postoperatively.
Statement of Ethics
The study was approved by the institutional research ethics committee and conducted in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki. A plain language statement was posted together with the survey. We attached a “postage-paid, self-addressed envelope” to encourage participation. Informed consent was obtained from all individual participants included in the study. In posting out of the “self-administered survey”, patients had the option of “non-participation”. This would be either explicit in the survey or implicit (non-return of the survey).
Conflict of Interest Statement
The Authors have no conflicts of interest to declare
Funding Sources
Not applicable
Author Contributions
R.S and I.L wrote the final manuscript, interpreted the results, and verified analytical methods.
I.L and D.S planned and carried out data collection. K.L and A.A conceived the original idea. K.L supervised the project. All authors provided critical feedback and helped shape the research, analysis, and manuscript.
References
- Dupree CE, Blair K, Steele SR, Martin JM (2014) Laparoscopic sleeve gastrectomy in patients with pre-existing gastroesophageal reflux disease: A national analysis. JAMA Surg 149(4):328-34.
- Rosenthal RJ, Diaz AA, Arvidsson D, Baker RS, Basso N, et al. (2012) International sleeve gastrectomy expert panel consensus statement: Best practice guidelines based on experience of >12,000 Cases. Surg Obes Relat Dis 8(1):8-19.
[Cross Ref] [Google Scholar] [Pubmed]
- Chiu S, Birch DW, Shi X, Sharma AM, Karmali S (2011) Effect of sleeve gastrectomy on gastro esophageal reflux disease: A systematic review. Surg Obes Relat Dis 7(4):510-5.
[Cross Ref] [Google Scholar] [Pubmed]
- Martin- Perez J, Arteaga-Gonzalez I, Matin-Malagon A, Diaz-Luis H, Cassanova-Trujillo C, et al (2014) Frequency of abnormal oesophageal acid exposure in patients eligible for bariatric surgery. Surg Obes Relat Dis 10(6):1176-80.
[Cross Ref] [Google Scholar] [Pubmed]
- Brockenmyer JR, Simon TE, Jacob RK, Husain F, Choi Y (2012) Upper gastrointestinal swallow study following bariatric surgery: Institutional review and review of the literature. Obes Surg 22:1039-43.
[Cross Ref] [Google Scholar] [Pubmed]
- Keidar A, Appelbaum L, Schweiger C, Elazary R, Baltasar A (2010) Dilated upper sleeve can be associated with severe postoperative gastro-oesophageal dysmotility and reflux. Obesity Surgery 20:140-7.
[Cross Ref] [Google Scholar] [Pubmed]
- Dakkak M, Bennett JR (1992) A new dysphagia score with objective validation. J Clin Gastroent 14(2):99-100.
[Cross Ref] [Google Scholar] [Pubmed]
- Rijnhart-De Jong HG, Draisma WA, Smout AJ, Broeders IA, Gooszen HG (2008) The visick score: A good measure for the overall effect of anti-reflux surgery. Scand J Gastro 43(7):787-93.
[Cross Ref] [Google Scholar] [Pubmed]
- Dent J, El-Serag HB, Wallander MA, Johansson S (2005) Epidemiology of gastro-oesophageal reflux disease: A systematic review. Gut 54(5):710-7.
[Cross Ref] [Google Scholar] [Pubmed]
- Zagari RM, Fuccio L, Wallander MA, Johansson S, Fiocca R, et al (2008) Gastro oesophageal reflux symptoms, oesophagitis and barrett’s oesophagus in the general population: Loiano- Monghidoro study. Gut 57(10):1354-59.
[Cross Ref] [Google Scholar] [Pubmed]
- Toro JP, Lin E, Patel AD, Davis SS Jr, Sanni A, et al (2014) Association of radiographic morphology with early GORD and satiety control after sleeve gastrectomy. J Am Coll Surg 219(3):430-8.
[Cross Ref] [Google Scholar] [Pubmed]
- Triantafyllidis G, Lazoura O, Sioka E, Tzovaras G, Antoniou A, et al (2011) A systematic review of staple-line reinforcement in laparoscopic sleeve gastrectomy. Obesity Surgery 21:473-478.
[Cross Ref] [Google Scholar] [Pubmed]
- Lazoura O, Zacharoulis D, Triantafyllidis G, Fanariotis M, Sioka E, et al. (2011) Symptoms Of gastro-oesophageal reflux following laparoscopic sleeve gastrectomy are related to final shape of the sleeve as depicted by radiology. Obesity Surgery 21:295-9.
[Cross Ref] [Google Scholar] [Pubmed]
- Chambers AP, Smith EP, Begg DP, Grayson BE, Sisley S, et al. (2014) Regulation of Gastric emptying rate and its role in nutrient induced GLP-1 secretions in rats after vertical sleeve gastrectomy. Am J Physiol Endocrinol Metab 306(4):424-432.
[Cross Ref] [Google Scholar] [Pubmed]
- Samakar K, McKenzie TJ, Tavakkoli A, Vernon AH, Robinson MK, et al (2016) The effect of laparoscopic sleeve gastrectomy with concomitant hiatal hernia repair on gastro-esophageal reflux disease in the morbidly obese. Obes Surg 26(1):61-66.
[Cross Ref] [Google Scholar] [Pubmed]
Citation: Suthakaran R, Lim I, So D, Lim K, Aly A (2022) Prediction of Gastro- Oesophageal Reflux Using the Shape of Sleeve Gastrectomy Observed On Postoperative Gastrografin Swallow. J Gastrointest Dig Syst.12:667 DOI: 10.4172/2161-069X.1000667
Copyright: © 2022 Suthakaran R, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 2488
- [From(publication date): 0-2022 - Feb 03, 2025]
- Breakdown by view type
- HTML page views: 2093
- PDF downloads: 395