Research Article Open Access
Predicting Risk of Anastomotic Leak in Patients Undergoing Neo-adjuvant Radiotherapy and Low Anterior Resection for Rectal Cancer
Byrne C*, Smith RA, Abdelrazeq A, Pranesh N, Taylor B, Tighe MJ and Rooney P
Department of Surgery, Warrington Hospital, Lovely Lane, Warrington, WA5 1QG, UK
- Corresponding Author:
- Caroline Byrne
Department of Surgery, Warrington Hospital
Lovely Lane, Warrington, WA5 1QG, UK
Tel: 447715339809
E-mail: carolinebyrne@doctors.org.uk
Received Date: February 09, 2015; Accepted Date: February 12, 2015; Published Date: February 20, 2015
Citation: Byrne C, Smith RA, Abdelrazeq A, Pranesh N, Taylor B, et al. (2015) Predicting Risk of Anastomotic Leak in Patients Undergoing Neoadjuvant Radiotherapy and Low Anterior Resection for Rectal Cancer. J Gastrointest Dig Syst 5:255. doi:10.4172/2161-069X.1000255
Copyright: © 2015 Byrne C, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Gastrointestinal & Digestive System
Abstract
Background: Anastomotic leakage is an important source of morbidity and mortality in patients undergoing neoadjuvant therapy and low anterior resection for rectal cancer despite use of concurrent defunctioning stoma. The objective of this study was to identify any preoperative haematological factors which may be associated with an increased risk of anastomotic leak in this patient group.
Methods: Retrospective data collection was undertaken for patients undergoing surgery between 2007 and 2012.
Results: 48 patients were identified of whom 13 (27%) developed an anastomotic leak. The median time interval from surgery to diagnosis of leak was 8 (IQR=2 to 14) weeks - only 5 leaks (10%) presented during the initial postoperative stay. A low preoperative platelet (logistic regression, p=0.027) and white cell count (p=0.049) were found to have a significant association with an increased risk of leak. It was possible to construct a simple additive risk score (white cell ≤ 4.5 x109/l=1/platelet count ≤ 250 x109/l=1) allowing a significant degree of risk stratification for anastomotic leak (score 0=1/19 (5% leak rate), score 1=5/18 (28%), score 2=7/11 (64%)).
Conclusion: Low platelet and white cell counts at the time of surgery may be associated with an increased risk of anastomotic leak in patients undergoing low anterior resection following preceding neoadjuvant therapy.
Keywords
Anastomotic leak; Low anterior resection; Neoadjuvant
Introduction
Anastomotic leakage represents an important source of postoperative morbidity and mortality following sphincter-preserving rectal resections for malignancy. Significant leaks may result in re-operation with the potential for permanent stoma formation, delayed administration of adjuvant therapy and potentially adverse oncological outcomes. Low pelvic anastomoses or coloanal anastomoses are known to be associated with an increased likelihood of leak with reported rates ranging from 10% to 20% in large patient series [1-5]. Administration of neoadjuvant radiotherapy and male gender are also well reported risk factors [1,6-8]. Previous meta-analyses have demonstrated that use of a defunctioning stoma significantly reduces the likelihood of both anastomotic leak and requirement for re-operation [9,10], thus defunctioning is commonly employed on a routine basis in this operative setting.
Despite these well recognised issues, anastomotic leakage following low anterior resection is an unpredictable clinical phenomenon which may occur in defunctioned patients without other obvious risk factors. Anastomotic leak may be clinically evident in the early postoperative period with the typical findings resulting from progressive pelvic sepsis. However, a contained anastomotic leak may present much later with only radiological evidence of a peri-anastomotic abscess cavity on subsequent contrast imaging with minimal or no clinical symptoms [11]. Even in the absence of symptoms or frank infective complications, this finding may delay or preclude subsequent stoma reversal.
Preoperative haematological indices have been widely investigated as prognostic markers for overall survival in resected colorectal cancer [12,13]. The objective of this study was to evaluate whether any pre-operative haematological parameters may be associated with increased risk of anastomotic leak in patients undergoing initial neoadjuvant therapy and subsequent low anterior resection with concurrent defunctioning stoma for rectal cancer.
Methods and Patients
All patients undergoing elective low anterior resection with curative intent and concurrent defunctioning stoma for histologically-confirmed rectal adenocarcinoma at a single institution between 2007 and 2012 were identified retrospectively from theatre records. Low anterior resection was defined as any low pelvic or coloanal anastomosis as documented by the performing surgeon. A total mesorectal excision (TME) with double-stapled anastomotic technique was utilised in all cases with routine use of pelvic drainage.
Staging comprised full colonoscopic assessment, computed tomography (CT) of chest, abdomen and pelvis along with pelvic magnetic resonance (MR) imaging. All patients had at least one year of follow-up to identify any intermediate and late outcomes following surgery. Anastomotic leak was defined as any anastomotic defect resulting in pathological communication between the intra- and extra-luminal spaces and classified according to the International Study Group of Rectal Cancer [14]. Patients who developed symptoms and/or signs of anastomotic leakage in the initial postoperative period were investigated with CT and intravenous contrast. Patients routinely underwent clinical assessment, contrast enema +/- endoscopic assessment in the subsequent postoperative period as a prelude to decision-making regarding reversal of defunctioning stoma. Clinico-pathological data were collected from hospital computer records including patient demographics, details of neoadjuvant therapy received, pre-operative haematological data, tumour histology, details of postoperative morbidity and requirement for re-operation. Preoperative haematological and biochemical results were collected immediately prior to the date of surgery. Short-course radiotherapy comprised 25 Gy in 5 daily fractions with surgery within one week. Long course chemoradiotherapy was 5-FU based and comprised 45 Gy in 25 daily fractions over 5-6 weeks with surgery typically occurring a further 6 weeks thereafter.
Statistical Analysis
Continuous data were analysed using median values and interquartile range (IQR) and compared using Mann-Whitney (2 groups) or Kruskal-Wallis testing (3 groups). Pre-operative factors associated with anastomotic leak were analysed using logistic regression and histological associations with preoperative haematological data were analysed using linear regression. Time-to-event data was analysed using Cox regression. Statview version 5 (SAS, California) statistical software was used for all analyses.
Results
60 patients underwent elective low anterior resection for rectal adenocarcinoma along with defunctioning stoma during the study period. 12 patients did not receive any form of neoadjuvant therapy leaving a study group of 48 patients. Table 1 demonstrates a breakdown of the patient characteristics, neoadjuvant therapy received, resected tumour histology and operative details. No cases of significant neoadjuavnt-related myelosuppression which delayed surgery were documented within this patient cohort. One patient (2%) died within 90 days of surgery having developed a contained anastomotic leak and concurrent pancreatic fistula in the early post-operative period which were managed with percutaneous drainage.
| Male (%) | 34 (71%) |
| Median age (IQR) | 64 (60 - 71) yrs |
| ASA 1/2/3 | 11 (23%)/26 (54%)/11 (23%) |
| Median BMI (IQR) | 27.5 (23 - 29.5) |
| Vascular co-morbidity (%) | 13 (27%) |
| Neoadjuvant therapy | |
| Short course radiotherapy (%) | 17 (35%) |
| Long course chemoradiotherapy (%) | 31 (65%) |
| T stage | |
| T0/T1 | 16 (33%) |
| T2 | 10 (21%) |
| T3 | 21 (44%) |
| T4 | 1 (2%) |
| N stage | |
| N0 | 32 (67%) |
| N1 | 13 (27%) |
| N2 | 3 (6%) |
| Median number of lymph nodes sampled (IQR) | 9 (7 - 15) |
| Median number of involved nodes (IQR) | 2 (1 - 3) |
| Median lymph node ratio in N+ve cases (IQR) | 0.16 (0.08 - 0.41) |
| Tumour differentiation | |
| Complete response | 6 (13%) |
| Well/Moderate | 41 (85%) |
| Poor | 1 (2%) |
| Median tumour size (IQR) | 17 (11 - 30) mm |
| R1 rate (%) | 1 (2%) |
| Circumferential margin involved | 1 (2%) |
| Distal margin involved | 0 |
| Mode of surgery | |
| Open | 43 (90%) |
| Laparoscopic | 5 (10%) |
| Type of defunctioning stoma | |
| Loop ileostomy | 47 (98%) |
| Loop transverse colostomy | 1 (2%) |
Table 1: Patient demographics and clinico-pathological data (n=48).
Anastomotic leak
Of the 48 patients who received neoadjuvant therapy, 13 anastomotic leaks were identified (27%). Table 2 demonstrates a breakdown of the timing of when leaks were diagnosed along with imaging modality utilised, ISGRC classification and clinical details. Only 4 of the 13 anastomotic leaks presented during the initial postoperative in-patient admission with the majority of cases identified on subsequent imaging prior to consideration for stoma reversal. The median time interval from surgery to diagnosis of radiologically confirmed leak was 8 (IQR=2 - 14) weeks. Only 3 of the 13 patients who developed a leak required some form of intervention in the short-term (transrectal or percutaneous drainage) and no patients required re-laparotomy. One patient went on to develop a recto-vaginal fistula necessitating surgical repair 13 months postoperatively and one patient developed an anastomotic stricture which ultimately required formation of end colostomy after 20 months. Of the 12 patients who did not receive any neoadjuvant therapy, only one patient (8%) developed a leak. There was no statistically significant difference in the leak rate between the two patient groups (ie. 13/48 (27%) vs. 1/12 (8%) - Fisher’s exact, p=0.161).
| Patient | Timing (wks) | Imaging | ISGRC group | Details |
|---|---|---|---|---|
| 1 | 38 | GGE | A | Small leak with pre-sacral cavity on routine GG enema |
| 2 | 14 | GGE | A | Small leak with pre-sacral cavity on routine GG enema |
| 3 | 29 | CT + clinical | A | Pre-sacral collection and palpable anastomotic defect |
| 4 | 8 | GGE + clinical | A | Small anastomotic defect palpable - confirmed on GGE |
| 5 | 2 | GGE + clinical | A | Small anastomotic defect palpable - confirmed on GGE |
| 6 | 6 | MR | B | Re-admission with pelvic abscess drained transrectally |
| 7 | 8 | GGE | A | Small leak with pre-sacral cavity on routine GG enema |
| 8 | 1 | CT | B | Pelvic collection - percutaneous + subsequent transrectal drain* |
| 9 | <1 | CT + clinical | A | Faeculant initial drain output - small leak on subsequent GGE |
| 10 | 2 | CT | B | Concurrent leak and pancreatic fistula - died 9wks post-op |
| 11 | 33 | GGE | A | Small leak with pre-sacral cavity on routine GG enema |
| 12 | 1 | CT | A | Small pelvic collection - recto-vaginal fistula 7 months post-op |
| 13 | 12 | GGE | A | Pre-sacral collection and palpable anastomotic defect |
Table 2: Details of anastomotic leaks (n=13). GGE=Gastrograffin Enema/ISGRC=International Study Group of Rectal Cancer (A=no active therapeutic intervention/B=active therapeutic intervention but managed without re-laparotomy/C=re-laparotomy required). *Patient went on to develop anastomotic stricture eventually requiring reversal of loop ileostomy and formation of permanent end colostomy 20 months following initial resection.
Factors associated with anastomotic leak
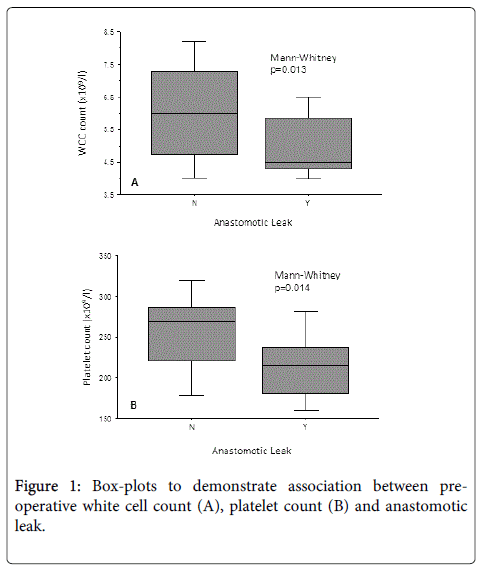
Table 3 demonstrates the results of the univariate logistic regression analysis. The results show that low preoperative platelet (p=0.027) and white cell counts (p=0.049) were significantly associated with an increased likelihood of anastomotic leak within this patient group. Figure 1 graphically demonstrates this association. None of the other variables investigated were significant.
| Variable | OR (95% CI) | Χ2 | p-value |
|---|---|---|---|
| Age | 1.005 (0.934 - 1.080) | 0.016 | 0.9 |
| Gender (M) | 0.640 (0.168 - 2.436) | 0.428 | 0.513 |
| Vascular co-morbidity (Y) | 0.750 (0.170 - 3.309) | 0.144 | 0.704 |
| BMI | 0.974 (0.842 - 1.125) | 0.132 | 0.717 |
| Long vs. short course RT | 0.535 (0.145 - 1.970) | 0.886 | 0.346 |
| ASA grade > 2 | 0.525 (0.097 - 2.837) | 0.56 | 0.454 |
| Pre-op Hb | 1.000 (0.972 - 1.030) | 0.001 | 0.993 |
| Pre-op WCC | 0.593 (0.352 - 0.999) | 3.853 | 0.049 |
| Pre-op neutrophils | 0.557 (0.297 - 1.044) | 3.333 | 0.068 |
| Pre-op lymphocytes | 0.723 (0.228 - 2.294) | 0.304 | 0.582 |
| Pre-op platelets | 0.984 (0.970 - 0.998) | 4.914 | 0.027 |
| Pre-op serum creatinine | 0.993 (0.956 - 1.031) | 0.129 | 0.719 |
Table 3: Univariate logistic regression - preoperative factors associated with leak (n=48). RT=Radiotherapy, Hb=Haemoglobin, WCC=White Cell Count. *Continuous independent variables were analysed on a continuous basis for the purposes of univariate logistic regression. The odds ratio (OR) in this situation reflects the proportional increased risk (OR>1) or reduced risk (OR<1) of leak associated with each incremental unit increase in the independent variable in question.
Risk Score Based on Pre-operative WCC and Platelet Count
The median preoperative white cell and platelet counts were 5.4 (IQR=4.5 to 6.9) × 109/l and 240 (IQR=204 to 284) × 109/l respectively.
One patient was leucopaenic (<3.5 × 109/l) at the time of surgery and no patients were thrombocytopaenic (<150 × 109/l). Table 4 demonstrates the results of a simple additive risk score using the preoperative white cell and platelet counts.
| Additive score based on: WCC ≤ 4.5=1/WC>4.5=0 Platelets ≤ 250=1/ Platelets> 250=0 | |
| Preoperative risk score | Frequency of anastomotic leak |
| 0 | 1/19 (5%) |
| 1 | 5/18 (28%) |
| 2 | 7/11 (64%) |
Table 4: Preoperative haematological score to risk stratify likelihood of anastomotic leak.
This suggests that concurrent use of both preoperative white cell and platelet counts may allow a significant degree of risk stratification for likelihood of anastomotic leak within this patient group.
Preoperative platelet count and tumour histology
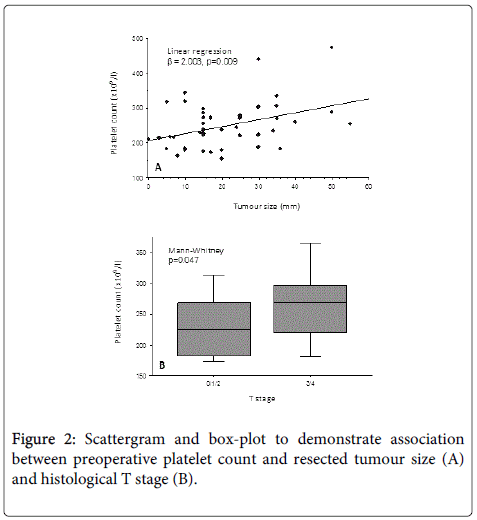
Figure 2 demonstrates the influence of resected tumour size and T stage on preoperative platelet count. This suggests that increasing tumour size and more advanced T stage were both associated with a trend towards a higher preoperative platelet count at the time of surgery.
On multiple linear regression, tumour size remained significantly associated with platelet count (t-value=2.462, p=0.018) while T stage was of borderline significance (t-value=1.826, p=0.075). No associations between the preoperative white cell count and resected histological tumour characteristics were demonstrated. There was no association between type of neoadjuvant therapy received (ie. long vs. short course) and preoperative white cell (Mann-Whitney, p=0.914) or platelet counts (Mann-Whitney, p=0.383).
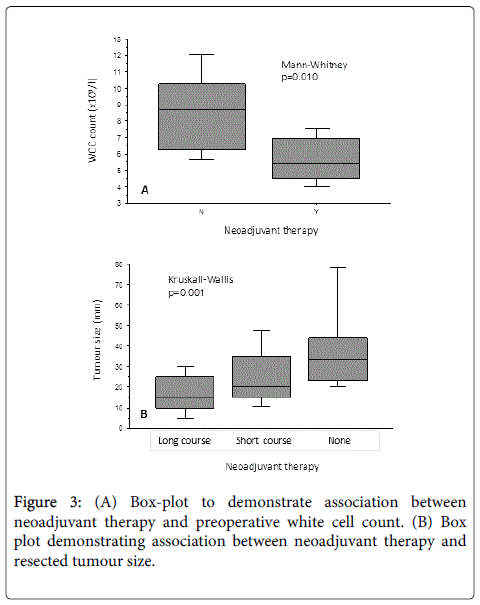
However, when comparing the preoperative full blood count between those cases who did (n=48) and did not (n=12) receive any form of neoadjuvant therapy, there was a significantly lower white cell count at the time of surgery in patients who received neoadjuvant treatment (Figure 3A - Mann-Whitney, p=0.010).
Figure 3B demonstrates the expected downstaging trend of smaller resected tumours in patients undergoing long-course chemoradiotherapy when compared with short-course and no neoadjuvant therapy.
Anastomotic leak and stoma reversal
Of the overall group of 60 patients (including those who did not receive neoadjuvant therapy) there were 14 anastomotic leaks in total (23%). Six of these 14 patients (43%) eventually required a permanent stoma compared with 8 (17%) of the remaining 46 patients who did not develop a leak (Fisher’s exact, p=0.058). The median time interval from surgery to reversal of stoma was 4.5 months. Of those patients whose stomas were reversed, anastomotic leak was strongly associated with a significantly prolonged time interval to stoma reversal (Cox regression, p<0.001).
Discussion
Anastomotic healing following low anterior resection is dependent on numerous physiological and surgical considerations. Despite optimal surgical technique, adequate vascularity and tension-free anastomotic construction, several preoperative and patient-related factors may conspire to impair healing and result in anastomotic breakdown. Neoadjuvant therapy has been associated with an increased risk of anastomotic complications in rectal surgery [1]. Mucosal hyperaemia and acute tissue oedema are common early findings following radiotherapy while the development of subsequent obliterative endartertitis and submucosal fibrosis can result in rectal wall thickening, reduced rectal compliance and relative tissue hypoxia [15] which may all contribute to impaired anastomotic healing. There is a strong evidence base to support the routine use of defunctioning stomas in low anterior resections. Previous meta-analyses have demonstrated reduced risk of anastomotic leak and early re-laparotomy when defunctioning is undertaken alongside resection [9,10]. However, use of defunctioning stomas are not without short-term complications [16] and can be associated with adverse quality of life outcomes for patients following rectal cancer surgery [17].
Previous studies evaluating risk of anastomotic leak following anterior resection typically comprise heterogenous cohorts of patients with regard to level of anastomosis, neoadjuavnt therapy and use of defunctioning stomas with reported anastomotic leakage rates typically in the region of 10% to 20%. In addition, most studies commonly only quote the rate of ‘symptomatic’ or ‘clinically relevant’ anastomotic leak. In the present study we sought to control for these confounding factors by only selecting patients undergoing both neoadjuvant therapy and subsequent defunctioning stoma alongside low anterior resection. When reviewing the rate of all cases of anastomotic leakage within our patient group (symptomatic and asymptomatic), the Figure of 27% appears to be above the upper range of what would be expected. However, it is important to appreciate that in the context of rectal surgery, cases of anastomotic leakage in reality comprise an entirely disparate spectrum of clinical entities ranging from major leak resulting in early life-threatening sepsis necessitating re-laparotomy, to an entirely asymptomatic pre-sacral collection which resolves with conservative management and is only diagnosed on follow-up contrast imaging. Of the anastomotic leaks identified within our study cohort (n=13), only a minority were diagnosed during the initial postoperative in-patient admission (n=5) and only three cases required intervention in the short term with no patients requiring re-laparotomy. It would, therefore, be reasonable to conclude that the symptomatic leak rate in this patient cohort rate was 10% (5/48). When considering the preponderance of male patients in this cohort, the fact that all cases received neoadjuvant treatment and the types of leak identified, the observed anastomotic leak rate is in line with published data for this high risk group. The median time interval from resection to diagnosis of leak was 8 weeks and previous studies have also demonstrated that anastomotic leakage is commonly only diagnosed at a late stage following surgery [11,18]. Although the majority of leaks we identified were asymptomatic, patients who developed any leak waited longer for subsequent stoma reversal and were more likely to end up requiring a permanent stoma in accordance with previous literature [19]. Therefore, identifying and reporting these asymptomatic cases is clinically relevant in any studies evaluating anastomotic leak as an end-point in rectal cancer surgery.
Several studies have demonstrated that early postoperative increases in serum inflammatory markers predict anastomotic leak following colorectal resections [20]. To our knowledge, this is the first study to investigate the potential impact of preoperative white cell and platelet counts on likelihood of anastomotic leak. Platelet degranulation and white cell migration represent key steps in early wound healing and it is an entirely intuitive finding that patients who developed an anastomotic leak exhibited a trend towards lower circulating levels of white cells and platelets at the time of surgery. However, only marginal differences within the normal reference range for each parameter were observed between the two groups and this potentially suggests that only a minor degree of myelosuppression at the time of surgery may contribute to impaired anastomotic healing. This assertion is also supported by the finding that the median preoperative white cell count was higher in the small patient cohort who did not receive neoadjuavnt therapy when compared with those who did.
Significant (grade 3 or 4) haematological toxicity is generally very unusual with short-course neoadjuvant radiotherapy and is only seen in around 5% of patients undergoing long-course chemoradiotherapy [21]. Within the present study group, only one patient was found to be leucopaenic at the time of surgery and no patients were thrombocytopaenic. Due to the retrospective nature of this study we were unable to reliably ascertain the degree of any significant haematological toxicity associated with neoadjuvant therapy. However, on reviewing all the clinical data available, no record of any delays in surgery were recorded as a result of toxicity secondary to neoadjuvant treatment.
Over recent years, several studies have investigated the prognostic impact of preoperative haematological indices on patient survival following colorectal resections for malignancy. Elevated circulating platelet and neutrophil counts along with lower lymphocyte counts are widely shown to be associated with adverse survival outcomes following colorectal cancer resections [12,13] and similar trends have been demonstrated in a number of other gastrointestinal malignancies. Thrombocytosis, neutrophilia and lymphocytopaenia represent components of the patient’s host inflammatory response to malignancy with a more marked inflammatory response reflecting adverse tumour biology. This is the result of a complex set of inflammatory and pro-thrombotic mechanisms occurring within the tumour micro-vasculature involving platelet-cancer cell and platelet-leucocyte interactions [22,23].
In the present study, histologically larger tumours were found to be associated with higher preoperative platelet counts and this finding is concordant with existing literature [24]. Given the previous association between low platelet counts and anastomotic leak, this implies the potential for a counter-intuitive situation of patients with smaller tumours being more likely to experience a leak. This pattern of findings, however, is most likely explained on the basis that patients with smaller tumours at the time of surgery experienced a greater degree of downstaging and that more extensive concurrent background fibrosis within the rectal wall is what results in impaired anastomotic healing in these cases. There was a clear trend towards increasing duration of neoadjuvant therapy being associated with smaller resected tumours indicating a more pronounced downstaging effect with long-course treatment as would be expected [25].
Given the additional anxiety that many rectal cancer patients experience regarding the prospect of managing stomas, it is essential to provide patients with an accurate and honest appraisal of the risks of anastomotic leak and consequent risk of permanent stoma as part of the consent process prior to surgery. The findings from this study indicate that patients undergoing low anterior resection with neoadjuvant radiotherapy represent a special group who are at particularly high risk of anastomotic complications despite use of defunctioning stomas. The results also suggest that preoperative white cell and platelet counts may allow a significant degree of risk stratification for anastomotic leak and may possibly facilitate the process of identifying a patient sub-group prior to surgery that would be most appropriately managed with a permanent colostomy as opposed to a low anastomosis. The relatively small patient cohort and retrospective nature of this study precludes any definitive conclusions being drawn. However, these findings merit prospective evaluation in a larger cohort of patients. Alongside this, an evaluation of additional preoperative inflammatory and thrombotic parameters which were not evaluable from the available data collected in the present study (ie. serum C-reactive protein, albumin, fibrinogen, etc) may possibly also yield supplementary predictive information as to the risk of anastomotic complications.
References
- Matthiessen P, Hallböök O, Andersson M, Rutegård J, Sjödahl R (2004) Risk factors for anastomotic leakage after anterior resection of the rectum.Colorectal Dis 6: 462-469.
- Peeters KC, Tollenaar RA, Marijnen CA, Klein Kranenbarg E, Steup WH, et al. (2005) Risk factors for anastomotic failure after total mesorectal excision of rectal cancer.Br J Surg 92: 211-216.
- Matthiessen P, Hallböök O, Rutegård J, Simert G, Sjödahl R (2007) Defunctioning stoma reduces symptomatic anastomotic leakage after low anterior resection of the rectum for cancer: a randomized multicenter trial.Ann Surg 246: 207-214.
- Paun BC, Cassie S, MacLean AR, Dixon E, Buie WD (2010) Postoperative complications following surgery for rectal cancer.Ann Surg 251: 807-818.
- Snijders HS, van den Broek CB, Wouters MW, Meershoek-Klein Kranenbarg E, Wiggers T, et al. (2013) An increasing use of defunctioning stomas after low anterior resection for rectal cancer. Is this the way to go?Eur J Surg Oncol 39: 715-720.
- Law WI, Chu KW, Ho JW, Chan CW (2000) Risk factors for anastomotic leakage after low anterior resection with total mesorectal excision.Am J Surg 179: 92-96.
- Kang CY, Halabi WJ, Chaudhry OO, Nguyen V, Pigazzi A, et al. (2013) Risk factors for anastomotic leakage after anterior resection for rectal cancer.JAMA Surg 148: 65-71.
- Park JS, Choi GS, Kim HR (2014) Multicentre analysis of risk factors for anastomotic leakage after laparoscopic rectal cancer excision: the Korean laparoscopic colorectal surgery study group. Ann Surg 257: 665-671.
- Hüser N, Michalski CW, Erkan M, Schuster T, Rosenberg R, et al. (2008) Systematic review and meta-analysis of the role of defunctioning stoma in low rectal cancer surgery.Ann Surg 248: 52-60.
- Tan WS, Tang CL, Shi L, Eu KW (2009) Meta-analysis of defunctioning stomas in low anterior resection for rectal cancer.Br J Surg 96: 462-472.
- Floodeen H, Hallböök O, Rutegård J, Sjödahl R, Matthiessen P (2013) Early and late symptomatic anastomotic leakage following low anterior resection of the rectum for cancer: are they different entities?Colorectal Dis 15: 334-340.
- Proctor MJ, Morrison DS, Talwar D, Balmer SM, Fletcher CD, et al. (2011) A comparison of inflammation-based prognostic scores in patients with cancer. A Glasgow Inflammation Outcome Study.Eur J Cancer 47: 2633-2641.
- Ishizuka M, Nagata H, Takagi K, Iwasaki Y, Kubota K (2013) Combination of platelet count and neutrophil to lymphocyte ratio is a useful predictor of postoperative survival in patients with colorectal cancer.Br J Cancer 109: 401-407.
- Rahbari NN, Weitz J, Hohenberger W, Heald RJ, Moran B, et al. (2010) Definition and grading of anastomotic leakage following anterior resection of the rectum: a proposal by the International Study Group of Rectal Cancer.Surgery 147: 339-351.
- Mancini ML, Sonis ST (2014) Mechanisms of cellular fibrosis associated with cancer regimen-related toxicities. Front Pharmacol 27: 51.
- Caricato M, Ausania F, Ripetti V, Bartolozzi F, Campoli G, et al. (2007) Retrospective analysis of long-term defunctioning stoma complications after colorectal surgery.Colorectal Dis 9: 559-561.
- Yau T, Watkins D, Cunningham D, Barbachano Y, Chau I, et al. (2009) Longitudinal assessment of quality of life in rectal cancer patients with or without stomas following primary resection.Dis Colon Rectum 52: 669-677.
- Caulfield H, Hyman NH (2013) Anastomotic leak after low anterior resection: a spectrum of clinical entities.JAMA Surg 148: 177-182.
- Lindgren R, Hallböök O, Rutegård J, Sjödahl R, Matthiessen P (2011) What is the risk for a permanent stoma after low anterior resection of the rectum for cancer? A six-year follow-up of a multicenter trial.Dis Colon Rectum 54: 41-47.
- Platt JJ, Ramanathan ML, Crosbie RA, Anderson JH, McKee RF, et al. (2012) C-reactive protein as a predictor of postoperative infective complications after curative resection in patients with colorectal cancer. Ann Surg Oncol 4168-4177.
- Sauer R, Becker H, Hohenberger W (2004) Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 351: 1731-1740.
- Smyth SS, McEver RP, Weyrich AS, Morrell CN, Hoffman MR, et al. (2009) Platelet functions beyond hemostasis.J Thromb Haemost 7: 1759-1766.
- Ware J, Corken A, Khetpal R (2013) Platelet function beyond hemostasis and thrombosis.Curr Opin Hematol 20: 451-456.
- Sasaki K, Kawai K, Tsuno NH, Sunami E, Kitayama J (2012) Impact of preoperative thrombocytosis on the survival of patients with primary colorectal cancer.World J Surg 36: 192-200.
- Den Dulk M, Smit M, Peeters KC (2007) A multivariate analysis of limiting factors for stoma reversal in patients with rectal cancer entered into the total mesorectal excision (TME) trial: a retrospective study. Lancet Oncol 8: 297-303.
Relevant Topics
- Constipation
- Digestive Enzymes
- Endoscopy
- Epigastric Pain
- Gall Bladder
- Gastric Cancer
- Gastrointestinal Bleeding
- Gastrointestinal Hormones
- Gastrointestinal Infections
- Gastrointestinal Inflammation
- Gastrointestinal Pathology
- Gastrointestinal Pharmacology
- Gastrointestinal Radiology
- Gastrointestinal Surgery
- Gastrointestinal Tuberculosis
- GIST Sarcoma
- Intestinal Blockage
- Pancreas
- Salivary Glands
- Stomach Bloating
- Stomach Cramps
- Stomach Disorders
- Stomach Ulcer
Recommended Journals
Article Tools
Article Usage
- Total views: 14689
- [From(publication date):
February-2015 - Jul 11, 2025] - Breakdown by view type
- HTML page views : 10107
- PDF downloads : 4582